Metal-organic frameworks (MOFs), which were assembled from inorganic metal connectors and organic linkers, have extremely-large surface areas, high porosity, controlled pore sizes, ordered crystalline structures and excellent mechanical stability [1]. All these merits encourage further study of using MOFs in chemical sensors [2]. However, most MOFs are insulators or semiconductors, and thus the applications based on the direct use of MOFs in electrochemical sensing were less [3]. A simple and efficient strategy to improve the conductivity is to use the mixture of MOFs and some conductive materials, such as metal nanoparticles [4], organic polymers [5], carbon [6] and so on. Recently, composites of metal-organic frameworks and carbon-based materials have attracted enormous attention because of the extraordinary characteristics of carbon-based materials, involving high mechanical and elastic strength, excellent chemical and thermal robustness, distinguished electronic and optical properties, low weight, low toxicity and sometimes low cost. Several MOF-modified carbon paste electrodes, which were made from graphite powder and nonconductive binder with an appropriate mixing ratio, have already been developed for metal ion and biomaterial sensing [7-9]. Zeolitic imidazolate frameworks (ZIFs), as a subclass of metal-organic frameworks, were found as the promising porous materials composed of inorganic metal nodes linked by imidazole or imidazolate bridging ligands [10]. They not only have the generic property advantages of most MOFs, but also possessed exceptional thermal and chemical stability [10] making ZIFs being a good candidate for gas adsorption [11], catalysis [12] and separation [13] applications. Meanwhile, the ultrahigh surface area and the high porosity of ZIFs have also triggered great interests in the area of electrochemical sensing [14]. However, the applications of ZIFs in electrochemical analysis were not widely studied.
Graphene, as one of the carbon-based materials, has attracted tremendous attention in recent years due to its high specific surface area, excellent electronic conductivity, exceptional electronic transport property, high mechanical strength and good chemical stability [15]. Graphene has been a powerful electrode modified material for preparing the electrochemical sensors or biosensors [16, 17]. However, the application performance of graphene was limited because the graphene usually tended to agglomerate or even restack to form graphite through strong-stacking interaction and vander Waals interactions [18]. To overcome this drawback, some molecules or polymers have been introduced to the surface of graphene layers, such as MOFs [19], poly (diallyldimethylammonium chloride) [20] and sulfonated polyaniline [21].
In our previous work, G-ZIF-8 nanocomposites were successfully synthesized with well-intergrown nanocrystals of zeolitic imidazolate frameworks (ZIF-8) supported on three-dimensional (3D) graphene by a counter diffusion technique [22]. The incorporation of ZIF-8 crystals overcomes the aggregation of the graphene nanosheets and thus greatly improves the surface area of the G-ZIF-8 composites. G-ZIF-8 composites with hierarchical pore structures showed high electrochemical capacitance and good stability. As is well known, composites of graphene with other materials have found a number of reports on electroanalysis owning to their good electronic properties and large specific surface area [23-25]. Herein, in order to explore the potential application of the G-ZIF-8 hybrid, in this paper, the advantage of GZIF-8 nanocomposites used in electroanalysis field was testified by using dopamine (DA) as an analyte. G-ZIF-8 nanocomposites were cast on a glassy carbon electrode to form G-ZIF-8 modified electrode and the electrode was used for the detection of DA. This work gives help for using graphene-MOFs composites to fabricate the electrochemical sensor.
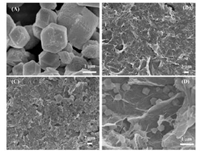
2. Results and discussion 2.1. Characterization of the G-ZIF8/GCEThe morphologies of ZIF8/GCE, G/GCE and G-ZIF8/GCE were investigated through scanning electron microscopy (SEM). As indicated in Fig. 1A, the pristine ZIF-8 crystal particles aggregated and showed hexagonal morphology. Fig. 1B shows that graphene was homogeneously coated on the surface of the electrode and wrinkled flake-like graphene sheets overlaid. Compared with ZIF-8 and G films, the SEM images of G-ZIF8/GCE (Fig. 1C and D) shows that ZIF-8 nanocrystals were homogeneously grown on the surface of graphene and graphene sheets were refrained from restacking to graphite, which implies the high accessible surface area and thus the high sensitivity by using in sensor for detection.
