Horseradish peroxidase (HRP), a sort of oxidoreductase found in the roots of horseradish, is used extensively in clinic medicine [1], chemical synthesis [2], effluent treatment [3], food industry [4] and other fields because of cheap production, relative stable property and wider variety of substrates compared with other enzymes. The heme-containing HRP can catalyze various of aromatic compounds oxidation in the presence of H2O2 to produce chromogenic reaction [5], which endows HRP with potential applications in enzyme linked immunosorbent assay (ELISA) and biosensors for diagnosis and biochemical detection [6-8]. Some pioneering studies have shown that the reaction mediums are the main factor of influencing HRP activity. For example, the HRP activity increases dramatically when the corresponding reaction occurs in emulsion/microemulsion [9-12], micelles/reverse micelles [13, 14], as well as gels [15-17]. It is clearly, surfactants are the significant components of these mediums and play important roles in enzymatic reaction. Kireyko et al. studied the mechanisms of peroxidase oxidation of o-dianisidine, 3, 3', 5, 5'-tetramethylbenzidine (TMB), and o-phenylenediamine in the presence of sodium dodecyl sulfate (SDS) and found the existence of SDS with certain concentration can stabilize intermediates formed in the peroxidase oxidation of these substrates due to the electrostatic interaction between positively charged intermediates and negatively charged surfactants [18]. Besides the electrostatic interaction, surfactant is amphiphilic and it can not only interact with species existed in the system by hydrophobic interaction but also enhance the solubility of hydrophobic species in solutions by forming micelles, which may affect the mechanism of enzymatic processes. Unfortunately, the intrinsical function and the mechanism of action for surfactant in these reactions are less involved. Obviously, TMB is an optimum substrate of HRP due to its excellent affinity with enzyme, low toxicity, and non-teratogenicity [19]. However, the intrinsic disadvantages of TMB, such as poor water solubility, short color developing time derived from the highly unstable chromogens, limit the scope of its practical application [20, 21]. Herein, the anionic surfactant SDS and ionic liquid 1-ethyl-3-methylimidazolium tetrafluoroborate ([Emim][BF4]) were combined together and used as the reaction medium of chromogenic catalysis of HRP-TMB-H2O2 aiming at creating a novel environment for both improving water solubility of TMB and stabilizing chromogens so that to obtain higher detection efficiency for HRP/TMB in biochemistry and to explore the mechanism of surfactant/ionic liquid in enzymatic processes deeply.
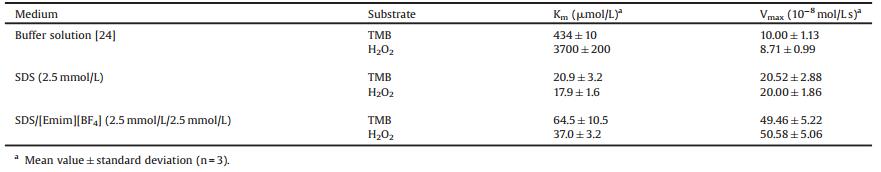
2. Results and discussion 2.1. The effect of different surfactants on HRP-TMB-H2O2 reactionIn this work, besides anionic surfactant SDS, cationic surfactant cetyltrimethylammonium bromide (CTAB) and non-ionic surfactant Tween 20 are involved in HRP-TMB-H2O2 reaction firstly for comparison. Here, we demonstrate that the stable blue chromogen (namely, diamine/diimine charge transfer complex) only can be obtained in the system containing SDS (Fig. 1), the anionic surfactants benefit the existence of blue diamine/diimine charge transfer complex (λm = 652 nm). However, the yellow diimine endproducts (λm = 450 nm) appear directly in cationic, nonionic surfactant solution as well as buffer solution (Fig. 1(b) and Chart S1 in Supporting information). The distinctions imply that the oxydate of TMB vary with the kind of surfactant. The stronger UV-vis absorbance at 652 nm for the sample containing both SDS and ionic liquid [Emim][BF4] suggests that the introduction of [Emim] [BF4] promoted the catalysis efficiency compared with that of sole SDS system. The blank color and the invisible UV-vis absorbance for samples F and G confirm that HRP and H2O2 are indispensable in the chromogenic catalysis of TMB and either SDS or [Emim][BF4] have no catalytic activity on substrate TMB.
