b Collaborative Innovation Center of Chemical Science and Engineering (Tianjin), Tianjin 300072, China;
c Tianjin Bohai Fine Chemical Co., Ltd., Tianjin 300072, China;
d Tianjin Engineering Research Center of Functional Fine Chemicals, Tianjin 300072, China
Organic fluorescent materials are under profound research and developing very rapidly owing to the diversity and flexibility of their chemical structures [1]. However, many fluorescent materials suffer from the notorious aggregation-caused quenching (ACQ) effect which seriously limits their applications [2]. Fortunately, aggregation-induced emission (AIE) effect was found by Tang at 2001 [3]. These AIE compounds were non-/weakly emissive in solution but strongly emissive upon aggregation. This exciting phenomenon can be attributed to the restriction of intramolecular rotation (RIR) [4-8]. Since then, AIE-active fluorescent materials were intensively investigated by virtue of their tunable luminescence upon external stimuli such as heat, solvent-vapor, pressure, light, etc. [9]. Therefore, they displayed wide practical applications, such as organic light-emitting devices, chemo/biosensors, bioimaging sensors, smart windows, information displays and memory devices [10-13]. In which, piezofluorochromic (PFC) materials have attracted more attention due to their special optical properties [14-20]. PFC materials are a kind of material which displays color change in response to mechanical force (pressing or grinding) [21, 22].
Recently, some AIE-active fluorophores such as silole [23, 24], triphenylethene derivatives [25-27], cyano-substituted diarylethenes [28-30], diphenyl-dibenzofulvenes [31], 1, 1, 2, 2-tetraphenylethene (TPE) [32-34], and 9, 10-divinylanthracenes [35-41], were reported as promising PFC candidates. Compared with other compounds, 9, 10-divinylanthracene and its derivatives could present more remarkable AIE effect and more obvious color changes upon external stimuli [40]. However, the development of these PFC materials was still at the initial stage and the understanding of PFC phenomenon was not sufficient. Therefore, it is significant to further study on the PFC properties of 9, 10-divinylanthracene derivatives.
So far, several 9, 10-divinylanthracene derivatives (e.g. FLA-Cn [38], DSC-OCn [42] and PT-Cn [43]) exhibiting alkyl lengthdependent PFC behaviors have been reported. Normally, the longer the alkyl chain was, the larger the PFC spectral shift exhibited.However, ACZn [44] with shorter alkyl chain afforded larger remarkable grinding-induced spectral shifts. This was a special phenomenon for N-alkyl length-dependent PFC materials without any reasonable explanation.
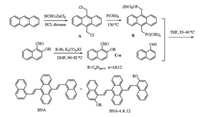
In this paper, a series of 9, 10-bis[2-(2-alkoxynaphthalen-1-yl) vinyl]anthracene derivatives (BNAs, Scheme 1) were designed and synthesized to examine the effect of steric hindrance of orthoposition alkoxyl chain on their PFC behaviors. UV-vis spectrophotometer and photoluminescence spectra were employed to characterize the fluorescence properties and PFC behaviors of BNAs. Moreover, the mechanism of BNAs' PFC behaviors was also studied and the influence of alkoxyl chain' length on their PFC behaviors were characterized by powder wide-angle X-ray diffraction (PXRD). Furthermore, the thermal properties were measured by thermal gravimetric analysis (TGA) and differential scanning calorimetry (DSC) experiments. Based on these measurements, we found BNAs possessed similar PFC behaviors as ACZn. Meanwhile, these results and the mechanism we put forward would be helpful to further understand the AIE-active PFC phenomenon and promote the applications of 9, 10-divinylanthracene derivatives in many fields.
|
Download:
|
| Scheme1. Synthesis for BNA, BNA-4, BNA-8, BNA-12. | |
2. Results and discussion 2.1. Synthesis of the BNAs
As described in Scheme 1, compounds B and C-n were synthesized according to the reported method [45]. Then the Wittig reaction of Wittig reagent B and the corresponding aromatic aldehydes in THF provided the target compounds (BNAs) [46]. Their structures were confirmed by 1H NMR, 13C NMR and MALDIMS analysis.
2.2. UV-vis absorption spectraAs shown in Fig. S1 in Supporting information, their UV-vis absorption bands were quite similar, the maximum UV-vis absorption peaks (λabmax) of BNA, BNA-4, BNA-8 and BNA-12 in THF solution were located at 413 nm, 410 nm, 412 nm and 412 nm respectively. This result indicated that the length of alkoxyl chain actually presented little effect on λabmax. However, due to the increase of n-π conjugation between auxochromous group (-OR) and the conjugated system, the peak occurring at 312 nm of BNA shifted to 341 nm with intensity decrease.
