Owing to the shortage of resources and global warming, sustainability is an emerging global issue of importance for humanity, and establishing a renewable chemical industry featuring renewable resources as the dominant feedstock rather than fossil resources is very important for the future [1, 2].
Nature produces 170 billion metric tons of biomass per year by photosynthesis, 75% of which can be assigned to carbohydrates. Surprisingly, only 3%-4% of these compounds are used by human for consumption and other purposes [3, 4]. Biomass is the most abundant renewable available resource, and is a promising alternative as a sustainable supply of biofuel and related valuable fine chemicals and is currently viewed as a feedstock for green chemical processes for the future [5]. Among available biomass resources, carbohydrates are regarded as some of the most important raw materials for the production of 5-HMF, a very versatile platform toward fuels and chemicals. Through selective oxidation, reduction, and rehydration of HMF, other useful intermediates, including bio-based polymers (2, 5-diformylfuran and 2, 5-furandicarboxylic acid), fuels (2, 5-dimethylfuran), and solvents such as levulinic acid (LA) can be obtained [6, 7]. In general, 5-HMF is produced from monosaccharides including fructose and glucose, which are not economical substrates because of their low availability and because their high costs limit their further applications. Therefore, the ability to produce 5-HMF from cellulose or other non-edible polysaccharides is desirable. Unfortunately, the low 5-HMF yields and expensive and toxic catalytic systems involved with using these polysaccharides as substrates are problems that remain unsolved. Sucrose (α-D-glucopyranosyl-(1→2)-β-D-fructofuranoside) is an inexpensive chemical produced in sugar cane and sugar beet cultivation, and consists of one fructose and one glucose moiety linked together by a glycosidic bond. Compared to cellulose, its bond is much more easily hydrolyzed and it produces higher 5-HMF yields when used as a substrate [8]. Although high yields of 5-HMF have been achieved with fructose [9, 10], chemical and/or biochemical transformations that convert sucrose to highly valuable synthetic intermediates such as 5-HMF, bioethylene, 1, 2-propylene glycol, and LA are more commercially feasible [11, 12]. There is no doubt that the direct use of abundantly available sucrose for the largescale production of 5-HMF would be more ideal. However, it has been observed in literature that less attention has been paid to the conversion of sucrose to platform chemicals than to the conversions of fructose and other sugars. Chun et al. achieved a 5-HMF yield of 82.0 ± 3.9 wt% using a reaction mixture containing 5 g of [MOIM]Cl and 5 mL of a 20% sucrose solution with 0.5 mol/L HCl and CrCl2 with a 30 min reaction time [13]. Jadhav et al. reported a 67.2% 5-HMF yield, which was achieved through dehydration of sucrose at 120 ℃ in 150 min using 2 equiv. of the ionic liquid [TetraEG(mim)2][OMs]2 as a catalyst [14]. Jain et al. reported 5-HMF synthesis from sucrose in a sealed reactor using zirconium phosphate (ZrP) as a catalyst; 52% 5-HMF yield was obtained in H2O-diglyme [6]. Jadhav et al. used 10 wt% acid-modified silverexchanged silicotungstic acid (AgSTA) as a catalyst, which resulted in 92% conversion of sucrose with 62.5% 5-HMF yield with a 160 min reaction time at 120 ℃ [5]. However, the strongly acidic of hydrochloride, the harmfulness of chromium ion to environment, the high cost of the catalyst such as AgSTA containing silver elements, limit the further applications for conversion of sucrose into 5-HMF. Therefore, it is strongly desired to use the catalyst which is cheap, environmentally friendly, and obtained higher 5-HMF yield from sucrose.