|
Download:
|
| Fig. 1. (a) Time-dependent absorbance (652 nm) for different samples and the corresponding color images. The HRP-TMB-H2O2 reaction occurred in buffer solution (A), 2.5 mmol/L CTAB (B), 2.5 mmol/L Tween 20 (C), 2.5 mmol/L SDS (D), 5 mmol/L SDS/[Emim][BF4] (2.5 mmol/L/2.5 mmol/L) (E) Experiments were carried out using 200 μmol/L TMB, 2 mmol/L H2O2 and 0.05 μg/mL HRP in the solutions at pH = 7.4 and T = 25 ± 0.2 ℃ unless otherwise stated. (F) and (G) represent TMB-H2O2 and HRP-TMB in 5 mmol/L SDS/[Emim][BF4] (2.5 mmol/L/2.5 mmol/L), respectively. (b) Schematic illustration of chromogenic catalysis of HRP-TMB-H2O2 in different mediums. | |
Furthermore, the effect of the other two anionic surfactants, sodium dodecyl sulfonate and sodium dodecyl benzene sulfonate, on the chromogenic catalysis have been examined, both of them exhibit the capability of stabilizing the blue diamine/diimine charge transfer complex (Fig. S1 in Supporting information). Compared with sodium dodecyl sulfonate and sodium dodecyl benzene sulfonate, the anionic surfactant SDS shows more excellent properties in promoting the formation of blue chromogen as can be seen in Fig. S1. In addition to its wide applications in foods and pharmaceuticals due to its harmless [22], the SDS is singled out as the studied object in the present work.
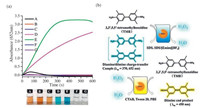
2.2. The effect of concentration of SDS and SDS/[Emim][BF4] combination on HRP-TMB-H2O2 reactionThe concentrations of surfactant and surfactant/ionic liquid in medium for chromogenic catalysis of TMB with HRP affect the color developing effect and reaction rate significantly (Fig. 2). In Fig. 2(a) and (b), the absorbance at 652 nm in 5 min corresponds to the accumulating concentration of the blue chromogens of TMB. We found the accumulations of blue chromogens in mediums of both SDS and SDS/[Emim][BF4] combination solutions undergo the courses from increase to decrease with the increase of SDS or SDS/ [Emim][BF4] concentration. For sole SDS solution, the optimum concentration corresponding to the highest peaks and deepest blue color is 2.5 mmol/L (Fig. 2(a)E), slightly higher than the critical micelle concentration (CMC) of SDS in buffer solution (Table S1 in Supporting information). The similar case also can be found in systems containing both SDS and [Emim][BF4]. The optimum total concentration for the combination of SDS/[Emim][BF4] is 5 mmol/L (2.5 mmol/L SDS and 2.5 mmol/L [Emim][BF4]) (Fig. 2(b)E). Interestingly, for the optimized medium with and without ionic liquid, the SDS concentration keeps constant even though the CMC of SDS decreases to 1.1 mmol/L after introducing the [Emim][BF4] to the system (Table S1). This suggests that the charge numbers carried by SDS aggregates maybe the key factor impacting the productivity of the blue chromogens. However, compared with the sole SDS system, the stronger UV-vis absorbance companied with deeper blue is observed for the samples prepared by SDS/[Emim] [BF4] combination. Moreover, the compounded solutions seems more competent in stabilizing the blue chromogens because there is no any green change in color can be detected here as can be seen in sole SDS solutions of 5.0 mmol/L and 10 mmol/L (F and G in Fig. 2(a)), which characterizes the coexistence of blue chromogen and yellow end-product in equilibrium (Fig. S2 in Supporting information) [18].These excellent chromogenesis presented in mediums of SDS/[Emim][BF4] combinations manifest the synergistic effect of SDS and [Emim][BF4] on the chromogenic catalysis of HRP-TMB-H2O2.
