b College of Materials and Energy, Institute of Biomaterial, South China Agricultural University, Guangzhou 510642, China
People still paly a great deal of attention on dinuclear copper complexes with two metal ions in close proximity. These complexes are particularly of interest in relation to their potential uses as catalysts, biological processes and inorganic materials [1-3]. Generally, efforts are made to magnetic interactions between copper ions, and such studies have served for understanding the spin-exchange mechanism in antiferro-or ferromagnetic coupling [4]. However, their potentials as new materials in liquid have been ignored. Inspired by that hydrogenase enzymes (nickel or iron complexes) can efficiently catalyze both the production and the oxidation of dihydrogen [5], and several molecular catalysts for the production of hydrogen from acid or water based on transition metal complexes have been developed [6-9], we also designed some molecular electrocatalysts by the assemblies of complexes [10-12]. It is widely regarded that transition metals capable of forming metal-hydride intermediates are essential to the HER mechanism. Based on this consideration, much effort has been made to design coordinatively unsaturated complexes supported by tetra-and pentadentate ligands for proton or water reduction [13-16]. As an expanded work, here we present the synthesis, structure, characterization and magnetic properties of one new copper(Ⅱ) complex, [(HL)CuCl-CuCl(HL)]HCl 1, as well as its electro-catalytic properties for proton and water reduction to hydrogen.
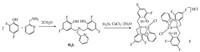
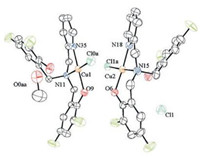
2. Results and discussion 2.1. General characterizationBased on the literature procedure, 2-(pyridylmethyl)amino-N, N-bis(2-methylene-4, 6-difluorophenol) (H2L) was prepared [17]. In the presence of triethylamine, the reaction of CuCl2·2H2O and H2L at one molar ratio set of 1:1 provides a novel Cu(Ⅱ) complex 1, [(HL)CuCl-CuCl(HL)]HCl (Scheme 1). As shown in Fig. 1, in solid complex 1 consists of two copper units. Each of the metal centers is coordinated by two nitrogen atoms and one oxygen atom from one deprotonated ligand (HL-). The observed Cu-N bond distances fall in the range 1.991(3)-2.078(4) Å and the bond distances of Cu—O fall in the range 1.946(3)-1.980(3) Å. Noteworthy is that the Cu—Cl bond distances are asymmetric at 2.2754(11) and 2.2844(12) Å, respectively. To test the state of complex 1 in liquid, we also did ESI-MS measurement in methanol. From Fig. S1 (Supporting information), Cl- ligand dissociates from copper center in methanol, which is in agreement with the result from ESI-MS measurement which exhibits one ion at a mass-to-charge ratio (m/z) of 454.0386, with the mass and isotope distribution pattern corresponding to that of [HL-Cu]+ (calcd. m/z of 454.824).
|
Download:
|
| Scheme 1. Schematic representation of the synthesis of [(HL)CuCl-CuCl(HL)]HCl 1. | |
|
Download:
|
| Fig. 1. ORTEP drawing of complex 1 with thermal ellipsoids on the 50% probability level (hydrogen atoms are not shown). Selected bond distances (Å): Cu(1)—Cl(4), 2.2844(12); Cu(1)—O(9), 1.980(3); Cu(1)—N(11), 2.062(3); Cu(1)—N(35), 2.013(3); Cu(2)—Cl(3), 2.2754(11); Cu(2)—O(6), 1.946(3); Cu(2)—N(15), 2.078(4); Cu(2)—N (18), 1.991(3). | |
2.2. Magnetic properties of complex 1
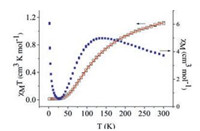
To explore its properties as one magnetic material in solid, the magnetic behavior of complex 1 was investigated in the temperature range 2-300 K. As shown in Fig. 2, the decrease of χMT from 1.122 cm3·K·mol-1 at 300 K to 0.0126 cm3·K·mol-1 at 2 K reveals a paramagnetic system with an antiferromagnetic interaction for 1. It is worthy to note that the χM values exhibit a complicated change when the temperature is lowered. The value increases to a maximum around 140 K, then decreases to a minimum around 28 K, finally increases abruptly cooling to 2 K (Fig. 2). The magnetic analysis was carried out using the a modified Bleaney-Bowers equation S1 = S2 = 1/2 spin coupled dimer model (H = -2JS1S2) [Eq. (1) [4, 18]
|
Download:
|
| Fig. 2. Plot of the temperature dependence of the cM and cMT for complex 1. | |
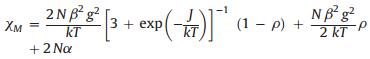
|
(1) |
In which N, g, β, k and T have their usual meanings; J is magnetic exchange coupling; ρ is the molar fraction of noncoupled species; Nα shows the contributions of the magnetic impurity and the sum of diamagnetic and van Vleck paramagnetic parts, respectively. The least-squares fits to the magnetic susceptibility data (the red solid line of Fig. 2) to give the parameters: J =-160 cm-1, ρ = 7.96 × 10-2, Nα = 1.1 ×10-4 cm3 mol-1 and R = 5.4 ×10-6 with g of 2.0 fixed. The J value indicates the moderately strong antiferromagnetic exchange coupling within the copper(Ⅱ) units. The magnetic behavior of complex 1 is well correlated with the previous work [19].