Ionic liquids (ILs) and heteropolyacids (HPAs) have been attracting widespread interest in biomass conversion during the past decades owing to their unique advantages [1, 15, 16]. ILs are nonflammable and have negligible vapor pressure, high thermal and chemical stability, and adjustable solvent power for organic and inorganic substances [17, 18]. They usually act as both solvents and catalysts in the dehydration of biomass to chemical intermediates. HPAs are protonic acids with strong Brønsted acidity, and have also been widely applied as solid acids in the conversion of biomass including monosaccharides and polysaccharides to 5-HMF and LA [1, 16, 19]. In this study, several types of ILs and solid acids were investigated as catalysts in the conversion of sucrose to 5-HMF respectively; the IL N-methylimidazolium hydrogen sulfate ([Hmim][HSO4]) and the cesium salt of dodecatungstophosphoric acid (Cs2.3H0.7PW12O40), showed excellent catalytic activity, respectively. Various reaction parameters, including reaction the temperature and time and the catalyst dosage, were optimized. Moreover, the dehydrations of fructose and glucose to 5-HMF in these reaction systems were studied (Scheme 1).
|
Download:
|
| Scheme1. Schematic illustration of the synthesis of 5-HMF from sucrose. | |
2. Results and discussion 2.1. Catalyst screening
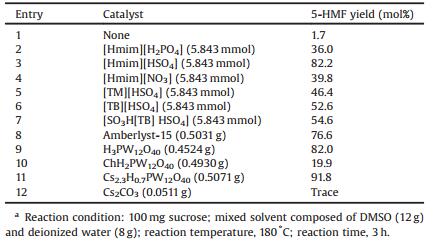
We examined the effects of different catalysts on the conversion of sucrose to 5-HMF, including several types of ILs and solid acids. The results were shown in Table 1. We initially screened catalysts by mixing 100 mg of sucrose with 20 g of a mixed solvent composed of DMSO (12 g) and deionized water (8 g) for 3 h at 180 ℃, and a very low 5-HMF yield (1.7%) was obtained in the absence of catalysts (Table 1, entry 1). Different ILs including imidazolium-type and thiazolium-type ILs were used as catalysts.It was found that the imidazolium cation was more suitable for promoting the conversion of sucrose to 5-HMF than the thiazolium cation, and [Hmim][HSO4] showed the best catalytic performance with an 82.2% 5-HMF yield (Table 1, entry 3). Meanwhile, with [Hmim] as the cation, the catalytic activity in the dehydration of sucrose in the presence of various anions decreased in the following order: HSO4- > NO3- > H2PO4- (Table 1, entries 2-4). HPAs have been attracting widespread interest in biomass conversion owing to their unique advantages. In this study, we used H3PW12O40, ChH2PW12O40, and Cs2.3H0.7PW12O40 as catalysts for the conversion of sucrose to 5-HMF (Table 1, entries 9-10). Interestingly, it was observed that Cs2.3H0.7PW12O40 and H3PW12O40 showed much better catalytic activity than ChH2PW12O40 although they have similar phosphotungstic acid structures. Cs2.3H0.7PW12O40 showed better catalytic activity in the conversion of sucrose to 5-HMF than H3PW12O40 did, which suggests that the Cs+ cation is more suitable for this process. However, no 5-HMF was obtained when Cs2CO3 (equimolar amount of cesium) was used as the catalyst separately (Table 1, entry 12), which indicated that Cs+ can only show good catalytic performance in the conversion of sucrose to 5-HMF when combined with phosphotungstic acid to form Cs2.3H0.7PW12O40. However, H3PW12O40 showed good catalytic activity despite being used as a single catalyst (81.97% 5-HMF yield). In contrast, 5-HMF has been obtained in low yield ( < 1%) from sucrose in ethanol-THF using H3PW12O40 as the catalyst [16]. This shows that the solvent also has a significant effect on the conversion of sucrose to 5-HMF.
|
|
Table 1 Results for the conversion of sucrose into 5-HMF with different catalystsa. |
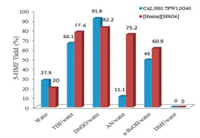
Dimethylformamide (DMF), tetrahydrofuran (THF), n-butyl alcohol (n-BuOH), DMSO, and acetonitrile (AN) are often used as additives in water phase to suppress side reactions during carbohydrate conversion reactions. The contributions of THF, DMSO, AN, n-BuOH, and DMF to the conversion of sucrose to 5-HMF were evaluated in the present study. As shown in Fig. 1, the solvent that consisted of water (8 g) and DMSO as the co-solvent (12 g) led to better performance in the conversion of sucrose to 5-HMF compared to THF, AN, n-BuOH, and DMF. 5-HMF yields of 27.9% and 20.0% were obtained using Cs2.3H0.7PW12O40 and [Hmim][HSO4] as catalysts, respectively, in pure water. With the addition of a co-solvent such as DMSO, the 5-HMF yield improved significantly. However, when the co-solvent was DMF, 5-HMF generation was obviously suppressed (Fig. 1).