|
Download:
|
| Fig. 2. UV-vis spectra and the corresponding color images for chromogenic catalysis of TMB-HRP-H2O2 occurs five minutes in sole SDS solutions (a) and SDS/[Emim][BF4] combinations (b). Time-dependent UV-vis absorbance (652 nm) for samples prepared by sole SDS solution (c) and by SDS/[Emim][BF4] combinations (d). The molar ratios for all SDS/[Emim][BF4] combinations are 1:1. | |
For the present reaction systems, the rate of formation of the blue chromogen the mean rate of formation within 200 s (Table S2 in Supporting information) varies with the concentration of SDS and SDS/[Emim][BF4] combination significantly (Fig. 2(c) and (d)) and reach maximum when SDS concentration is 2.5 mmol/L in both systems with and without ionic liquids. This consistency suggests that the charge numbers carried by SDS aggregates may also influence the reaction kinetics. It is worth mentioning that the blue chromogen is both the product and the reactant (namely intermediate) of consecutive reaction (TMB → blue chromogens →yellow diimine end-products) in buffer solution, therefore the maximum of the concentration of the blue chromogens appear (Fig. 2(c)A and (d)A), which is the most remarkable peculiarity of the consecutive reaction. However, the peculiarity of the consecutive reaction disappears gradually with the increase of the introduced SDS. Obviously, the SDS greatly stabilized the blue chromogens by their electrostatic interaction and thus leading to the weakening and even end of the reaction from blue chromogens to yellow diimine end-products. In this view, SDS increases the rate of formation of the blue chromogen. Furthermore, the negatively charged SDS may facilitate the electron transfer between HRP and substrates and also can conduce to the increase of the rate of formation of blue chromogen with the increase of SDS concentration. However, when SDS concentration is over 2.5 mmol/L, the stronger interaction between SDS and HRP prevails and cause the denaturation of HRP gradually. The sharp decline in UV-vis absorbance of the samples containing higher concentration SDS and SDS/[Emim][BF4] should also be attributed to HRP denaturation (destabilization of HRP helical structure) under the irritation of SDS, which has been verified by the weakening of negative peak of HRP in CD spectroscopy (Fig. S3 in Supporting information).
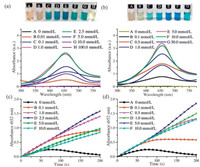
2.3. The respective effect of SDS and [Emim][BF4] on the reaction of HRP-TMB-H2O2We explore the respective functions of SDS and [Emim][BF4] in the chromogenic catalysis further. Firstly, by fixing SDS concentration at the optimum concentration of 2.5 mmol/L and then increasing the [Emim][BF4] concentration in the medium, the rate of formation of the blue chromogen (the beginning linear portion in Fig. 3(a) and (b), namely νmax in following section) increases dramatically (Fig. 3(a)). Then, keep the concentration of [Emim] [BF4] constant but increase the SDS concentration in the medium, there is no obvious changes observed for the rate of formation of the blue chromogen regardless of the variation of SDS concentration, as shown in Fig. 3(b). Obviously, the [Emim][BF4] in the mediums is the main factor of promoting rate of formation of the blue chromogen. Further observation we found that the UV-vis absorbance of the samples prepared by SDS/[Emim][BF4] combination increases significantly with the increases of SDS concentration when the reaction reaches equilibrium, which reconfirms that the SDS is responsible for the stabilization of the blue chromogen due to the electrostatic attraction between positively charged blue chromogen and negatively charged surfactant. It is worth mentioning that [Emim][BF4] is in favor of increasing the rate of formation but is incapable of stabilizing the blue chromogen that tends to decompose quickly in sole [Emim][BF4] solution evidenced by UV-vis absorbance decreases at 652 nm but increases at 450 nm as well as the faded color during the course of reaction (Fig. S4 in Supporting information).

|
Download:
|
| Fig. 3. Time-dependent UV-vis absorbance of different samples. (a) (A-E: the mediums are fixed SDS concentration at 2.5 mmol/L and varied [Emim][BF4] concentration, F: buffer solution); (b) (A-D: the mediums are fixed [Emim][BF4] concentration at 2.5 mmol/L and varied SDS concentration, D: sole [Emim][BF4] (2.5 mmol/L), E: buffer solution). Dash lines mark off the initial reaction period. TEM images of before (c) and after (d) reaction of HRP-TMB-H2O2 in 5 mmol/L SDS/[Emim][BF4] (2.5 mmol/L/ 2.5 mmol/L). | |
The blue chromogens are the distinct chromogens of TMB, we captured their morphology by TEM. Fig. 3(c) and (d) provides the images before and after reaction in the medium of SDS/[Emim] [BF4] (2.5 mmol/L/2.5 mmol/L) solution respectively. It is clearly, the typical spherical SDS micelles disperse in the solution before the reaction. However, the rod-like crystal with about 200 nm diameters and 3-4 μm lengths were observed after the reaction, which is diamine/diimine charge-transfer complexes exactly confirmed by the disappearance of UV-vis absorbance at 652 nm after the reaction solution was filtrated (Fig. S5 in Supporting information). Further observation we found that the rod-like products were covered by numerous spherical species that should be the resultant of further rearrangement of surfactant SDS during the course of reaction. Just the attachment of negatively charged SDS aggregates on diamine/diimine charge-transfer complexes maintains the stability of the blue chromogens.