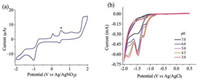
From ESI-MS analysis (Fig. S1 in Supporting information), the title complex provides the species of [HL-Cu]+ in methanol. To study its function as an electrocatalyst in liquid, the electrochemical behavior of 1 was investigated in a DMF solution with [(n-Bu)4N]ClO4 as the supporting electrolyte. As shown in Fig. 3a, the CV of complex 1 shows a quasi-reversible redox peak at -0.86 V versus Ag/AgNO3, which is assigned to the couple of CuⅡ/CuⅠ.
|
Download:
|
| Fig. 3. (a) Cyclic voltammogram (CV) for 0.44 mmol/L solution of complex 1 in 0.10 mol/L of [n-Bu4N]ClO4 acetonitrile solution at a glassy carbon electrode and a scan rate of 100 mV/s. Ferrocene internal standard (*). (b) CVs of 0.424 mmol/L complex 1 with varying pH values. | |
To test possible electrocatalytic activity of this copper complex, CVs of complex 1 were measured in the presence of acetic acid (proton source). From Fig. S2 (Supporting information) it can be seen that the catalytic current near the couple of CuⅡ/Ⅰ (-0.86 V versus Ag/AgNO3) increased with increasing acetic acid concentration from 0.00 mmol/L to 1.40 mmol/L. This indicates that hydrogen evolution electrocatalyzed by complex 1 requires the reduction of Cu(Ⅱ) to Cu(Ⅰ), Cu(Ⅰ) to Cu(0) and protonation. Interestingly, upon addition of acetic acid, a new quasi-reversible redox peak and an irreversible reduction wave were observed near -1.55 (CuⅠ/Cu0) and -0.78 V versus Ag/AgNO3, respectively. Further addition of acid from 0.0 to 1.4 mmol/L results in a positive shift from -0.65 V to -0.42 V versus Ag/AgNO3, and an increase in the peak current, consistent with a catalytic process [20]. Based on the above observations, the couples of CuⅡ/CuⅠ and CuⅠ/Cu0 are devoted to proton reduction, which were observed in the reported copper complexes [10, 15].
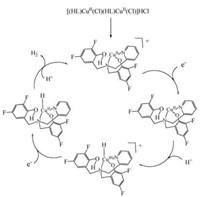
On the basis of above analyses and literature precedents [15, 21], we also tried to propose the catalytic cycle depicted in Scheme 2 for the generation of hydrogen from acetic acid mediated by 1. Oneelectron reduction of [(HL)CuⅡ]+ provides a putative [(HL)CuⅠ] species. Addition of hydrogen proton to [(HL)CuⅠ]- gives the CuⅢ-H species. Then one-electron reduction of the CuⅢ-H species affords H2, and further regenerates the starting complex 1. Although the relative contributions are indistinguishable in this analysis, we suspect that these processes are complementary H2 evolution pathways.