2.3. AIE effectIt was found that these compounds emitted strong yellow light under 365 nm UV lamp in solid state, but emitted no visible fluorescence in pure THF. This indicated that they were typical AIEactive materials. In order to study the AIE effect and fluorescent behaviors of BNAs, THF and water were used as a good and poor solvent, respectively. As described in Fig. 1, when water fraction (fw) ≤ 50%, BNA solution was practically non-fluorescent with almost unchanged photoluminescence (PL) intensity. It could be explained by the free rotation of naphthalene ring around C—C single bond, leading to a non-radiative relaxation pathway [47, 48]. With the increase of fw, the fluorescence intensity of BNA solution was gradually enhanced and reached the maximum at fw = 70%, which was about 15-fold higher than that in pure THF. The results could be ascribed to the RIR mechanism [4, 5]. With the increase of water fraction, BNA molecules began to aggregate into nanoparticles which hindered the free rotation of naphthalene ring. This process could further lead to radiative relaxation and increase fluorescence intensity. The other BNAs displayed similar behaviors. The fluorescence intensity of BNA-4 also achieved the maximum at fw = 70%, and approximately 40-fold higher than that in pure THF. Similarly, the PL intensity of BNA-8 at fw = 70% was 88-fold higher while the PL intensity of BNA-12 at fw = 60% was18-fold higher than that in pure THF.

|
Download:
|
| Fig. 1. PL spectra of the compounds in water/THF with different water fractions (a) BNA; (b) BNA-4; (c) BNA-8; (d) BNA-12; (concentration, 10 μmol/L; excitation wavelength, BNA:413 nm; BNA-4: 410 nm; BNA-8: 412 nm; BNA-12: 412 nm). The insets depict the changes in PL peak intensity and emission images of the compounds in different water fraction under 365 nm UV illumination. | |
When adding water into the THF solution, molecules were ready to orderly pack into crystalline and result in boosted light emission. However, further increasing the water fraction might make the solute molecules aggregate in a fast way. The crystal particles might be accompanied by the formation of amorphous particles to yield a decreased PL intensity. In this regard, the PL intensity was influenced by the ratio of crystalline and amorphous states of these compounds [48-54]. This assumption could be supported by scanning electronic microscopy (SEM) images of BNA. As showed in (b) of Fig. 2, the molecules mainly aggregated into acicular crystal at 60% water fraction, while amorphous particles at 90% water fraction described in (c).

|
Download:
|
| Fig. 2. SEM images of BNA formed from THF/water mixtures. (a) fw = 0%; (b) fw = 60%; (c) fw = 90%. | |
2.4. PFC behaviors
The solid-state fluorescence responses of BNAs towards external pressure were illustrated in Fig. 3. The insets showed the fluorescence images of BNA, BNA-4, BNA-8, BNA-12 under 365 nm UV lamp. It was found that BNA and BNA-8 emitted green-yellow light originally while BNA-4 and BNA-12 presented yellow light. It was obvious that all of them changed to orange after writing a letter "T" by a spatula. The results indicated that these samples were sensitive to external force stimuli and exhibited a red-shifted fluorescence upon external force. In general, AIE compounds usually adopted twisted conformation and formed a loose packing mode. Therefore, the destruction of crystal lattice by mechanical force could lead to the release of binding energy, the planarization of the molecular conformation and the increase of intermolecular π-π overlap. This procedure could result in the red shift of the PL spectrum [55, 56].

|
Download:
|
| Fig. 3. Normalized fluorescence emission spectra of (a) BNA, (b) BNA-4, (c) BNA-8, (d) BNA-12 mixed with KBr upon pressing, annealing. The insets depict the fluorescence images of BNA, BNA-4, BNA-8, BNA-12 under 365 nm UV lamp after pressing and annealing. | |
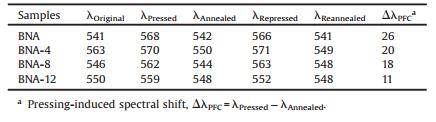
According to the spectra shown in Fig. 3 and the corresponding spectroscopic data summarized in Table 1, when the samples were pressed, the red shifts of BNAs' fluorescence emission wavelength occurred to some extent. However, the maximum emission peaks of BNA, BNA-8 and BNA-12 were recovered to their original state after annealing, except for BNA-4. This phenomenon could be explained by the metastable state of BNA-4. When the pristine samples of BNAs were synthesized and precipitated from THF, BNA, BNA-8 and BNA-12 were in microcrystalline states while BNA-4 would have a small portion of amorphous particles, which meant BNA-4 were in a metastable state. When the pressed samples of BNAs were annealed, the pressed amorphous state should be totally recrystallized and recovered to micro-crystalline state including the initially amorphous parts of BNA-4 [9, 37, 38]. Therefore, the fluorescent peak of BNA-4 exhibited a blue shift compared with the original curve.
|
|
Table 1 Peak wavelengths (l/nm) of BNAs under various external stimuli. |
As described in Table 1, BNA exhibited the most remarkable bathochromic shift (△λPFC = λPressed -λAnnealed = 26 nm). Nevertheless, the △λPFC became smaller with the increase of alkoxyl chain' length (20 nm, 18 nm and 11 nm for BNA-4, BNA-8 and BNA-12, respectively). Based on these results, we found that the length of alkoxyl chain was quite important to their PFC behaviors. This phenomenon was possibly ascribed to the steric effect generated from the alkoxyl chain. Moreover, the steric effect could also inhibit the planarization and intermolecular π-π overlap of BNAs. Therefore, it was more difficult for the micro-crystalline of BNA-12 to transform into amorphous state than that of BNA under mechanical stimulus [2, 42-44, 56, 57].