2.4. Kinetic assay of HRP-TMB-H2O2 in mediums with SDS and SDS/ [Emim][BF4] combinationThe optimized SDS/[Emim][BF4] combination (the mixed solution of 2.5 mmol/L SDS and 2.5 mmol/L [Emim][BF4]) is specified as the medium for chromogenic catalysis of HRP-TMBH2O2 and the dynamics of the catalytic reaction is investigated quantitatively. Table 1 summarized the kinetic parameters in different mediums obtained by Michaelis-Menten curves (Fig. S6 in Supporting information) and Michaelis equation as followed Eq. (1), where, v0 is initial velocity, vmax is maximum initial velocity, [S] is the concentration of the substrate, Km is the apparent Michaelis-Menten constant usually identified as an indicator of enzyme affinity to substrates, whose value just relates to the pH, temperature, and ionic strength of surroundings, but irrelevant to the concentration of substrates or enzymes for the specific enzymatic reaction. A lower Km value means a stronger affinity and vice versa [23, 24].
|
|
Table 1 The kinetic parameters for catalytic reaction of HRP-TMB-H2O2 in different mediums. |
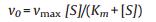
|
(1) |
Among the kinetic parameters of three different reaction systems, the Km values of TMB and H2O2 obtained in mediums containing SDS or SDS/[Emim][BF4] combinations are much lower than that of in buffer solution, indicating the enzymes have higher binding affinities to substrates in SDS and SDS/[Emim][BF4] solutions compared with that in buffer solution. This may be attributed to the immobilization of HRP on the surface of negatively charged SDS micelles that encapsulate the hydrophobic TMB. It is known that the ionic liquid [Emim][BF4] carried an imidazole ring that has the similar structure as histidine that originally bind to the iron center of the heme [25] and thus enable [Emim][BF4] to compete with substrates for bonding to heme. This maybe the main reason for the decrease of the affinity between HRP and substrates in the system containing SDS/[Emim][BF4] combination. Accordingly, the Km value obtained in solution of SDS/[Emim][BF4] combination is higher than that of in sole SDS solution. Additionally, the strong electrostatic interaction between the imidazolium cation and the carboxyl on HRP leads to more hemes exposure in the solution [26], which is verified by the significant increase of peak intensity of Soret band of heme at 403 nm in [Emim][BF4] aqueous solution (Fig. S7 in Supporting information). Cyclic voltammetry curves of HRP demonstrated that the characteristic reductive peak current of porphyrinatoiron (Ⅲ) in [Emim][BF4] aqueous solution is more negative (Fig. S8 in Supporting information), which reconfirmed that more hemes are exposed between solution and the electrodes [26]. Correspondingly, the catalytic capacity of HRP is enhanced remarkably in system containing [Emim][BF4] and therefore the vmax increases significantly.
2.5. Mechanism of the catalytic reaction of HRP-TMB-H2O2 in SDS/ [Emim][BF4] combinationThe catalytic mechanism for TMB with HRP in common medium has been proposed in previous work [27]. However, the introduction of SDS and [Emim][BF4] affects the reaction rate and products. Obviously, the catalytic mechanism and processes maybe changed. Based on the above discussion the possible catalytic mechanism for TMB with HRP in SDS/[Emin][BF4] combination medium was proposed as follow:
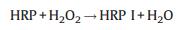
|
(1) |
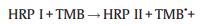
|
(2) |

|
(3) |
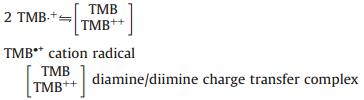
|
(4) |
It is obviously that the surficial protein of HRP (pI = 4.2) carries with negative charges in PBS (pH 7.4), which enable HRP interacts with imidazolium cation of ILs easily. The strong electrostatic interaction lead to more hemes exposure in the solution and therefore benefits H2O2 binding to heme to generate HRP Ⅰ that is the highly oxidation state intermediate comprising a Fe (Ⅳ) and a porphyrin-based cation radical in Reaction (1) [28]. Subsequently, the positively charged HRP Ⅰ tends to absorb on the surface of the existing SDS micelles that encapsulate the hydrophobic TMB. The existence of negatively charged SDS micelles not only provides a location for immobilizing HRP Ⅰ but also facilitates the electron transfer between TMB and HRP Ⅰ. Correspondingly, the cation radical TMB·+ and HRP Ⅱ formed during the course of Reaction (2). The produced HRP Ⅱ is further reduced to HRP by reacting with TMB, and the other cation radical TMB·+ is generated simultaneously as can be seen in Reaction (3). Two active cation radicals TMB·+ then will polymerize to form diamine/diimine charge transfer complex further (Reaction (4)). The diamine/diimine charge transfer complex, namely blue chromogen, is stabilized by the strong electrostatic interaction between SDS and blue chromogen in the studied system. In a word, the combination of [Emim][BF4] and SDS not only improve the efficiency of the catalysis of HRP-TMB-H2O2 but also maintain the stability of the blue chromogen.