|
Download:
|
| Fig. 1. SEM images of ZIF-8 (A), G (B) and G-ZIF-8 composite (C and D) on the surface of GCE at different magnifications. | |
The BET surface areas of ZIF-8, G and G-ZIF-8 materials were measured by nitrogen sorption experiments (Fig. 2). As shown in Fig. 2A, the N2 adsorption of the pure ZIF-8 exhibited typical type-Ⅰ profile, which indicated that ZIF-8 owned microporous structure. The surface area of the pure ZIF-8 obtained from the curves was 1895.1 m2/g. Additionally, from Fig. 2B we could see the pore size distributions (PSD) of ZIF-8 was mainly focused on 1.23 nm. As can be seen from Fig. 2C, the isotherm of G-ZIF-8, which had a steep increase at low relative pressure and quickly reached balance, was a little similar to Type-Ⅰ behavior, suggesting that the G-ZIF-8 was dominated by microporous characteristic. The BET surface area of G-ZIF-8 was 756.9 m2/g, which was greatly larger than the BET surface area of 157.5 m2/g for the 3D graphene (Fig. 2E). In addition, Fig. 2D and F revealed that the PSDs of G-ZIF-8 were mainly in 1.25 nm, while that of graphene were mainly focused on 1.85 nm and 2.77 nm. The increased specific surface area of the G-ZIF-8 further testified that the incorporation of ZIF-8 crystals to graphene sheets not only refrained graphene sheets from restacking but also considerably improved the surface area of the G-ZIF-8 composites.

|
Download:
|
| Fig. 2. N2 adsorption-desorption isotherms and pore size distributions for ZIF-8 (A, B) G-ZIF-8 (C, D) and G (E, F). (STP = standard temperature and pressure). | |
The electrochemical characterization of G-ZIF8/GCE was performed using the well characterized outer-sphere electron transfer redox probe hexaammineruthenium(Ⅲ) chloride (Ru(NH3)6Cl3). Fig. 3A depicts typical cyclic voltammograms of bare GCE, ZIF8/GCE, G/GCE, G-ZIF8/GCE at a scan rate of 50 mV/s. A pair of well-defined redox waves with the average value of redox peak potentials (EPa + EPc)/2, known as formal potential (E0'), was exhibited to be 0.22 V at all the electrodes. In addition, a smaller redox peak-to-peak separation (△EP) of 56 mV was obtained at GZIF8/GCE than those of 63 mV, 65 mV, 63 mV for G/GCE, ZIF8/GCE and GCE, respectively. This is indicative of a fast electron transfer rate. The peak current of ZIF8/GCE is smaller than that of bare GCE, which is ascribed to the modification of poor conductive ZIF-8 at GCE. The peak current of G/GCE is almost similar to that of GCE. Furthermore, the response peak current is increased remarkably for G-ZIF8/GCE compared with the other three electrodes (bare, ZIF-8, G modified GCE). These results could be attributed to the synergistic effect of ZIF-8 and graphene. The incorporation of ZIF-8 crystals refrains graphene sheets from overlying and thus improves accessible surface area, which is favorable for the electron transfer of the analyte. Fig. 3B shows the cyclic voltammograms of G-ZIF8/GCE at different scan rates. As can be seen, the redox peak current was enhanced as the scan rate increasing from 20 mV/s to 400 mV/s. Meanwhile, from the inset of Fig. 3B, both anodic and cathodic peak currents increased linearly with the square root of scan rate, indicating a diffusion-controlled process [26].