|
Download:
|
| Scheme 2. The possible catalytic mechanism for proton reduction by [(HL)CuCl-CuCl(HL)]HCl. | |
To further test the electro-catalytic activity of complex 1, bulk electrolysis of a DMF solution containing 3.18 μmol/L complex 1 was conducted in the presence of 1.4 mmol/L acetic acid at variable applied potential using a glassy carbon plate electrode in a doublecompartment cell. Fig. S3a (Supporting information) shows the total charge of bulk electrolysis of complex 1 in the presence of acid, when the applied potential was -1.45 V versus Ag/AgNO3, the maximum charge reached 32 mC during 2 min of electrolysis, accompanying evolution of a gas, which was confirmed as H2 by gas chromatography (GC). A controlled-potential electrolysis (CPE) experiment under the same potential without complex 1 only gave a charge of 11 mC (Fig. S3b, Supporting information), showing that this complex does serve an effective hydrogen production under such conditions. According to Fig. S4 (Supporting information), ~0.021 mL of H2 was produced over an electrolysis period of 2 h. By using Eqs. (2) [22] and (3) [23], TOF was calculated to be 16.3 moles of hydrogen per mole of catalyst per hour at an OP of 941.6 mV (Eq. S1 and Fig. S5, Supporting information).
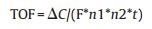
|
(2) |

|
(3) |
Where, ΔC is the charge from the catalyst solution during CPE minus the charge from solution without catalyst during CPE; F is Faraday's constant, n1 is the number of moles of electrons required to generate one mole of H2, n2 is the number of moles of catalyst in solution, and t is the duration of electrolysis.
To study the electrochemical behavior of complex 1 in aqueous medium, CVs were measured in buffered aqueous solutions where pH = 3.0-7.0, which is the range associated with catalytic water reduction. As observed in Fig. 3b, in a pH 7.0 solution, an irreversible CuⅡ/CuⅠ wave was observed at -1.48 V versus Ag/AgCl. And the strength of peak current increased markedly with decreasing pH values, which reflects the shifts in the thermodynamic potential for electrocatalysis [24]. As shown in Fig. S6 (Supporting information), in the absence of complex 1, a catalytic current was not apparent until a potential of -1.60 V versus Ag/ AgCl was attained. With addition of 0.354 mmol/L complex 1, the onset of catalytic current was observed at about -0.96 V versus Ag/ AgCl, and the current strength increased significantly with increasing concentrations of complex 1 from 0.00 mmol/L to 0.354 mmol/L.
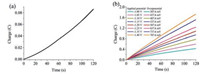
To further get the evidence for the electro-catalytic activity in aqueous media by complex 1, the bulk electrolysis of a 3.18 mmol/L complex 1 was conducted in a 0.25 mol/L buffer solution under variable applied potentials. When the applied potential was -1.45 V versus Ag/AgCl, the maximum charge was only 86 mC during 2 min of electrolysis in absence of complex 1 (Fig. 4a). Under the same conditions, the charge reached 1809 mC with addition of complex 1 (Fig. 4b), accompanying a large amount of gas bubble appeared, which was confirmed to be H2 by GC analysis. The evolved H2 was analyzed by gas chromatography, Fig. S7a (Supporting information), which gave 4.56 mL of H2 over an electrolysis period of 1 h with a Faradaic efficiency of 99% for H2 (Fig. S7b in Supporting information). According to Equations (2) and (4) [6, 24], we also calculated the TOF for the catalyst as reaching a maximum of 1415.6 moles of hydrogen per mole of catalyst per hour at an overpotential (OP) of 787.6 mV (pH 7.0) (Eq. S2 and Fig. S8 in Supporting information), where
|
Download:
|
| Fig. 4. | |

|
(4) |
Based on the TOF values, under the similar conditions, the electro-catalytic activity of complex 1 is higher than several copper complexes, including a copper complex supported by a tetradentate amine phenol ligand with a TOF of 104.3 moles of H2 per mole of catalyst per hour at an OP of 839 mV [15], a copper complex with dicyano acetic acid methyl ester ligand that shows 285 mol of hydrogen per mole of catalyst per hour at an OP of 787.6 mV [25], and a dinuclear copper complex with 6-(3-aminomethylpropanol)-2-tert-buty-4-methylphenol ligand that shows a TOF of 650 mol of hydrogen per mole of catalyst per hour at an OP of 836.7 mV [21].
3. ConclusionThe reaction of 2-(pyridylmethyl)amino-N, N-bis(2-methylene-4, 6-difluorophenol) (H2L) and Cl2·2H2O affords the copper(Ⅱ) complex, [(HL)CuCl-CuCl(HL)]HCl, which has been characterized by physics-chemical and spectroscopic methods. In solid state, this complex exhibits a magnetic exchange interaction between copper (Ⅱ) ions. In liquid, it acts as an electrocatalyst for hydrogen generation both from acetic acid and aqueous buffer. This discovery affords a new method for designing material that shows different performance in different states.