2.5. The PFC mechanismTo further investigate the PFC mechanism of BNAs, PXRD experiments were conducted. As shown in Fig. 4, the sharp and intense reflections of the four original compounds indicated that they were in the micro-crystalline state [46-48]. Moreover, the ground BNAs displayed broad and weak diffraction peaks, indicating that some micro-crystalline was transformed into amorphous phase by grinding. However, the PXRD curves of BNA-4, BNA-8 and BNA-12 showed slight difference between the original and ground samples. The slight difference could be attributed to the steric effect endowed by the alkoxyl chain, which could partly hinder the transformation between the crystalline and amorphous state. Besides, the alkoxyl chain at ortho-position could impede the extent of intermolecular π-π overlap and the planarization of molecular conformation between the core anthracene ring and naphthalene rings when the samples were pressed. Because of this, the extent of red shift was reduced with the increasing of alkoxyl chain, especially for BNA-12. After the annealing process, some peaks of these curves were partly recovered [2, 46, 57]. In fact, during the PFC process, only the packing mode of AIE-active PFC materials changed rather than their chemical structure. In other words, mechanical stimuli only altered the intermolecular stacking mode and π-π interaction in the state of aggregation [21, 22]. These results demonstrated that the packing mode had a remarkable effect on PFC properties.

|
Download:
|
| Fig. 4. PXRD patterns of (a) BNA; (b) BNA-4; (c) BNA-8; (d) BNA-12; (1) original; (2) ground; (3) annealed. | |
2.6. Thermal performance
As we can see in Fig. S2 in Supporting information, these compounds exhibit nice thermostability before 350 ℃, but loss 10% weight at around 370-390 ℃. As illustrated in Fig. 5, DSC measurements showed that BNA and BNA-12 had only one endothermic peaks in the high-temperature zone which were considered as the isotropic melt transition (Tm), while BNA-4 had three and BNA-8 had two peaks. The Tm of these samples decreased from 310 ℃ to 127 ℃ with alkoxyl length increasing. Moreover, the Tm peaks of BNA, BNA-4, BNA-8 and BNA-12 were found to remain almost unchanged before and after grinding. However, the endothermic peaks of BNA-4 at 181 ℃ and BNA-8 at 107 ℃ were recognized as phase transition from crystalline to liquid crystal (TL) [2, 42, 45], and they shifted to 173 ℃ and 100 ℃ respectively after grinding. The profiles also became slightly flat and broad, which possibly meant they may locate in a metastable state. Based on these results, we found that the phase transition and its change upon grinding were not in order.

|
Download:
|
| Fig. 5. DSC curves of (a) BNA; (b) BNA-4; (c) BNA-8; (d) BNA-12; (1) ground; (2) original. | |
3. Conclusion
Four 9, 10-bis[2-(2-alkoxynaphthalen-1-yl)vinyl]anthracene derivatives, BNA, BNA-4, BNA-8 and BNA-12 were designed and synthesized. All of them were found to be AIE-active organic luminescent materials with PFC behaviors, in which, BNA displayed the largest PFC spectral shift (△λPFC = 26 nm). Moreover, the PFC properties were distinctly alkoxyl-length dependent. For BNAs, the longer the alkoxyl chain, the more remarkable PFC property they displayed. In other words, the length of alkoxyl chain at ortho-position could affect the planarization of molecular conformation and influence the intermolecular π-π overlap in aggregate state. Therefore, the length of alkoxyl chain could be a powerful tool to tune the PFC properties of 9, 10-bis[2-(2-alkoxynaphthalen-1-yl)vinyl]anthracene derivatives.
4. Experimental 4.1. Measurements1H NMR spectra and 13C NMR spectra were recorded on a Bruker Avance 400 MHz spectrometer with CDCl3 as the solvent.High-resolution mass spectra were acquired on the solariX MALDI-FTMS. Infrared Spectroscopy (IR) were recorded on a Nicolet 22 AVATAR 370 FT-IR spectrometer. The melting point was detected on TYT-3. UV-vis spectra were measured on the Evolution 300 UV-vis spectrophotometer. Photoluminescence spectra were recorded with a Hitachi F-2500 spectrophotometer and photoluminescence spectra of solid state were measured by Horiba Jobin Yvon Fluorolog-3 spectrophotometer. Scanning electronic microscopy (SEM) was taken on S4800 (Japan). Powder wide angle X-ray diffraction (PWXD) measurements were performed on a Miniflex 600 Powder X-ray diffractometer of Rigaku, operating at 40 V, 15 A, a rate of 4° min-1. Thermal gravimetric analysis (TGA) were acquired on TGA 128 and differential scanning calorimetry (DSC) experiments were carried out on a Perkin-Elmerat, both were with a heating rate of 10 ℃/min in a nitrogen atmosphere.