|
Download:
|
| Fig. 3. (A) Cyclic voltammograms of bare GCE, ZIF8/GCE, G/GCE and G-ZIF8/GCE at a scan rate of 50 mV/s. (B) Cyclic voltammograms of G-ZIF8/GCE with different scan rates: 20, 50, 100, 150, 200, 250, 300 and 400 mV/s (from inner to outer). Electrolyte: 5.0 × 10-3 mol/L Ru(NH3)6Cl3/0.1 mol/L KCl solution. Inset: plots of the anodic and cathodic peak currents (IPa and IPc) vs. the square root of scan rate. | |
2.2. Electrochemical behavior of DA at G-ZIF8/GCE
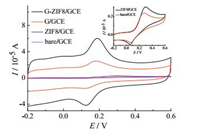
Fig. 4 depicts the cyclic voltammetric performances of 50 μmol/L DA in 0.1 mol/L PBS (pH 7.0) at bare GCE, ZIF8/GCE, G/GCE and GZIF8/GCE. At the bare GCE and ZIF8/GCE, the cyclic voltammograms show a pair of redox waves with △EP of 206 mV and 239 mV, respectively, while for the G/GCE and G-ZIF8/GCE, △EP' is remarkably reduced to only 66 mV and 63 mV, indicating that G/GCE and G-ZIF8/GCE have a better electrocatalytic activities and a fast electron transfer for DA. Meanwhile, G-ZIF8/GCE shows a large anodic peak current, which are about 3 times, 10 times and 14 times of G/GCE, ZIF8/GCE and bare GCE, respectively. These results could be attributed to the synergistic effect of ZIF-8 and graphene, which makes the nanocomposite large electrode surface area, high conductivity and thus good electrochemical activity [27].
|
Download:
|
| Fig. 4. Cyclic voltammograms of 50 μmol/L DA at bare GCE, ZIF8/GCE, G/GCE and GZIF8/GCE in 0.1 mol/L PBS (pH 7.0). Scan rate: 50 mV/s. Insert: magnification of CV curves of ZIF8/GCE and bare GCE. | |
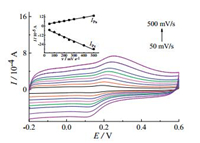
The effect of the scan rate (υ) on the electrochemical behavior of DA at G-ZIF8/GCE was investigated. As shown in Fig. 5, both anodic and cathodic peak currents enhance gradually with the increase of the scan rate from 50 to 500 mV/s. The relationships of oxidation and reduction peak currents (IPa and IPc) with the scan rate can be seen from the inset of Fig. 5. The redox peak currents increase linearly with the scan rate, which suggests that the electrochemical response of DA on G-ZIF8/GCE is an adsorption-controlled process, which is expected for a thin-layer effects with DA possessing conjugated region for π-π interaction trapped in the 3D G-ZIF-8 nanocomposite network [28].
|
Download:
|
| Fig. 5. Cyclic voltammograms of G-ZIF8/GCE in pH 7.0 PBS containing of 50 mmol/L DA at different scan rates (as the arrow direction): 50, 100, 150, 200, 250, 300, 400 and 500 mV/s. Inset: calibration plot for corresponding anodic and cathodic peak currents versus the scan rate. | |
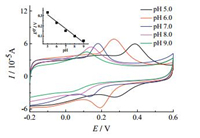
The influence of pH values on the electrochemical behavior of DA at G-ZIF8/GCE was investigated in 0.1 mol/L PBS with the values of pH ranging from 5.0 to 9.0. As depicted in Fig. 6, the redox peak potentials shifted towards the negative, which indicates that proton takes part in the electrochemical reaction [29]. The inset of Fig. 6 clearly shows that E0' has a good linear dependence with pH in the pH range from 5.0 to 9.0. The slope value of -67.7 mV/pH is found to be very close to the theoretical value of -59 mV/pH, indicating that the electrochemical redox process of DA undergoes an equal number of electron and proton transfer process. This is in agreement with the two electrons and two protons transfer for DA [30]. In the meantime, the oxidation peak current achieved its largest value at pH 7.0. Thus, 0.1 mol/L pH 7.0 PBS was selected for the subsequent experiments.