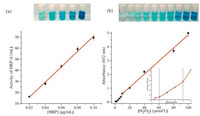
2.6. Detection of HRP activity and H2O2 concentration using SDS/ [Emim][BF4] combination as mediumBased on the above analysis, the colorimetric strategies for detection of HRP activity and H2O2 concentration were explored based on the SDS/[Emim][BF4] (2.5 mmol/L/2.5 mmol/L) combination as medium of chromogenic catalysis of HRP-TMB-H2O2. The activity of HRP is defined as the increment of the blue chromogen concentration in unit interval catalyzed by per-volume HRP, it can be calculated by Eq. (2) (Supporting information). The activity assay (Fig. 4(a)) shows good linearity between activity and mass concentrations of HRP, which implies the reliability of the method. Moreover, the chromogenic system also exhibits higher sensitivity in determining the concentration of H2O2. The low detection limit of 1 μmol/L and two linear ranges of low concentration from 2 μmol/L to 8 μmol/L and 10 μmol/L to 100 μmol/L (Fig. 4(b), Eq. (3) in Supporting information) are comparable to other methods (Table S3 in Supporting information) [23, 29]. Obviously, the introduce of SDS/[Emim][BF4] combination in the medium endows the system some novel advantages in detection of HRP activity and H2O2 concentration, such as simple and safety operation, non-toxic, higher sensitivity etc. Furthermore, the studied system is potential to be applied in the detection of the H2O2-mediated oxidation of glucose, cholesterol in blood [23, 30, 31] or in the refined Elisa kits.
|
Download:
|
| Fig. 4. (a)The variation of activity with mass concentration (0.02μg/mL -0.10 μg/mL) for HRP, (b) the linear calibration plots for H2O2 (1 μmol/L -100 μmol/L) in medium of 5 mmol/L SDS/[Emim][BF4] (2.5 mmol/L/2.5 mmol/L) combination and the corresponding color images. The error bars represent the standard error derived from three repeated measurements. | |
3. Conclusion
In summary, a novel efficient medium for chromogenic catalysis of TMB with HRP in presence of H2O2 was proposed by combination SDS and [Emim][BF4] properly. The possible catalytic mechanism for TMB with HRP in SDS/[Emin][BF4] combination medium was proposed. [Emim][BF4] contributes to the enhancement of HRP catalytic capacity and the surfactant SDS mainly expert at stabilizing the blue chromogens. Based on the superior combination of SDS and [Emim][BF4], the colorimetric assay for detecting HRP activity and H2O2 concentration was established. This work demonstrates a novel efficient medium for chromogenic catalysis with potential applications in biosensors and clinical diagnosis.
4. Experimental 4.1. Materials3, 3', 5, 5'-tetramethlybenzidene and horseradish peroxidase (HRP, EC1.11.1.17, 206 U/mg) were purchased from Sigma-Aldrich (St. Louis, MO) and stored in a refrigerator at 4 ℃. Sodium dodecyl sulfate (SDS), 1-ethy-3-methyllmidazolium tetrafluoroborate ([Emim][BF4]), DMSO-d6 and guanidine hydrochloride were purchased from Aladdin Industrial Co., Ltd. Sodium dihydrogen phosphate dehydrate, Sodium phosphate dibasic dodecahydrate and 30% hydrogen peroxide solution (H2O2) were purchased from Shanghai Lingfeng Chemical Reagent Co., Ltd. Ultrapure water with a resistivity of 18.2 MV cm from Millipore Simplicity water purification system was used for preparing phosphate buffer solutions (1 mmol/L, pH 7.4) that were the solvents for all experiments. All other chemicals were of analytical reagent grade and used without further purification.