4.2. SynthesisCompound A: A mixture of anhydrous 1, 4-dioxane (100 mL), anthracene (8.90 g, 50.00 mmol) and paraformaldehyde (7.50 g, 250.00 mmol) was stirred at room temperature, then 200 mL concentrated hydrochloric acid was added dropwise to the reaction mixture over a period of 1 h. Then, the suspension was heated to refluxing for 3 h under nitrogen, after that, it was allowed to stand for 16 h to yield yellow precipitate, which was filtered and washed with dioxane (30 mL × 3), followed by recrystallization in toluene to afford compound A (8.44 g, yield: 60%). 1H NMR (400 MHz, CDCl3): δ 8.40-8.38 (m, 4H), 7.67-7.66 (m, 4H), 5.61 (s, 4H).
Compound B: Compound A (3.00 g, 11.00 mmol) and triethylphosphate (12 mL) were added into a flask and then vigorously stirred at 150 ℃ for 12 h. Then, it was cooled to room temperature and separated by column chromatography (EA: PE = 1:2) to obtain fine light yellow powder (3.31 g, yield: 62%). 1H NMR (400 MHz, CDCl3): δ 8.41-8.39 (m, 4H), 7.60-7.58 (m, 4H), 4.29 (s, 2H), 4.24 (s, 2H), 3.92-3.91 (m, 4H), 3.86-3.81 (m, 4H), 1.08 (t, 12H, J = 7.20 Hz).
Compound C-4: n-Butyl bromide (1.3 mL, 14.00 mmol) was added dropwise into a mixture of 2-hydroxy-1-naphthaldehyde (2.06 g, 12.00 mmol), K2CO3 (1.39 g, 15.00 mmol), N, N-dimethylformamide (30 mL) and KI (catalytic amount) over a period of 15 min. The reaction mixture was stirred and heated at 90-94 ℃ for 8 h, then cooled to room temperature, transferred to 500 mL beaker, diluted with 50 mL deionized water and extracted by dichloromethane (30 mL × 3). The organic phases were combined and washed by 1% NaOH (30 mL × 3) and deionized water (30 mL × 3), respectively. After that, the organic phase was dried over anhydrous MgSO4 and concentrated to reddish brown liquid (1.07 g, yield: 39%). C-8 and C-12 were similarly synthesized.
BNA: A suspension of compound B (0.30 g, 0.63 mmol), 1-naphthaldehyde (0.28 g, 1.80 mmol) and t-BuOK (1.80 g, 16.20 mmol) in THF (20 mL) was stirred under a nitrogen atmosphere at 35 ℃ for 12 h. The resulting precipitate was collected by filtration and washed with methanol (30 mL) to afford the yellow product (0.13 g, yield: 43%). Melting point: 309.4-311.8 ℃. 1H NMR (400 MHz, CDCl3): δ 8.58-8.55 (m, 4H), 8.23-8.21 (m, 2H), 8.15 (d, 2H, J = 6.80 Hz), 8.06 (d, 2H, J = 16.00 Hz), 7.94-7.96 (m, 4H), 7.82 (d, 2H, J = 16.40 Hz), 7.67 (t, 2H, J = 7.20 Hz), 7.57-7.54 (m, 8H); 13C NMR (100 MHz, CDCl3): δ 144.7, 133.9, 129.7, 128.9, 128.6, 127.7, 126.5, 126.1, 125.4, 124.0, 123.9, 122.5. MALDI-MS: calcd. for C38H26: 482.20290, [M]+ found 482.20286.
BNA-4: A mixture of compound B (0.30 g, 0.63 mmol), compound C-4 (0.43 g, 1.80 mmol) and t-BuOK (1.80 g, 16.20 mmol) in THF (20 mL) was stirred under a nitrogen atmosphere at 35 ℃ for 12 h. The product was filtrated and washed with methanol (30 mL) to give the orange-yellow product (0.18 g, yield: 46%). Melting point: 198.2-200.8 ℃. 1H NMR (400 MHz, CDCl3): δ 8.75-8.73 (m, 4H), 8.42 (d, 2H, J = 8.80 Hz), 8.08 (d, 2H, J = 16.80 Hz), 7.90 (d, 4H, J = 8.80 Hz), 7.58-7.56 (m, 6H), 7.45-7.40 (m, 6H), 4.33 (t, 4H, J = 6.50 Hz), 2.02-1.98 (m, 4H), 1.65-1.59 (m, 4H), 1.00 (t, 6H, J = 7.20 Hz); 13C NMR (100 MHz, CDCl3): δ 154.5, 133.9, 132.9, 131.7, 131.0, 129.7, 129.2, 128.9, 128.4, 126.9, 126.7, 125.1, 124.2, 123.6, 120.8, 114.4, 69.2, 31.9, 19.5, 13.9. MALDI-MS: calcd. for C46H42O2: 626.31793, [M]+ found 626.31792.