|
Download:
|
| Fig. 6. Cyclic voltammograms obtained at G-ZIF8/GCE in 0.1 mol/L PBS containing 50 μmol/L DA with various pH value: 5.0 (black line), 6.0 (red line), 7.0 (blue line), 8.0 (purple line), 9.0 (green line). Scan rate: 50 mV/s. Inset: plot of E0' against pH value. | |
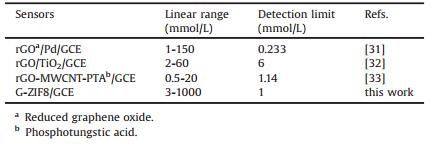
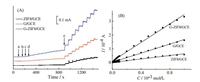
Fig. 7A presents the amperometric responses of ZIF8/GCE, G/GCE and G-ZIF8/GCE towards DA by successively adding different amount of DA to a continuous stirring PBS at the controlled constant potential of 0.3 V. It can be seen that a fast and sensitive response for each addition of DA was attained at three electrodes and obvious increases in the oxidation current with the increased concentrations of DA were obtained. Fig. 7B shows the corresponding calibration curves. Response current was increased linearly over the DA concentration ranging from 3.0 μmol/L to 1.0 μmol/L for three electrodes. The detection sensitivity for GZIF8/GCE is 0.34 μA/μmol/L, for ZIF8/GCE is 0.063 μA/μmol/L and for G/GCE is 0.16 μA/μmol/L. The detection limit of G-ZIF8/GCE for DA was estimated to be 1.0 μmol/L at a signal-to-noise ratio (S/N) of 3, which was lower than that of ZIF8/GCE (2.5 μmol/L) and the same as that of G/GCE (1.0 μmol/L). The high sensitivity and low detection limit for G-ZIF8/GCE may be ascribed to the synergistic effect from the conductivity of graphene and the high electrode surface area of G-ZIF8/GCE. A comparison of electroanalytical characteristics of some modified electrodes to the determination of DA was listed in Table 1. It showed that G-ZIF8/GCE has the wide linear concentration range and a comparable detection limit compared with that of rGOa/Pd/GCE [31], rGO/TiO2/GCE [32] and rGO-MWCNT-PTAd/GCE [33].
|
Download:
|
| Fig. 7. (A) Current-time response curves of ZIF8/GCE, G/GCE and G-ZIF8/GCE upon successive additions of (a) 3.0' 10-6 mol/L, (b) 2.0 ×10-6 mol/L, (c) 5 × 10-6 mol/L, (d) 1.0 × 10-5 mol/L and (e) 1.0 × 10-4 mol/L DA in pH 7.0 PBS with a constant stirring rate at an applied potential of 0.30 V. (B) Calibration plots for response current versus the concentration of DA. | |
|
|
Table 1 Comparison of several graphene modified electrodes for the determination of DA. |
The selectivity of DA determination was carefully studied by using some common interfering species in biological samples including hydrogen peroxide (H2O2), uric acid (UA), glucose and ascorbic acid (AA). Fig. 8 shows the typical amperometric response of G-ZIF8/GCE for DA and the interfering species. It could be seen that the presence of 2-fold uric acid and 100-fold glucose, 4.4-fold hydrogen peroxide and 0.15-fold ascorbic acid did not cause significant interference in the determination of DA (20 μmol/L).

|
Download:
|
| Fig. 8. Amperometric response curve of G-ZIF8/GCE upon successive additions of (a) 0.20 mmol/L DA, (b) 0.40 mmol/L uric acid, (c) 20mmol/L glucose, (d) 0.88 mmol/L hydrogen peroxide, (e) 30 μmol/L ascorbic acid and (f) 0.20 mmol/L DA to a stirred 0.1 mol/L pH 7.0 PBS at a potential of 0.30 V. | |
The stability of G-ZIF8/GCE was evaluated by detecting the cyclic voltammogram of 50 μmol/L DA and the electrode was stored in PBS (pH 7.0) at the ambient temperature when not in use. It was noted that the oxidation current of DA maintained as high as 98% for 12 hours, 88% for three days. In addition, the reproducibility of the electrode was investigated. Six G-ZIF8/GCEs were used to examine the CV of 50 μmol/L DA and a relative standard deviation (RSD) of 2.5% was obtained.
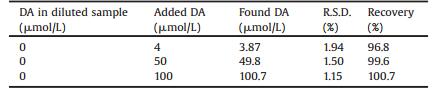
To evaluate the practical feasibility of G-ZIF8/GCE, recovery test was conducted in cow serum samples. The cow serum sample was diluted to 100 times with 0.1 mol/L PBS before measurement and the concentration of DA was analyzed under optimized conditions by i-t amperometry [34]. The analytical results were listed in Table 2. As can be observed, the recovery was between 96.8% and 100.7% and the RSD was less than 2.0%, revealing the developed G-ZIF8/GCE could be potentially applied for the determination of DA in real samples.