4.2. ApparatusUV-2550 UV-vis spectrophotometer (Shimadzu UV-2550, Japan) and a 1 mL measurement cell was used for absorbance spectrums and kinetics experiments. CMC of SDS was measured by the dynamic contact angle measuring instrument and tensiometer DCAT11 (Dataphysics, GER). CD spectra of HRP in far-ultraviolet (UV) region (190-260 nm) was recorded on a J-810 CD spectrometer (Jasco, Japan) using a 1.0 mm quartz cuvette. Cyclic voltammetry experiment was performed on a CHI 630C Electrochemical Analyzer (Shanghai Chenhua Co., China). The three-electrode system was composed of a working electrode (GCE), a platinum wire counter electrode, and a silver chloride reference electrode. Transmission electron microscopy (TEM) images were obtained on a JEM-2100F (JEOL, Japan) instrument at accelerating voltage of 200 kV.
4.3. Samples preparationFirstly, stock solutions (0.01 mol/L) of different surfactants and [Emim][BF4] were prepared in the phosphate buffer solution and then equilibrated at 25 ± 0.2 ℃ for at least 24 h until reach equilibrium. Then the stock solution of SDS (or CTAB, Tween 20) was diluted to 2.5 mmol/L. Stock solutions of SDS and [Emim][BF4] were mixed together and then diluted to prepare mixed solutions with different compositions. The above mediums were treated in ultrasonic bath until dispersing uniformly and then placed in an incubator at 25 ± 0.2 ℃.
4.4. BioassayThe following experiments were measured under the same conditions of 25 ± 0.2 ℃ and pH = 7.4. UV-vis spectra were performed by monitoring the absorbance at a specific time when the reaction occurs 5 min. Time-dependent curves were carried out in time course mode by monitoring the absorbance change at 652 nm. The concentration of HRP, TMB and H2O2 is 0.05 μg/mL, 200 μmol/L and 2 mmol/L respectively for all catalytic experiment unless otherwise stated. Firstly, 0.05 μg/mL HRP solutions incubated for 1 h in different reaction mediums (pH = 7.4, 25 ± 0.2 ℃), then added 8 μL TMB (0.2 mol/L) stock solution prepared in DMSO-d6 and 2 μL H2O2 (8.0 mmol/L) into the incubation mediums to obtain 8 mL reaction solutions, took 3 mL sample for absorbance measurements.
The kinetic assays of HRP-TMB-H2O2 were further conducted in the mediums containing SDS (2.5 mmol/L) and SDS/[Emim][BF4] (2.5 mmol/L/2.5 mmol/L) combination, kinetic parameters were obtained by the enzyme kinetics theory and methods. The typical Michaelis-Menten curve was obtained by fitting the Menten equation. Kinetic assays were carried on fixing HRP concentration at 0.05 mg/mL and varying concentrations of H2O2 and TMB.
The activity assay of HRP was realized as follows. Firstly, 24μL TMB stock solution (0.2 mol/L), 2 μL H2O2 (8.0 mol/L) were added into the mediums containing SDS/[Emim][BF4] (2.5 mmol/L/ 2.5 mmol/L) combinations, then added HRP solutions into the mixtures to obtain reaction solutions (8 mL) with different mass concentrations of HRP (10-50 mg/mL)and mixed quickly. Finally, took 3 mL sample for recording the absorbance at 652 nm within 1 min.
H2O2 detection was measured by the following steps. Firstly, 8 μL TMB stock solution (0.2 mol/L), different volumes of H2O2 were added into the mediums containing SDS/[Emim][BF4] (2.5 mmol/L/2.5 mmol/L) combinations to obtain mixtures with different H2O2 concentration (1 μmol/L-100 μmol/L). Then added 4 mL HRP solution (0.1 mg/mL) into the mixtures to obtain reaction solutions (8 mL) and mixed quickly. Finally, took 3 mL sample for recording the absorbance at 652 nm at the specific time when the reaction occurred 3 min.
AcknowledgmentWe thank the National Natural Science Foundation of China (Nos. 21476072, 91334203) for supporting this work.