|
Download:
|
| Fig. 1. Results of the conversion of sucrose to 5-HMF with different co-solvents. | |
2.2. Possible reaction pathway
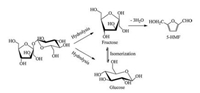
Sucrose consists of one fructose and one glucose moiety linked together by a glycosidic bond. A possible reaction pathway for the dehydration of sucrose into 5-HMF is shown in Scheme 2. It is well known that the conversion of sucrose to 5-HMF involves two steps. First, sucrose rapidly hydrolyzes to monosaccharides glucose and fructose, and then fructose and glucose dehydrate into 5-HMF [7, 18]. We speculated that the mechanism through which sucrose is converted to 5-HMF in this reaction system is similar to those in previous reports [8, 20]. In the first step, new hydrogen bonds are formed through coordination of HSO4-/H2PO4-/H2PW12O40- with -OH. Meanwhile, H+, Cs+, and electron-rich imidazolium cation will weaken the glycosidic bond to facilitate hydrolysis. We propose that glucose and fructose are produced in this way. Moreover, in the presence of a catalyst, the active catalytic site has the potential to initiate glucose isomerization via an intramolecular hydride shift, the glucose with Cs+ or electronrich imidazolium cation complex gives a favorable transition via the ene-diol intermediate, then loses its Cs+ or electron-rich imidazolium cation and forms free fructose [8]. The mechanism of 5-HMF formation from fructose has been debated over the years; both a pathway with cyclic intermediates and an open-chain mechanism have been approved by most researchers. Finally, 5-HMF is obtained via acidic hydrolysis of 4-hydroxy-5-hydroxymethyl-4, 5-dihydrofuran-2-carbaldehyde, and the structure of this intermediate was clearly confirmed by a combination of 1H and 13C NMR data [21].

|
Download:
|
| Scheme2. Proposed mechanism for the transformation of sucrose into 5-HMF. | |
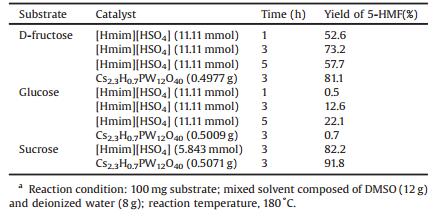
In this work, the conversions of d-fructose and glucose to 5-HMF in these reaction systems were also studied. As shown in Table 2, our results were consistent with previous results, where it was also found that the 5-HMF yield from d-fructose was much higher than that from glucose. Meanwhile, as the reaction time increased, the 5-HMF yields from d-fructose increased up to 3 h and then slowly decreased. In contrast, the 5-HMF yields from glucose increased continuously as reaction time increased to 5 h. This suggests that d-fructose generates 5-HMF more easily than glucose does and requires shorter reaction times to achieve higher 5-HMF yields. Interestingly, it was observed that the 5-HMF yield from d-fructose was dramatically lower than that from sucrose; we speculate this result may be caused by follow reasons, on one hand, when using sucrose as the substrate, fructose was constantly replenished by sucrose hydrolysis, meanwhile, the isomerization of glucose also production of fructose. And then fructose quickly converted into the 5-HMF, fructose is less directly exposed in the reaction system avoid more side-reaction, and obtained higher 5-HMF yield. On the other hand, more by-products were obtained when use D-fructose as substrate and were detected by HPLC.
|
|
Table 2 Comparison of 5-HMF production from different substrates under hydrothermal conditionsa. |
2.3. Effects of various reaction parameters on the conversion of sucrose to 5-HMF
Various reaction parameters such as reaction temperature and time, catalyst dosage, and co-solvent mixture have significant effects on the dehydration of sucrose to 5-HMF. In this study, the effects of the reaction parameters on the conversion of sucrose to 5-HMF were examined by using [Hmim][HSO4] as a model catalyst for the optimization of reaction conditions owing to its unique advantages and stronger solvent adaptability.