BNA-8 and BNA-12 were similarly synthesized.
BNA-8, yellow product (0.27 g, yield: 58%). Melting point: 135.9-137.0 ℃. 1H NMR (400 MHz, CDCl3): δ 8.75-8.72 (m, 4H), 8.41 (d, 2H, J = 8.80 Hz), 8.09 (d, 2H, J = 16.80 Hz), 7.90 (d, 4H, J = 8.80 Hz), 7.58-7.55 (m, 6H), 7.44 (m, 6H), 4.31 (t, 4H, J = 6.80 Hz), 2.04-1.95 (m, 4H), 1.40-1.21 (m, 20H), 0.83 (t, 6H, J = 6.80 Hz); 13C NMR (100 MHz, CDCl3): δ 154.5, 133.9, 132.9, 131.8, 130.9, 129.7, 129.2, 128.9, 128.5, 126.9, 126.7, 125.1, 124.2, 123.6, 120.8, 114.5, 69.6, 31.8, 29.9, 29.4, 29.2, 26.3, 22.6, 14.0. MALDI-MS: calcd. for C54H58O2: 738.44313, [M]+ found 738.44324.
BNA-12, orange-yellow product (0.26 g, yield: 48%). Melting point: 126.6-128.1 ℃. 1H NMR (400 MHz, CDCl3): δ 8.75-8.73 (m, 4H), 8.41 (d, 2H, J = 8.40 Hz), 8.09 (d, 2H, J = 16.80 Hz), 7.90 (d, 4H, J = 8.40 Hz), 7.58-7.55 (m, 6H), 7.47-7.41 (m, 6H), 4.31 (t, 4H, J = 6.40 Hz), 2.04-1.97 (m, 4H), 1.39-1.21 (m, 36H), 0.87 (t, 6H, J = 6.80 Hz); 13C NMR (100 MHz, CDCl3): δ 154.5, 133.9, 133.0, 131.8, 131.0, 129.7, 129.2, 128.9, 128.5, 126.9, 126.7, 125.1, 124.2, 123.5, 120.8, 114.4, 69.6, 31.9, 29.9, 29.6, 29.5, 29.4, 26.3, 22.7, 14.1. MALDIMS: calcd. for C62H74O2: 850.56833, [M]+ found 850.56893.
AcknowledgmentWe are grateful for the financial support from the National Natural Science Foundation of China (No. 21576194).
Appendix A. Supplementary dataSupplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.cclet.2017.02.018.
| [1] | G.F. Zhang, H.F. Wang, M.P. Aldred, et al., General synthetic approach toward geminal-substituted tetraarylethene fluorophores with tunable emission properties: X-ray crystallography, aggregation-induced emission and piezofluorochromism. Chem. Mater. 26 (2014) 4433–4446. DOI:10.1021/cm501414b |
| [2] | X.Q. Zhang, Z.G. Chi, B.J. Xu, et al., End-group effects of piezofluorochromic aggregation-induced enhanced emission compounds containing distyrylanthracene. J. Mater. Chem. 22 (2012) 18505–18513. DOI:10.1039/c2jm33140c |
| [3] | J.D. Luo, Z.L. Xie, J.W.Y. Lam, et al., Aggregation-induced emission of 1-methyl-1, 2, 3, 4, 5-pentaphenylsilole. Chem. Commun. (2001) 1740–1741. |
| [4] | R. Misra, T. Jadhav, B. Dhokale, et al., Reversible mechanochromism and enhanced AIE in tetraphenylethene substituted phenanthroimidazoles. Chem. Commun. 50 (2014) 9076–9078. DOI:10.1039/C4CC02824D |
| [5] | L.Q. Yan, Z.N. Kong, Y. Xia, et al., A novel coumarin-based red fluorogen with AIE, self-assembly, and TADF properties. New J. Chem. 40 (2014) 7061–7067. |
| [6] | Q. Zhao, L. Li, F.Y. Li, et al., Aggregation-induced phosphorescent emission (AIPE) of iridium (Ⅲ) complexes. Chem. Commun. 6 (2008) 685–687. |
| [7] | F. Wang, X. Li, S. Wang, et al., New p-conjugated cyanostilbene derivatives: synthesis, characterization and aggregation-induced emission. Chin. Chem. Lett. 27 (2016) 1592–1596. DOI:10.1016/j.cclet.2016.04.020 |
| [8] | K. Duraimurugan, J. Sivamani, M. Sathiyaraj, et al., Piezoflurochromism and aggregation induced emission properties of 9, 10-bis (trisalkoxystyryl) anthracene derivatives. J. Fluoresc. 26 (2016) 1211–1218. DOI:10.1007/s10895-016-1805-4 |
| [9] | R.R. M, C.W. Liao, W.L. Su, et al., Quinoxaline based D-A-D molecules: high contrastreversible solid-state mechano-and thermo-responsive fluorescent materials. J. Mater. Chem. C 1 (2013) 5491–5501. DOI:10.1039/c3tc31179a |
| [10] | J.W. Chung, S.J. Yoon, S.J. Lim, et al., A general single-source route for the preparation of hollow nanoporous metal oxide structures. Angew. Chem. Int. Ed. 48 (2009) 7030–7034. DOI:10.1002/anie.v48:38 |