Appendix A. Supplementary dataSupplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.cclet.2017.03.012.
| [1] | L.K. Folkes, P. Wardman. Oxidative activation of indole-3-acetic acids to cytotoxic species— a potential new role for plant auxins in cancer therapy1. Biochemi. Pharmacol. 61 (2001) 129–136. DOI:10.1016/S0006-2952(00)00498-6 |
| [2] | P. Domínguez de María. Nonsolvent applications of ionic liquids in biotransformations and organocatalysis. Angew. Chem. Int. Ed. 47 (2008) 6960–6968. DOI:10.1002/anie.v47:37 |
| [3] | A. Bhunia, S. Durani, P.P. Wangikar. Horseradish peroxidase catalyzed degradation of industrially important dyes. Biotechnol. Bioeng. 72 (2001) 562–567. DOI:10.1002/(ISSN)1097-0290 |
| [4] | M. Gerard, A. Chaubey, B.D. Malhotra. Application of conducting polymers to biosensors. Biosens. Bioelectron. 17 (2002) 345–359. DOI:10.1016/S0956-5663(01)00312-8 |
| [5] | G.I. Berglund, G.H. Carlsson, A.T. Smith, H. Szöke, A. Henriksen, J. Hajdu. The catalytic pathway of horseradish peroxidase at high resolution. Nature 417 (2002) 463–468. DOI:10.1038/417463a |
| [6] | J. Jia, B. Wang, A. Wu, G. Cheng, Z. Li, S. Dong. A method to construct a thirdgeneration horseradish peroxidase biosensor: self-assembling gold nanoparticles to three-dimensional sol-gel network. Anal. Chem. 74 (2002) 2217–2223. DOI:10.1021/ac011116w |
| [7] | D. Tang, R. Yuan, Y. Chai. Ultrasensitive electrochemical immunosensor for clinical immunoassay using thionine-doped magnetic gold nanospheres as labels and horseradish peroxidase as enhancer. Anal. Chem. 80 (2008) 1582–1588. DOI:10.1021/ac702217m |
| [8] | G. Liu, Y. Wan, V. Gau, J. Zhang, L. Wang, S. Song, C. Fan. An enzyme-based EDNA sensor for sequence-specific detection of femtomolar DNA targets. J. Am. Chem. Soc. 130 (2008) 6820–6825. DOI:10.1021/ja800554t |
| [9] | M. Moniruzzaman, N. Kamiya, M. Goto. Biocatalysis in water-in-ionic liquid microemulsions: a case study with horseradish peroxidase. Langmuir 25 (2008) 977–982. |
| [10] | M. Moniruzzaman, N. Kamiya, K. Nakashima, M. Goto. Water-in-ionic liquid microemulsions as a new medium for enzymatic reactions. Green Chem. 10 (2008) 497–500. DOI:10.1039/b802501k |
| [11] | I.V. Pavlidis, D. Gournis, G.K. Papadopoulos, H. Stamatis. Lipases in water-inionic liquid microemulsions: structural and activity studies. J. Mol. Catal. B: Enzym. 60 (2009) 50–56. DOI:10.1016/j.molcatb.2009.03.007 |
| [12] | L. Xue, H. Qiu, Y. Li, L. Lu, X. Huang, Y. Qu. A novel water-in-ionic liquid microemulsion and its interfacial effect on the activity of laccase. Colloids Surf. B: Biointerfaces 82 (2011) 432–437. DOI:10.1016/j.colsurfb.2010.09.016 |
| [13] | D. Mandal, M. Ghosh, S. Maiti, K. Das, P.K. Das. Water-in-oil microemulsion doped with gold nanoparticle decorated single walled carbon nanotube: Scaffold for enhancing lipase activity. Colloids Surf. B: Biointerfaces 113 (2014) 442–449. DOI:10.1016/j.colsurfb.2013.09.047 |
| [14] | S. Maiti, M. Ghosh, P.K. Das. Gold nanorod in reverse micelles: a fitting fusion to catapult lipase activity. Chem. Commun. 47 (2011) 9864–9866. DOI:10.1039/c1cc12940f |
| [15] | D. Das, S. Roy, S. Debnath, P.K. Das. Surfactant-stabilized small hydrogel particles in oil: hosts for remarkable activation of enzymes in organic solvents. Chem.-Eur. J. 16 (2010) 4911–4922. DOI:10.1002/chem.200903205 |
| [16] | Q. Wang, Z. Yang, Y. Gao, W. Ge, L. Wang, B. Xu. Enzymatic hydrogelation to immobilize an enzyme for high activity and stability. Soft Matter 4 (2008) 550–553. DOI:10.1039/b715439a |