|
|
Table 2 Determination of DA in cow serum sample (n = 3). |
3. Conclusion
G-ZIF8/GCE was prepared. SEM results shows that ZIF-8 nanocrystals were homogeneously intergrown on the surface of graphene and thus refrained graphene sheets from overlying, implying improved accessible surface area of G-ZIF-8 composites. The BET results further testified that G-ZIF-8 composites had a larger surface area than 3D graphene. The electrochemical characterization of G-ZIF8/GCE was performed by using Ru (NH3)6Cl3 and the large current was observed at G-ZIF8/GCE compared with those at bare GCE, ZIF8/GCE and G/GCE. The electrochemical behavior of dopamine was investigated and GZIF8/GCE exhibited excellent electroanalytical performance for DA. These results can be ascribed to the synergistic effect from the conductivity of graphene and the high electrode surface area of GZIF8/GCE. It is believable that the structure characteristic of G-ZIF-8 nanocomposite is favorable for using MOFs to fabricate highly sensitive electrochemical sensor system.
4. Experimental 4.1. ReagentsDopamine hydrochloride (DA) was procured from Alfa Aesar and used as received. The solution of DA was freshly prepared in double distilled water prior to use each time. All the other chemicals (analytical-reagent grade) were used as supplied without any further purification. 0.1 mol/L K2HPO4 and 0.1 mol/L NaH2PO4 were used to prepare the phosphate buffer solution (PBS) (0.1 mol/L) with different pH. Doubly-distilled water, which was provided by a Milli-Q purification system (Millipore), was used for preparing all solutions in the experiment. All experiments were conducted at the ambient temperature and highly pure nitrogen was used for deaeration throughout the electrochemical experiments.
4.2. ApparatusAll the electrochemical experiments were accomplished using a CHI 760E Electrochemical Workstation (Shanghai Chenhua Instrument Corporation, China). A conventional three-electrode system was employed with an unmodified or modified glassy carbon electrode (GCE) (3 mm in diameter) as the working electrode, a saturated calomel electrode (SCE) and a platinum electrode as the reference and the counter electrodes, respectively. All electrode potentials were reported to SCE. The FEI Quanta 250 FEG scanning electron microscopy (SEM) (America) was employed for characterizing the morphology of the modified electrodes. The specific surface areas and pore size distributions were measured using a TriStarII3020 (Micromeritics Instrument Corporation) apparatus at 77 K. Before analysis, the samples were vacuumed and degassed at 473 K for 3 h.
4.3. Fabrication of the modified electrodesG-ZIF-8 was synthesized and characterized according to the same procedure documented in our previous work [22]. 2 mg of GZIF-8 was dispersed in 1 mL double-distilled water to achieve homogeneous suspension by bath sonication when used. Prior to the modification, GCE was polished using 0.3 μm Al2O3 powder and rinsed thoroughly with double distilled water, and then sonicated in double distilled water, ethanol and double distilled water each for about 1 minute in turn. The cleaned GCE was then dried in air. G-ZIF-8 modified electrode (denoted as G-ZIF8/GCE) was made by dropping 7 mL G-ZIF-8 suspension onto GCE surface and dried under an infrared lamp. For comparison, ZIF-8 and graphene modified electrodes (denoted as ZIF8/GCE and G/GCE, respectively) were made in the same way except by dropping ZIF-8 and graphene suspensions, respectively.
AcknowledgmentThis work was financially supported by the National Natural Science Foundation of China (Nos. 21575014 and 21175013).