As shown in Fig. 2a, the effect of reaction temperature on the conversion of sucrose to 5-HMF was investigated. Reactions were conducted at 150, 160, 170, 180, 190, and 200 ℃. It was previously reported that the activation energy is higher for 5-HMF formation than for 5-HMF disappearance; thus, the maximum obtainable concentration increases with increasing temperature [22]. In this study, reaction temperature had a significant effect on the conversion of sucrose to 5-HMF, and the optimal reaction temperature was 180 ℃. When the temperature was increased further (above 190 ℃), the 5-HMF yield decreased because more by-products such as LA were generated at higher temperatures. Higher temperatures increased the rate of dehydration of sucrose to 5-HMF, but also accelerated the decomposition of 5-HMF into by-products. Hence, for the highest 5-HMF selectivity, the optimum temperature was 180 ℃. It is well known that there is a reciprocal relationship between the reaction temperature and the time needed for proper conversion. Fig. 2b shows the effect of reaction time on the conversion of sucrose to 5-HMF at 180 ℃. There was an interesting phenomenon; the 5-HMF yield from sucrose did not behave like that from fructose, which decreased rapidly with the increase in reaction time (Table 2). This may be because fructose was constantly replenished by sucrose hydrolysis and because of glucose isomerization when using sucrose as the substrate. Fructose is less directly exposed in the reaction system, and thus, the amount of by-products generated is reduced compared to when fructose is used as the substrate directly. Therefore, the 5-HMF yield from sucrose decreases slowly as the reaction time increases, as shown in Fig. 2b. Because the highest 5-HMF yield was obtained in 3 h and longer reaction times resulted in the generation of more by-products, 3 h was the optimal reaction time for 5-HMF production.

|
Download:
|
| Fig. 2. Effects of various reaction parameters on the conversion of sucrose (100 g) to 5-HMF. (a) temperature; (b) time; (c) mass ratios of DMSO/water; (d) catalyst amount. | |
The introduction of DMSO as a co-solvent increased the 5-HMF yield from sucrose when [Hmim][HSO4] was used as the catalyst. Moreover, the effect of different DMSO/water mass ratios on the conversion of sucrose to 5-HMF was also investigated. As shown in Fig. 2c, the yield increased to a maximum of 82.2% at 12 g of added DMSO in 20 g of mixed solvent. 5-HMF yields of 20.0% and 56.6% were obtained when using water and DMSO as the solvent, respectively. This suggests that DMSO as a co-solvent in this reaction system played a key role in the conversion of sucrose to 5-HMF, and caused the dramatic breakage of the water-water hydrogen bonds and associated with water molecules [23, 24]. Meanwhile, water and DMSO can both push the reaction toward 5-HMF formation and inhibit undesired side reactions [25].
A 5-HMF yield of 1.7% was obtained when no catalyst was added (Table 1, entry 1). As shown in Fig. 2d, 5-HMF yield increased dramatically to 51.7% when 0.56 mmol of [Hmim][HSO4] was added. This shows that [Hmim][HSO4] has excellent catalytic activity in the conversion of sucrose to 5-HMF. When the catalyst dosage was increased from 0.56 to 5.84 mmol, the 5-HMF yield increased from 51.7% to 82.2%. However, when the amount of catalyst was increased further, 5-HMF yield decreased. A possible reason is that a high catalyst loading not only accelerated the conversion of sucrose to 5-HMF, but also promoted other side reactions [26].
It is obvious that the yield of 5-HMF is highly dependent on the operation parameters such as the catalyst amount used. Therefore, it is meaningful to normalize the apparent yields to the 5-HMF formation rates with the unit of [mol(5-HMF)/mol(cat)], and make a comparison among the values obtained in this study together with those previously reported.