| [17] | Q. Wang, Z. Yang, L. Wang, M. Ma, B. Xu. Molecular hydrogel-immobilized enzymes exhibit superactivity and high stability in organic solvents. Chem. Commun. (2007) 1032–1034. |
| [18] | A. Kireyko, I. Veselova, T. Shekhovtsova. Mechanisms of peroxidase oxidation of o-dianisidine, 3, 3', 5, 5'-tetramethylbenzidine, and o-phenylenediamine in the presence of sodium dodecyl sulfate. Russ. J. Bioorg. Chem. 32 (2006) 71–77. DOI:10.1134/S1068162006010079 |
| [19] | V. Holland, B. Saunders, F. Rose, A. Walpole. A safer substitute for benzidine in the detection of blood. Tetrahedron 30 (1974) 3299–3302. DOI:10.1016/S0040-4020(01)97504-0 |
| [20] | M.W. Trucksess. Determination of Cry9C protein in corn-based foods by enzyme-linked immunosorbent assay: interlaboratory study. J. AOAC Int. 84 (2001) 1891–1901. |
| [21] | P. Ni, Y. Sun, H. Dai, J. Hu, S. Jiang, Y. Wang, Z. Li. Highly sensitive and selective colorimetric detection of glutathione based on Ag[Ⅰ] ion-3, 3', 5, 5'-tetramethylbenzidine (TMB). Biosens. Bioelectron. 63 (2015) 47–52. DOI:10.1016/j.bios.2014.07.021 |
| [22] | N. Salehi, A. Moosavi-Movahedi, L. Fotouhi, S. Yousefinejad, M. Shourian, R. Hosseinzadeh, N. Sheibani, M. Habibi, - Rezaei. Heme degradation upon production of endogenous hydrogen peroxide viainteraction of hemoglobin with sodium dodecyl sulfate. J. Photochem. Photobiol. B: Biol. 133 (2014) 11–17. DOI:10.1016/j.jphotobiol.2014.02.014 |
| [23] | K. Zhao, W. Gu, S. Zheng, C. Zhang, Y. Xian. SDS-MoS2 nanoparticles as highlyefficient peroxidase mimetics for colorimetric detection of H2O2 and glucose. Talanta 141 (2015) 47–52. DOI:10.1016/j.talanta.2015.03.055 |
| [24] | L. Gao, J. Zhuang, L. Nie, J. Zhang, Y. Zhang, N. Gu, T. Wang, J. Feng, D. Yang, S. Perrett. Intrinsic peroxidase-like activity of ferromagnetic nanoparticles. Nat. Nanotech. 2 (2007) 577–583. DOI:10.1038/nnano.2007.260 |
| [25] | H. Gharibi, Z. Moosavi-Movahedi, S. Javadian, K. Nazari, A.A. MoosaviMovahedi. Vesicular mixed gemini-sds-hemin-imidazole complex as a peroxidase-like nano artificial enzyme. J. Phys. Chem. B 115 (2011) 4671–4679. DOI:10.1021/jp112051t |
| [26] | L. Lu, Y. Hu, X. Huang, Y. Qu. A bioelectrochemical method for the quantitative description of the Hofmeister effect of ionic liquids in aqueous solution. J. Phys. Chem. B 116 (2012) 11075–11080. |
| [27] | P.D. Josephy, T. Eling, R.P. Mason. The horseradish peroxidase-catalyzed oxidation of 35, 3'. 5'-tetramethylbenzidine. Free radical and charge-transfer complex intermediates, J. Biol. Chem. 257 (1982) 3669–3675. |
| [28] | N.C. Veitch. Horseradish peroxidase: a modern view of a classic enzyme. Phytochemistry 65 (2004) 249–259. DOI:10.1016/j.phytochem.2003.10.022 |
| [29] | Y. Liu, M. Yuan, L. Qiao, R. Guo. An efficient colorimetric biosensor for glucose based on peroxidase-like protein-Fe 3 O 4 and glucose oxidase nanocomposites. Biosens. Bioelectron. 52 (2014) 391–396. DOI:10.1016/j.bios.2013.09.020 |
| [30] | Q. Liu, L. Zhang, H. Li, Q. Jia, Y. Jiang, Y. Yang, R. Zhu. One-pot synthesis of porphyrin functionalized gamma-Fe2O3 nanocomposites as peroxidase mimics for H2O2 and glucose detection. Mater. Sci. Eng. C Mater. Biol. Appl. 55 (2015) 193–200. DOI:10.1016/j.msec.2015.05.028 |
| [31] | N.R. Nirala, S. Abraham, V. Kumar, A. Bansal, A. Srivastava, P.S. Saxena. Colorimetric detection of cholesterol based on highly efficient peroxidase mimetic activity of graphene quantum dots. Sens. Actuators B: Chem. 218 (2015) 42–50. DOI:10.1016/j.snb.2015.04.091 |
 2017, Vol. 28
2017, Vol. 28