| [11] | T. Mutai, H. Tomoda, T. Ohkawa, et al., Switching of polymorph-dependent ESIPT luminescence of an imidazo[1, 2-a]pyridine derivative. Angew. Chem. Int. Ed. 47 (2008) 9522–9524. DOI:10.1002/anie.v47:49 |
| [12] | Y. Dong, J.W.Y. Lam, A. Qin, et al., Switching the light emission of (4-biphenylyl) phenyldibenzofulvene bymorphological modulation: crystallization-induced emission enhancement. Chem. Commun. (2007) 40–42. |
| [13] | Y. Zhao, H. Gao, Y. Fan, et al., Thermally induced reversible phase trasformations accompanied by emission switching between different colors of two aromatic-amine compounds. Adv. Mater. 21 (2009) 3165–3169. DOI:10.1002/adma.v21:31 |
| [14] | Y. Sagara, T. Kato. Mechanically induced luminescence changes in molecular assemblies. Nat. Chem. 1 (2009) 605–610. DOI:10.1038/nchem.411 |
| [15] | W. Christoph. Mechanoresponsive materials. J. Mater. Chem. 21 (2011) 8235–8236. DOI:10.1039/c1jm90068d |
| [16] | Z.G. Chi, X.Q. Zhang, B.J. Xu, et al., Recent advances in organic mechanofluorochromic materials. Chem. Soc. Rev. 41 (2012) 3878–3896. DOI:10.1039/c2cs35016e |
| [17] | K. Mizuguchi, H. Nakano. Mechanofluorochromism of 4-[bis(9, 9-dimethylfluoren-2-yl)amino]benzaldehyde. Dyes Pigm. 96 (2013) 76–80. DOI:10.1016/j.dyepig.2012.08.006 |
| [18] | Y. Ooyama, G. Ito, H. Fukuoka, et al., Mechanofluorochromism of heteropolycycl-icdonorep-acceptor type fluorescent dyes. Tetrahedron 66 (2010) 7268–7271. DOI:10.1016/j.tet.2010.07.018 |
| [19] | J. Liang, F. Hu, X.Y. Lv, et al., Synthesis, characterization and mechanochromic behavior of binuclear gold (Ⅰ) complexes with various diisocyano bridges. Dyes Pigm. 95 (2012) 485–490. DOI:10.1016/j.dyepig.2012.06.014 |
| [20] | K. Mizuguchi, H. Kageyama, H. Nakano. Mechanochromic luminescence of 4-[bis(4-methylphenyl)amino]benzaldehyde. Mater. Lett. 65 (2011) 2658–2661. DOI:10.1016/j.matlet.2011.05.068 |
| [21] | W. Liu, Y.L. Wang, M.X. Sun, et al., Alkoxy-position effects on piezofluorochromism and aggregation-induced emission of 9, 10-bis(alkoxystyryl) anthracenes. Chem. Commun. 49 (2013) 6042–6044. DOI:10.1039/c3cc42636j |
| [22] | G.F. Zhang, P. Matthew, Aldred, et al., Efficient green-red piezofluorochromism of bisanthracene-modified dibenzofulvene. RSC Adv. 5 (2015) 1079–1082. DOI:10.1039/C4RA10067K |
| [23] | Y.N. Hong, A. Jacky, W.Y. Lama, et al., Aggregation-induced emission: phenomenon, mechanism and applications. Chem. Commun. (2009) 4332–4353. |
| [24] | J.Z. Liu, W. Jacky, Y. Lam, et al., Aggregation-induced emission of silole molecules and polymers: fundamental and applications. J. Inorg. Organomet. Polym. Mater. 19 (2009) 249–285. DOI:10.1007/s10904-009-9282-8 |
| [25] | J.W. Chen, B. Xu, X.Y. Ouyang. Aggregation-induced emission of cis, cis-1, 2, 3, 4-tetraphenylbutadiene from restricted intramolecular rotation. J. Phys. Chem. A 108 (2004) 7522–7526. DOI:10.1021/jp048475q |
| [26] | H. Tong, Y.Q. Dong, M. Haussler, et al., Tunable aggregation-induced emission of diphenyldibenzofulvenes. Chem. Commun. (2006) 1133–1135. |
| [27] | Z.J. Ning, Z. Chen, Q. Zhang, et al., Aggregation-induced emission (AIE)-active starburst triarylamine fluorophores as potential non-doped red emitters for organic light-emitting diodes and Cl2 gas chemodosimeter. Adv. Funct. Mater. 17 (2007) 3799–3807. DOI:10.1002/(ISSN)1616-3028 |