| [1] | Q.L. Zhu, Q. Xu. Metal-organic framework composites. Chem. Soc. Rev. 43 (2014) 5468–5512. DOI:10.1039/C3CS60472A |
| [2] | W. Liu, X.B. Yin. Metal-organic frameworks for electrochemical applications. Trac Trend Anal. Chem. 75 (2016) 86–96. DOI:10.1016/j.trac.2015.07.011 |
| [3] | A. Morozan, F. Jaouen. Metal organic frameworks for electrochemical applications. Energy Environ. Sci. 5 (2012) 9269–9290. DOI:10.1039/c2ee22989g |
| [4] | H.R. Moon, D.W. Lim, M.P. Suh. Fabrication of metal nanoparticles in metal-organic frameworks. Chem. Soc. Rev. 42 (2013) 1807–1824. DOI:10.1039/C2CS35320B |
| [5] | Z. Zhang, H.T.H. Nguyen, S.A. Miller, S.M. Cohen. PolyMOFs: a class of interconvertible polymer-metal-organic-framework hybrid materials. Angew. Chem. Int. Edit. 54 (2015) 6152–6157. DOI:10.1002/anie.201502733 |
| [6] | Z. Xiang, Z. Hu, D. Cao, et al., Metal-organic frameworks with incorporated carbon nanotubes: improving carbon dioxide and methane storage capacities by lithium doping. Angew. Chem. Int. Ed. 50 (2011) 491–494. DOI:10.1002/anie.201004537 |
| [7] | Y. Li, C. Huangfu, H. Du, et al., Electrochemical behavior of metal-organic framework MIL-101 modified carbon paste electrode: an excellent candidate for electroanalysis. J. Electroanal. Chem. 709 (2013) 65–69. DOI:10.1016/j.jelechem.2013.09.017 |
| [8] | Y. Wang, Y. Wu, J. Xie, X. Hu. Metal-organic framework modified carbon paste electrode for lead sensor. Sens. Actuators B: Chem. 177 (2013) 1161–1166. DOI:10.1016/j.snb.2012.12.048 |
| [9] | Q. Xu, Y. Wang, G. Jin, et al., Photooxidation assisted sensitive detection of trace Mn2+ in tea by NH2-MIL-125(Ti) modified carbon paste electrode. Sens. Actuators B: Chem. 201 (2014) 274–280. DOI:10.1016/j.snb.2014.05.017 |
| [10] | K.S. Park, Z. Ni, A.P. Côté, et al., Exceptional chemical and thermal stability of zeolitic imidazolate frameworks. Proc. Natl. Acad. Sci. U. S. A. 103 (2006) 10186–10191. DOI:10.1073/pnas.0602439103 |
| [11] | S.R. Venna, M.A. Carreon. Highly permeable zeolite imidazolate framework-8 membranes for CO2/CH4 separation. J. Am. Chem. Soc. 132 (2009) 76–78. |
| [12] | C. Chizallet, S. Lazare, D. Bazer-Bachi, et al., Catalysis of transesterification by a nonfunctionalized metal-organic framework: acido-basicity at the external surface of ZIF-8 probed by FTIR and ab initio calculations. J. Am. Chem. Soc. 132 (2010) 12365–12377. DOI:10.1021/ja103365s |
| [13] | H. Bux, F. Liang, Y. Li, et al., Zeolitic imidazolate framework membrane with molecular sieving properties by microwave-assisted solvothermal synthesis. J. Am. Chem. Soc. 131 (2009) 16000–16001. DOI:10.1021/ja907359t |
| [14] | S.L. Li, Q. Xu. Metal-organic frameworks as platforms for clean energy. Energy Environ. Sci. 6 (2013) 1656–1683. DOI:10.1039/c3ee40507a |
| [15] | A.K. Geim, K.S. Novoselov. The rise of graphene. Nat. Mater. 6 (2007) 183–191. DOI:10.1038/nmat1849 |
| [16] | M. Pumera. Graphene in biosensing. Mater. Today 14 (2011) 308–315. DOI:10.1016/S1369-7021(11)70160-2 |
| [17] | D. Lu, Y. Zhang, L. Wang, et al., Sensitive detection of acetaminophen based on Fe3O4 nanoparticles-coated poly (diallyldimethylammonium chloride)-functionalized graphene nanocomposite film. Talanta 88 (2012) 181–186. DOI:10.1016/j.talanta.2011.10.029 |
| [18] | D. Li, M.B. Mueller, S. Gilje, R.B. Kaner, G.G. Wallace. Processable aqueous dispersions of graphene nanosheets. Nat. Nanotechnol. 3 (2008) 101–105. DOI:10.1038/nnano.2007.451 |