4. ExperimentalSynthesis of the complex [(HL)CuCl-CuCl(HL)]HCl 1: To a solution of H2L (0.393 g, 1 mmol) and triethylamine (0.10 g, 1 mmol) in acetonitrile (20 mL), Cl2·2H2O (0.171 g, 1 mmol) in methanol was added and the mixture was stirred for 30 min. Single crystals were obtained from the filtrate which was allowed to stand at room temperature for several days, collected by filtration, and dried in vacuo (0.681 g, 67%). The elemental analysis results (Found C, 47.87; H, 3.08; N, 5.56. C40H31Cl3Cu2F8N4O4 requires C, 47.24; H, 3.07; N, 5.51) were in agreement with the formula of the sample used for X-ray analysis.
AcknowledgmentThis work was supported by the National Natural Science Foundation of China (Nos. 20971045, 21271073).
Appendix A. Supplementary dataSupplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.cclet.2017.03.027.
| [1] | M. Taki, S. Itoch, S. Fukuzumi. Oxo-transfer reaction from a bis(μ-oxo)dicopper (Ⅲ) complex to sulfides. J. Am. Chem. Soc. 124 (2002) 998–1002. DOI:10.1021/ja016023a |
| [2] | J. Chen, X. Wang, Y. Shao, et al., A trinuclear copper(Ⅱ) complex of 2, 4, 6-tris(di-2-pyridylamine)-1, 3, 5-triazine shows prominent DNA cleavage activity. Inorg. Chem. 46 (2007) 3306–3312. DOI:10.1021/ic0614162 |
| [3] | D. Mandal, M. Chauhan, F. Arjmand, G. Arom, D. Ray. Interaction with DNA of a heteronuclear [Na2Cu4] coordination cluster obtained from the assembly of two hydroxo-bridged [Cu2Ⅱ] units by a dimeric sodium nitrate template. Dalton Trans. 38 (2009) 9183–9191. |
| [4] | O. Kahn. Molecular Magnetism, New York: VCH, 1993 . |
| [5] | J.C. Fontecilla-Camps, A. Volbeda, C. Cavazza, Y. Nicolet. Structure/function relationships of[NiFe]-and [FeFe]-hydrogenases. Chem. Rev. 107 (2007) 4273–4303. DOI:10.1021/cr050195z |
| [6] | Y. Sun, J.P. Bigi, N.A. Piro, et al., Molecular cobalt pentapyridine catalysts for generating hydrogen from water. J. Am. Chem. Soc. 133 (2011) 9212–9215. DOI:10.1021/ja202743r |
| [7] | M.E. Carroll, B.E. Barton, T.B. Rauchfuss, P.J. Carroll. Synthetic models for the active Site of the [FeFe]-hydrogenase: catalytic proton reduction and the structure of the doubly protonated intermediate. J. Am. Chem. Soc. 134 (2012) 18843–18852. DOI:10.1021/ja309216v |
| [8] | N. Wang, M. Wang, Y. Wang, et al., Catalytic activation of H2 under mild conditions by an[FeFe]-hydrogenase model via an active μ-hydride species. J. Am. Chem. Soc. 135 (2013) 13688–13691. DOI:10.1021/ja408376t |
| [9] | M.A. Gross, A. Reynal, J.R. Durrant, E. Reisner. Versatile photocatalytic systems for H2 generation in water based on an efficient DuBois-type nickel catalyst. J. Am. Chem. Soc. 136 (2014) 356–366. DOI:10.1021/ja410592d |
| [10] | J.P. Cao, T. Fang, L.Z. Fu, L.L. Zhou, S.Z. Zhan. First mononuclear copper(Ⅱ) electro-catalyst for catalyzing hydrogen evolution from acetic acid and water. Int. J. Hydrogen Energy 39 (2014) 13972–13978. DOI:10.1016/j.ijhydene.2014.07.030 |
| [11] | L.L. Zhou, T. Fang, J.P. Cao, et al., A dinuclear copper(Ⅱ) electrocatalyst both water reduction and oxidation. J. Power Sources 273 (2015) 298–304. DOI:10.1016/j.jpowsour.2014.09.075 |