Catalyst recycling is an important goal in terms of green and sustainable chemistry, the reusability of the catalyst [Hmim] [HSO4] and Cs2.3H0.7PW12O40 were tried. After the reaction, most of the 5-HMF in the aqueous solution was removed by ethyl acetate extraction until no 5-HMF was detected in the ethyl acetate. The water in the aqueous phase was completely removed through vacuum evaporation. The remaining [Hmim][HSO4] and DMSO were used directly in the next run by adding a fresh sucrose sample and water under the same reaction conditions. However, the unreacted sucrose is very difficult to remove completely from the mixture of DMSO and ionic liquid. If we ignore the sucrose which unreacted in the initial reaction, this processes was repeated 4 times, use [Hmim][HSO4] as catalyst obtaining 5-HMF yields of 90.7%, 88.4%, 86.9%, 80.4%, respectively. Therefore, it can be concluded that the catalyst was stable in this system, although the unreacted sucrose is not remove completely.
3. ConclusionEfficient conversion of sucrose to 5-HMF was investigated in a DMSO/water mixed solvent, and [Hmim][HSO4]/Cs2.3H0.7PW12O40 showed excellent catalytic activity and selectivity. This one-pot synthesis of 5-HMF from sucrose, which is an inexpensive chemical produced in sugar cane and sugar beet cultivation, instead of fructose increases the promising potential of large-scale production of 5-HMF from carbohydrates.
4. Experimental 4.1. MaterialsN-Methylimidazole was purchased from Nanjing Xiezun Chemical Co., Ltd. (Nanjin, China). 5-HMF (purity > 97%) was purchased from Heowns Biochem Technologies LLC (Tianjin, China). Amberlyst-15 was purchased from purchased from Aladdin Co. (Shanghai, China). H3PW12O40 and Cs2CO3 were purchased from Tianjin Guangfu Fine Chemical Research Institute (Tianjin, China). Methanol (HPLC grade) was purchased from Tianjin Kermel Chemical Reagent Co., Ltd. Vitriolic acid, phosphoric acid, hydrochloric acid, and nitric acids were purchased from Tianjin Chemical Reagent No. 5 Plant (Tianjin, China). All other chemicals were supplied by local suppliers and used without further purification.
4.2. Catalyst preparationBrønsted acidic [Hmim][HSO4], 1-methylimidazolium dihydric phosphate ([Hmim][H2PO4]) and 1-methylimidazolium nitrate ([Hmim][NO3]) were synthesized in our laboratory via a neutralization reaction and developed on the basis of a previous report [27-29]. 4, 5-dimethylthiazole hydrosulfate ([TM][HSO4]) and 2-isobutylthiazole hydrosulfate ([TB][HSO4]) were synthesized using similar procedures, 4, 5-dimethylthiazole (or 2-isobutylthiazole) was combined with sulfuric acid (50% w/w) in a 1:1 molar ratio and refluxed for 12 h. 4, 5-dimethyl-3-(4-sulfonic acid butyl)thiazolium hydrosulfate ([SO3H[TB] HSO4]) was synthesized in our laboratory and developed on the basis of a previous report [30]. All of the ionic liquids were characterized by 1H NMR spectrum, moreover, the ionic liquids which had not been reported previously were also characterized by HPLC-MS (Supplementary material).
Cesium salt of dodecatungstophosphoric acid (Cs2.3H0.7PW12O40) was prepared by titration of an aqueous solution of H3PW12O40 with an aqueous solution of Cs2CO3 according to the procedure reported elsewhere [19, 31]. First, Cs2CO3 was calcinated at 450 ℃ for 2 h to remove the adsorbed water before use. A series of acidic cesium salts, were prepared by a titration method with cesium content ranging from 1 to 3. The appropriate amounts of a 0.10 mol/L Cs2CO3 aqueous solution were added dropwise with a constant rate to a 0.08 mol/L aqueous solution of H3PW12O40 at room temperature with constant stirring. The Cs content, x in CsxH3-xPW12O40, was adjusted by the amount of Cs2CO3 solution added. After the resultant milky suspensions were aged at room temperature overnight, the solutions were slowly heated at 50 ℃ to obtain white solid powers, the resulting solid was ground into white powder. The ChxH3-xPW12O40 was combined with H3PW12O40 and choline chloride, the ChH2PW12O40 was synthesized on the basis of a previous report [1].