| [19] | J. Yang, F. Zhao, B. Zeng. One-step synthesis of a copper-based metal-organic framework-graphene nanocomposite with enhanced electrocatalytic activity. RSC Adv. 5 (2015) 22060–22065. DOI:10.1039/C4RA16950F |
| [20] | K. Liu, J. Zhang, G. Yang, C. Wang, J.J. Zhu. Direct electrochemistry and electrocatalysis of hemoglobin based on poly (diallyldimethylammonium chloride) functionalized graphene sheets/room temperature ionic liquid composite film. Electrochem. Commun. 12 (2010) 402–405. DOI:10.1016/j.elecom.2010.01.004 |
| [21] | H. Bai, Y. Xu, L. Zhao, C. Li, G. Shi. Non-covalent functionalization of graphene sheets by sulfonated polyaniline. Chem. Commun. (2009) 1667–1669. |
| [22] | C. Li, C. Hu, Y. Zhao, et al., Decoration of graphene network with metal-organic frameworks for enhanced electrochemical capacitive behavior. Carbon 78 (2014) 231–242. DOI:10.1016/j.carbon.2014.06.076 |
| [23] | Q. Zhou, G. Li, Y. Zhang, et al., Highly selective and sensitive electrochemical immunoassay of Cry1C using nanobody and π-π stacked graphene oxide/thionine assembly. Anal. Chem. 88 (2016) 9830–9836. DOI:10.1021/acs.analchem.6b02945 |
| [24] | Z. Zhou, Q. Shang, Y. Shen, et al., Chemically modulated carbon nitride nanosheets for highly selective electrochemiluminescent detection of multiple metal-ions. Anal. Chem. (2016) . |
| [25] | Z. Zhou, Y. Shen, Y. Li, et al., Chemical cleavage of layered carbon nitride with enhanced photoluminescent performances and photoconduction. ACS Nano 9 (2015) 12480–12487. DOI:10.1021/acsnano.5b05924 |
| [26] | X. Zhang, J. Bai, H.M. Zhang. Synthesis of nanosized LDHs by Au colloidal nanoparticles as nuclei and its application for electroanalysis. Appl. Clay Sci. 119 (2016) 410–416. DOI:10.1016/j.clay.2015.10.030 |
| [27] | Q. Lian, A. Luo, Z. An, et al., Au nanoparticles on tryptophan-functionalized graphenefor sensitive detection of dopamine. Appl.Surf.Sci. 349 (2015) 184–189. DOI:10.1016/j.apsusc.2015.04.217 |
| [28] | P.M. Hallam, C.E. Banks. A facile approach for quantifying the density of defects (edge plane sites) of carbon nanomaterials and related structures. Phys. Chem. Chem. Phys. 13 (2011) 1210–1213. DOI:10.1039/C0CP01562H |
| [29] | D. Nematollahi, H. Shayani-Jam, M. Alimoradi, S. Niroomand. Electrochemical oxidation of acetaminophen in aqueous solutions: kinetic evaluation of hydrolysis, hydroxylation and dimerization processes. Electrochim. Acta 54 (2009) 7407–7415. DOI:10.1016/j.electacta.2009.07.077 |
| [30] | X. Wang, Z. You, H. Sha, et al., Sensitive electrochemical detection of dopamine with a DNA/graphene bi-layer modified carbon ionic liquid electrode. Talanta 128 (2014) 373–378. DOI:10.1016/j.talanta.2014.04.078 |
| [31] | S. Palanisamy, S. Ku, S.M. Chen. Dopamine sensor based on a glassy carbon electrode modified with a reduced graphene oxide and palladium nanoparticles composite. Microchim. Acta 180 (2013) 1037–1042. DOI:10.1007/s00604-013-1028-1 |
| [32] | G.T.S. How, A. Pandikumar, H.N. Ming, L.H. Ngee. Highly exposed {001} facets of titanium dioxide modified with reduced graphene oxide for dopamine sensing. Sci. Rep. U.K. 4 (2014) 1–8. |
| [33] | Y.Y. Ling, Q.A. Huang, M.S. Zhu, et al., A facile one-step electrochemical fabrication of reduced graphene oxide-mutilwall carbon nanotubes-phospotungstic acid composite for dopamine sensing. J. Electroanal. Chem. 693 (2013) 9–15. DOI:10.1016/j.jelechem.2013.01.001 |
| [34] | L. Wu, L. Feng, J. Ren, X. Qu. Electrochemical detection of dopamine using porphyrin-functionalized graphene. Biosens. Bioelectron. 34 (2012) 57–62. DOI:10.1016/j.bios.2012.01.007 |
 2017, Vol. 28
2017, Vol. 28