| [12] | T. Fang, L.Z. Fu, L.L. Zhou, S.Z. Zhan. A water-soluble dinuclear copper electrocatalyst:[Cu(oxpn)Cu(OH)2] for both water reduction and oxidation. Electrochim. Acta 161 (2015) 388–394. DOI:10.1016/j.electacta.2015.02.101 |
| [13] | Z. Han, L. Shen, W.W. Brennessel, P.L. Holland, R. Eisenberg. Nickel pyridinethiolate complexes as catalysts for the light-driven production of hydrogen from aqueous solutions in noble-metal-free systems. J. Am. Chem. Soc. 135 (2013) 14659–14669. DOI:10.1021/ja405257s |
| [14] | D.Z. Zee, T. Chantarojsiri, J.R. Long, C.J. Chang. Metal-polypyridyl catalysts for electro-and photochemical reduction of water to hydrogen. Acc. Chem. Res. 48 (2015) 2027–2036. DOI:10.1021/acs.accounts.5b00082 |
| [15] | T. Fang, W. Li, S.Z. Zhan, X. Wei. Synthesis and electrocatalytic properties of a dinuclear copper (Ⅱ) complex for generating hydrogen from acetic acid or water. J. Coord. Chem. 68 (2015) 573–585. DOI:10.1080/00958972.2014.998657 |
| [16] | R. Tatematsu, T. Inomata, T. Ozawa, H. Masuda. Electrocatalytic hydrogen production by a nickel(Ⅱ) complex with a phosphinopyridyl ligand. Angew. Chem. Int. Ed. 55 (2016) 5247–5250. DOI:10.1002/anie.201511621 |
| [17] | L.Z. Fu, L.L. Zhou, L.Z. Tang, et al., A molecular iron(Ⅲ) electrocatalyst supported by amine-bis(phenolate) ligand for water reduction. Int. J. Hydrogen Energy 40 (2015) 8688–8694. DOI:10.1016/j.ijhydene.2015.05.026 |
| [18] | Y. Yong, D.R. Zhu, K.L. Zhang, et al., Magnetic properties of two 1D complexes with mixed bridging ligands. Polyhedron 19 (2000) 1461–1464. DOI:10.1016/S0277-5387(00)00402-2 |
| [19] | Y. Han, L.B. Liang, W.Q. Chen, et al., Synthesis, crystal structure, and magnetic properties of a salt containing[Cu2Cl7]3- and 4-nitrobenzyl-4'-dimethylaminopyridinium. J. Coord. Chem. 64 (2011) 4182–4190. DOI:10.1080/00958972.2011.637168 |
| [20] | A.M. Appel, D.L. Dubois, M.R. Dubois. Molybdenum-sulfur dimers as electrocatalysts for the production of hydrogen at low overpotentials. J. Am. Chem. Soc. 127 (2005) 12717–12726. DOI:10.1021/ja054034o |
| [21] | Q.X. Peng, C.N. Lin, Y.X. Zhang, S.Z. Zhan, C.L. Ni. Synthesis structure, magnetic and electrochemical properties of one dinuclear copper complex. Z. Anorg. Allg. Chem. 642 (2016) 860–865. DOI:10.1002/zaac.v642.15 |
| [22] | L. Tong, R. Zong, R.P. Thummel. Visible light-driven hydrogen evolution from water catalyzed by a molecular cobalt complex. J. Am. Chem. Soc. 136 (2014) 4881–4884. DOI:10.1021/ja501257d |
| [23] | G.A.N. Felton, R.S. Glass, D.L. Lichtenberger, D.H. Evans. Iron-only hydrogenase mimics. Thermodynamic aspects of the use of electrochemistry to evaluate catalytic efficiency for hydrogen generation. Inorg. Chem. 45 (2006) 9181–9184. DOI:10.1021/ic060984e |
| [24] | H.I. Karunadasa, C.J. Chang, J.R. Long. A molecular molybdenuμ-oxo catalyst for generating hydrogen from water. Nature 464 (2010) 1329–1333. DOI:10.1038/nature08969 |
| [25] | C.N. Lin, L.Z. Tang, S.T. Ren, et al., Synthesis, characterization and properties of a copper complex with dicyano acetic acid methyl ester ligand derived from tetracyanoethylene. Polyhedron 121 (2017) 13–18. DOI:10.1016/j.poly.2016.09.054 |
 2017, Vol. 28
2017, Vol. 28 