4.3. Conversion of sucrose to 5-HMFA typical catalytic reaction for the hydrolysis of sucrose using [Hmim][HSO4] or acidic cesium salt Cs2.3H0.7PW12O40 as a catalyst was performed in a 50 mL stainless steel vessel with a Teflon lining that was sealed with a screw cap. Into the reactor, 0.1 g of sucrose and different catalysts were introduced, and then 20 g of a mixed solvent composed of an organic reagent and deionized water was added. Finally, the reactor was immersed in a preheated oil bath, and the reaction mixture was stirred for a given time. Time zero was recorded when the reactor was immersed in the preheated oil bath. After the desired reaction time, the reactor was placed immediately in an ice-water bath to quench the reaction. The mixture was filtered to remove any insoluble solid residue and 1 mL of the filtrate was diluted with methanol up to a volume of 5.0 mL in a volumetric flask. The solution was sonicated for 1 min to dissolve the sample and then injected into a glass tube after passage through a 0.22 mm polytetrafluoroethylene filter to remove the solid residue completely. The aqueous solution was analyzed by high-performance liquid chromatography (HPLC). All experiments were performed at least three times, and the experimental error was ±1%.
4.4. Determination of 5-HMF yieldQuantitative and qualitative analyses of 5-HMF were performed by HPLC and gas chromatography and mass spectrometry (GC-MS). The concentration of 5-HMF was quantified using the calibration curve obtained with known concentrations of the standard substance. Chromatographic analysis was performed using a HPLC instrument (LC3000, Beijing Chuangxin Tongheng Science and Technology Co., Ltd.) with a Kromasil C18 reversed phase column (5 μm, 250 mm × 4.6 mm) and a UV detector. The mobile phase consisted of methanol and water (23/77, v/v) with a flow rate of 0.5 mL/min. The column temperature was maintained at 30 ℃ and the detection wavelength set to 284 nm. The volume for each injection was 20 μL. The 5-HMF as staple and the peak in the HPLC spectrogram appeared at 11.00 min. The 5-HMF yield was determined by high performance liquid chromatography (HPLC)of these aqueous solutions, using a standard curve (Fig. S1 in Supporting information) in order to quantify the amount.
The yields of 5-HMF (Y5-HMF) from sucrose/fructose/glucose was calculated as follows:
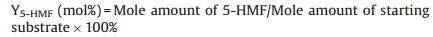
|
The qualitative analysis of 5-HMF (Fig. S2 in Supporting information) was conducted by GC-MS using a Bruker SCIONSQ/436-GC gas chromatograph from Agilent Technologies (USA) equipped with a HP-5MS capillary column (30 m × 0.25 mm × 0.25 mm), as well as a MS single quadrupole detector. The column temperature was initially held at 50 ℃ for 2 min, then programmed to increase to 230 ℃ at a rate of 15 ℃/min with a hold time of 6 min. Helium was used as the carrier gas at a linear velocity of 1.0 mL/min. Mass spectrometric measurements were performed using electron impact ionization (EI) at 70 eV and a scan range of m/z 50-500, at a rate of 1 scan/s.
AcknowldgementsThe authors would like to acknowledge the financial support from National Natural Science Foundation of China (No.21406166) and the Municipal Natural Science Foundation of Tianjin (No. 15JCQNJC13900).
Appendix A. Supplementary dataSupplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.cclet.2017.02.016.