| [28] | S.J. Yoon, S.Y. Park. Polymorphic and mechanochromic luminescence modulation in the highly emissive dicyanodistyrylbenzene crystal: secondary bonding interaction in molecular stacking assembly. J. Mater. Chem. 21 (2011) 8338–8346. DOI:10.1039/c0jm03711g |
| [29] | Y. Zhang, G. Zhuang, M. Ouyang, et al., Mechanochromic and thermochromic fluorescent properties of cyanostilbene derivatives. Dyes Pigm. 98 (2013) 486–492. DOI:10.1016/j.dyepig.2013.03.017 |
| [30] | X. Zhang, Z. Ma, Y. Yang. Fine-tuning the mechanofluorochromic properties of benzothiadiazole-cored cyano-substituted diphenylethene derivatives through D-A effect. J. Mater. Chem. C 2 (2014) 8932–8938. DOI:10.1039/C4TC01457J |
| [31] | X. Luo, J. Li, C. Li, et al., Reversible switching of the emission of diphenyldibenzofulvenes by thermal and mechanical stimuli. Adv. Mater. 23 (2011) 3261–3265. DOI:10.1002/adma.201101059 |
| [32] | X. Zhou, H. Li, Z. Chi, et al., Piezofluorochromism and morphology of a new aggregation-induced emission compound derived from tetraphenylethylene and carbazole. New J. Chem. 36 (2012) 685–693. DOI:10.1039/C1NJ20782B |
| [33] | N. Zhao, Z. Yang, J.W. Lam. Benzothiazolium-functionalized tetraphenylethene: an AIE luminogen with tunable solid-state emission. Chem. Commun. 48 (2012) 8637–8639. DOI:10.1039/c2cc33780k |
| [34] | B. Xu, J. He, Y. Mu, et al., Very bright mechanoluminescence and remarkable mechanochromism using a tetraphenylethene derivative with aggregationinduced emission. Chem. Sci. 6 (2015) 3236–3241. DOI:10.1039/C5SC00466G |
| [35] | L. Bu, M. Sun, D. Zhang, et al., Solid-state fluorescence properties and reversible piezochromic luminescence of aggregation-induced emission-active 9, 10-bis [(9, 9-dialkylfluorene-2-yl)vinyl]anthracenes. J. Mater. Chem. C 1 (2013) 2028–2035. DOI:10.1039/c3tc00017f |
| [36] | X. Zhang, Z. Chi, J. Zhang, et al., Piezofluorochromic properties and mechanism of an aggregation-induced emission enhancement compound containing Nhexyl-phenothiazine and anthracene moieties. J. Phys. Chem. B 115 (2011) 7606–7611. DOI:10.1021/jp202112e |
| [37] | W. Liu, J. Wang, Y.Y. Gao, et al., 2, 6, 9, 10-Tetra(p-dibutylaminostyryl)anthracene as a multifunctional fluorescent cruciform dye. J. Mater. Chem. C 2 (2014) 9028–9034. DOI:10.1039/C4TC01680G |
| [38] | M. Zheng, D.T. Zhang, M.X. Sun, et al., Solid-state fluorescence properties and reversible piezochromic luminescence of aggregation-induced emissionactive 9, 10-bis[(9, 9-dialkylfluorene-2-yl)vinyl]anthracenes. J. Mater. Chem. C 2 (2014) 1913–1920. DOI:10.1039/c3tc32035a |
| [39] | X. Zhang, Z. Chi, X. Zhou, et al., Influence of carbazolyl groups on properties of piezofluorochromic aggregation-enhanced emission compounds containing distyrylanthracene. J. Phys. Chem. C 116 (2012) 23629–23638. DOI:10.1021/jp306452n |
| [40] | Y.J. Dong, B. Xu, J.B. Zhang, et al., Piezochromic luminescence based on the molecular aggregation of 9, 10-bis((E)-2-(pyrid-2-yl)vinyl)anthracene. Angew. Chem. Int. Ed. 51 (2012) 10782–10785. DOI:10.1002/anie.v51.43 |
| [41] | H. Li, X. Zhang, Z. Chi, et al., New thermally stable piezofluorochromic aggregation-induced emission compounds. Org. Lett. 13 (2011) 556–559. DOI:10.1021/ol102872x |
| [42] | W. Liu, Y.L. Wang, L.Y. Bu, et al., Chain length-dependent piezofluorochromic behavior of 9, 10-bis(p-alkoxystyryl)anthracenes. J. Lumin. 143 (2014) 50–55. |
| [43] | M. Zheng, M.X. Sun, Y.P. Li, et al., Piezofluorochromic properties of AIE-active 9, 10-bis(N-alkylphenothiazin-3-yl-vinyl-2) anthracenes with different length of alkyl chains. Dyes Pigm. 102 (2014) 29–34. DOI:10.1016/j.dyepig.2013.10.020 |
| [44] | Y.L. Wang, W. Liu, L.Y. Bu, et al., Reversible piezochromic luminescence of 9, 10-bis[(N-alkylcarbazol-3-yl)vinyl]anthracenes and the dependence on N-alkyl chain length. J. Mater. Chem. C 1 (2013) 856–862. DOI:10.1039/C2TC00470D |
| [45] | Y. Xiong, X.L. Yan, Y.W. Ma, et al., Regulating piezofluorochromism of 9, 10-bis (butoxystyryl)anthracenes by isomerization of butyl group. Chem. Commun. 51 (2015) 3403–3406. DOI:10.1039/C4CC10196K |
| [46] | C.X. Niu, Y. You, L. Zhao, et al., Solvatochromism, reversible chromism and selfassembly effects of heteroatom-assisted aggregation-induced enhanced emission (AIEE) compounds. J. Ouyang Chem. Eur. J. 21 (2015) 13983–13990. DOI:10.1002/chem.201501902 |
| [47] | X. Yao, J.X. Ru, C. Xu, et al., Multistimuli-responsive luminescence of naphthalazine based on aggregation-induced emission. ChemistryOpen 4 (2015) 478–482. DOI:10.1002/open.v4.4 |
| [48] | Y.Z. Liu, Y.X. Lei, F. Li, et al., Indene-1, 3-dionemethylene-4H-pyran derivatives containing alkoxy chains of various lengths: aggregation-induced emission enhancement, mechanofluorochromic properties and solvent-induced emission changes. J. Mater. Chem. C 4 (2016) 2862–2870. DOI:10.1039/C5TC02932E |
| [49] | X.Y. Shen, Y.J. Wang, E.G. Zhao, et al., Effects of substitution with donoracceptor groups on the properties of tetraphenylethene trimer: aggregationinduced emission, solvatochromism, and mechanochromism. J. Phys. Chem. C 117 (2013) 7334–7347. DOI:10.1021/jp311360p |
| [50] | Y.L. Lin, G. Chen, L.F. Zhao, et al., Diethylamino functionalized tetraphenylethenes: structural and electronic modulation of photophysical properties, implication for the CIE mechanism and application to cell imaging. J. Mater. Chem. C 3 (2015) 112–120. DOI:10.1039/C4TC02161D |
| [51] | Q.Y. Lu, X.F. Li, J. Li, et al., Influence of cyano groups on the properties of piezofluorochromic aggregation-induced emission enhancement compounds derived from tetraphenylvinyl-capped ethane. J. Mater. Chem. C 3 (2015) 1225–1234. DOI:10.1039/C4TC02165G |
| [52] | T. Jadhav, B. Dhokale, S.M. Mobin, et al., Aggregation induced emission and mechanochromism in tetraphenylethene substituted pyrazabole. RSC Adv. 5 (2015) 68187–68191. DOI:10.1039/C5RA12697E |
| [53] | M.D. Yang, D.L. Xu, W.G. Xi, et al., Aggregation-induced fluorescence behavior of triphenylamine-based schiff bases: the combined effect of multiple forces. J. Org. Chem. 78 (2013) 10344–10359. DOI:10.1021/jo401719c |
| [54] | Na Zhao, M. Li, Y.L. Yan, et al., A tetraphenylethene-substituted pyridinium salt with multiple functionalities: synthesis, stimuli-responsive emission, optical waveguide and specific mitochondrion imaging. J. Mater. Chem. C 1 (2013) 4640–4646. DOI:10.1039/c3tc30759j |
| [55] | X.Q. Zhang, Z.G. Chi, J.Y. Zhang, et al., Piezofluorochromic properties and mechanism of an aggregation-induced emission enhancement compound containing n-hexyl-phenothiazine and anthracene moieties. J. Phys. Chem. B 115 (2011) 7606–7611. DOI:10.1021/jp202112e |
| [56] | G.H. Yin, Y.W. Ma, Y. Xiong, et al., Enhanced AIE and different stimuli-responses in red fluorescent (1, 3-dimethyl)barbituric acid-functionalized anthracenes. J. Mater. Chem. C 4 (2016) 751–757. |
| [57] | Q.K. Qi, Y.F. Liu, X.F. Fang, et al., AIE (AIEE) and mechanofluorochromic performances of TPE-methoxylates: effects of single molecular conformations. RSC Adv. 3 (2013) 7996–8002. DOI:10.1039/c3ra40734a |
 2017, Vol. 28
2017, Vol. 28