| [1] | X. Zhang, D. Zhang, Z. Sun, et al., Highly efficient preparation of hmf from cellulose using temperature-responsive heteropolyacid catalysts in cascade reaction. Appl. Catal. B-Environ. 196 (2016) 50–56. DOI:10.1016/j.apcatb.2016.05.019 |
| [2] | C.H. Christensen, J. Rass-Hansen, C. Marsden, et al., The renewable chemicals industry. Chemsuschem. 1 (2008) 283–289. DOI:10.1002/(ISSN)1864-564X |
| [3] | A. Corma, S. Iborra, A. Velty. Chemical routes for the transformation of biomass into chemicals. Chem. Rev. 107 (2007) 2411–2502. DOI:10.1021/cr050989d |
| [4] | H. Röper. Renewable raw materials in Europe-industrial utilisation of starch and sugar. Starch-St?rke 54 (2002) 89–99. DOI:10.1002/1521-379X(200204)54:3/4<89::AID-STAR89>3.0.CO;2-I |
| [5] | A.H. Jadhav, H. Kim, I.T. Hwang. An efficient and heterogeneous recyclable silicotungstic acid with modified acid sites as a catalyst for conversion of fructose and sucrose into 5-hydroxymethylfurfural in superheated water. Bioresour. Technol. 132 (2013) 342–350. DOI:10.1016/j.biortech.2013.01.030 |
| [6] | A. Jain, A.M. Shore, S.C. Jonnalagadda, et al., Conversion of fructose, glucose and sucrose to 5-hydroxymethyl-2-furfural over mesoporous zirconium phosphate catalyst. Appl. Catal. A-Gen. 489 (2015) 72–76. DOI:10.1016/j.apcata.2014.10.020 |
| [7] | A.A. Rosatella, S.P. Simeonov, R.F.M. Frade, et al., Cheminform abstract: 5-hydroxymethylfurfural (hmf) as a building block platform: biological properties, synthesis and synthetic applications. Green Chem. 13 (2011) 754–793. DOI:10.1039/c0gc00401d |
| [8] | T. Ståhlberg, S. Rodriguez-Rodriguez, P. Fristrup, et al., Metal-free dehydration of glucose to 5-(hydroxymethyl)furfural in ionic liquids with boric acid as a promoter. Chem. Eur. J. 17 (2011) 1456–1464. DOI:10.1002/chem.v17.5 |
| [9] | T. Okano, K. Qiao, Q. Bao, et al., Dehydration of fructose to 5-hydroxymethylfurfural (hmf) in an aqueous acetonitrile biphasic system in the presence of acidic ionic liquids. Appl. Catal. A-Gen. 451 (2013) 1–5. DOI:10.1016/j.apcata.2012.11.004 |
| [10] | G. Yong, Y. Zhang, J. Ying. Efficient catalytic system for the selective production of 5-hydroxymethylfurfural from glucose and fructose. Angew. Chem. Int. Ed. 47 (2008) 9345–9348. DOI:10.1002/anie.200803207 |
| [11] | A. Chinnappan, A.H. Jadhav, W.J. Chung, et al., Conversion of sugars (sucrose and glucose) into 5-hydroxymethylfurfural in pyridinium based dicationic ionic liquid ([C10(EPy)2]2Br-) with chromium chloride as a catalyst. Ind. Crop Prod. 76 (2015) 12–17. DOI:10.1016/j.indcrop.2015.05.085 |
| [12] | S. Peters, T. Rose, M. Moser. Sucrose: a prospering and sustainable organic raw material. Top. Curr. Chem. 294 (2010) 1–23. DOI:10.1007/978-3-642-14837-8 |
| [13] | J.A. Chun, J.W. Lee, Y.B. Yi, et al., Catalytic production of hydroxymethylfurfural from sucrose using 1-methyl-3-octylimidazolium chloride ionic liquid. Korean J. Chem. Eng. 27 (2010) 930–935. DOI:10.1007/s11814-010-0167-x |
| [14] | A.H. Jadhav, H. Kim, I.T. Hwang. Efficient selective dehydration of fructose and sucrose into 5-hydroxymethylfurfural (hmf) using dicationic room temperature ionic liquids as a catalyst. Catal. Commun. 21 (2012) 96–103. DOI:10.1016/j.catcom.2012.02.007 |
| [15] | W. Liu, Y. Wang, W. Li, et al., Polyethylene glycol-400-functionalized dicationic acidic ionic liquids for highly efficient conversion of fructose into 5-hydroxymethylfurfural. Catal. Lett. 145 (2015) 1080–1088. DOI:10.1007/s10562-015-1485-8 |
| [16] | Y. Yang, M.M. Abu-Omar, C. Hu. Heteropolyacid catalyzed conversion of fructose sucrose, and inulin to 5-ethoxymethylfurfural, a liquid biofuel candidate. Appl. Energy 99 (2012) 80–84. DOI:10.1016/j.apenergy.2012.04.049 |
| [17] | S. Hu, Z. Zhang, Y. Zhou, et al., Conversion of fructose to 5-hydroxymethylfurfural using ionic liquids prepared from renewable materials. Green Chem. 10 (2008) 1280–1283. DOI:10.1039/b810392e |
| [18] | M.E. Zakrzewska, E. Bogel-Ƚukasik, R. Bogel-Ƚukasik. Ionic liquid-mediated formation of 5-hydroxymethylfurfural a promising biomass-derived building block. Chem. Rev. 111 (2011) 397–417. DOI:10.1021/cr100171a |
| [19] | J. Tian, C. Fang, M. Cheng, et al., Hydrolysis of cellulose over CsxH3-xPW12O40(X=1-3) heteropoly acid catalysts. Chem. Eng. Technol. 34 (2011) 482–486. DOI:10.1002/ceat.201000409 |
| [20] | T. Ståhlberg, W. Fu, J.M. Woodley, et al., Synthesis of 5-(hydroxymethyl)furfural in ionic liquids: paving the way to renewable chemicals. Chemsuschem. 4 (2011) 451–458. DOI:10.1002/cssc.201000374 |
| [21] | A.S. Amarasekara, L.T.D. Williams, C.C. Ebede. Mechanism of the dehydration of d fructose to 5-hydroxymethylfurfural in dimethyl sulfoxide at 150 ℃: an NMR study. Carbohydr. Res. 343 (2008) 3021–3024. DOI:10.1016/j.carres.2008.09.008 |
| [22] | B.F.M. Kuster. 5-Hydroxymethylfurfural (HMF). A review focussing on its manufacture. Starch-Stärke 42 (1990) 314–321. DOI:10.1002/(ISSN)1521-379X |
| [23] | M. Kimura, et al., Effect of water on hydrolytic cleavage of non-terminal α-glycosidic bonds in cyclodextrins to generate monosaccharides and their derivatives in a dimethyl sulfoxide-water mixture. J. Phys. Chem. A 118 (2014) 1309–1319. |
| [24] | R.M. Musau, R.M. Munavu. The preparation of 5-hydroxymethyl-2-furaldehyde (HMF) from D-fructose in the presence of DMSO. Biomass 13 (1987) 67–74. DOI:10.1016/0144-4565(87)90072-2 |
| [25] | X. Cao, S.P. Teong, D. Wu, et al., An enzyme mimic ammonium polymer as a single catalyst for glucose dehydration to 5-hydroxymethylfurfural. Green Chem. 17 (2015) 2348–2352. DOI:10.1039/C4GC02488E |
| [26] | S. Xiao, B. Liu, Y. Wang, et al., Efficient conversion of cellulose into biofuel precursor 5-hydroxymethylfurfural in dimethyl sulfoxide?ionic liquid mixtures. Bioresour. Technol. 151 (2014) 361–366. DOI:10.1016/j.biortech.2013.10.095 |
| [27] | S.W. Liu, S.T. Yu, F.S. Liu, et al., Reactions of a-pinene using acidic ionic liquids as catalysts. J. Mol. Catal. A: Chem. 279 (2008) 177–181. DOI:10.1016/j.molcata.2007.06.026 |
| [28] | A. Mirjafari, N. Mobarrez, R.A. Oa€?Brien, et al., Microwave-promoted one-pot conversion of alcohols to oximes using 1-methylimidazolium nitrate[Hmim] [NO3], as a green promoter and medium. C.R. Chim. 14 (2011) 1065–1070. DOI:10.1016/j.crci.2011.06.003 |
| [29] | H. Ohno, M. Yoshizawa. Ion conductive characteristics of ionic liquids prepared by neutralization of alkylimidazoles. Solid State Ionics 154 (2002) 303–309. |
| [30] | Q. Yan, H. Zang, C. Wu, et al., Synthesis, characterization and catalytic application of novel ionic liquids based on thiazolium cation. J. Mol. Liq. 204 (2015) 156–161. DOI:10.1016/j.molliq.2015.01.016 |
| [31] | T. Okuhara, H. Watanabe, T. Nishimura, et al., Microstructure of cesium hydrogen salts of 12-tungstophosphoric acid relevant to novel acid catalysis. Chem. Mater. 12 (2000) 2230–2238. DOI:10.1021/cm9907561 |
 2017, Vol. 28
2017, Vol. 28