b College of Marine Sciences, Shanghai Ocean University, Shanghai 201306, China;
c School of Life and Environmental Sciences, Deakin University, Geelong, VIC 3217, Australia
Titrimetry is popular in both chemical laboratories and the control of various industrial processes [1] because of its intrinsic natures-simplicity, speed and cost-effectiveness [2]. Analytical chemists have extensively employed it for the measurement of major, minor and even trace constituents in complex matrices. Generally, optical-and electrochemical-methods are used to identify the equivalence point. The former is based on color change at the end point [3, 4], and has some distinct advantages (particularly when optical instrumentations are employed), such as satisfactory accuracy and sensitivity and risk-free contamination of head stage. While, optical methods face some challenges: 1) in most case sophisticated optical components and special indicators are required [5, 6]; 2) indicators are rarely completely selective [3]; 3) the need for transparent solutions and vessels is quite strict. The latter is based on the change of electrochemical parameters, such as voltage [7, 8] and current [1, 9] at the working electrodes. In contrast to optics-based devices, photo-electric conversion is not needed for electrochemical devices [10], allowing them to be more easily miniaturized and affordable [11]. However, electrode deterioration is unavoidable [1] and results in erratic measurements that decrease the accuracy because that the tips of the working electrodes must be immersed in the titration solution rather than a noninvasive test [9].
Ciprofloxacin is an antibacterial agent of the fluoroquinolones, and is considered as a broad-spectrum antibiotic used in the treatment of urinary and respiratory tract infections as well as in gastrointestinal and sexually transmitted diseases [12]. High performance liquid chromatography (HPLC) is the official method for determining ciprofloxacin in many countries [12-16]. However, apart from the requirement of expensive instrumentation, this technique for routine analysis are often time consuming. Recently, numerous methods based on optical [16-20] and electrochemical [12, 13, 16, 21-24] techniques have been reported. Among them, those approaches by titration exhibited outstanding performance, and have been used successfully [12, 24]. However, these methods based on either optical techniques or electrochemical techniques, still are candidates longing for further development due to their flaw mentioned above.
Capacitivelycoupled contactless conductivity detection (C4D) is an attractive alternative of conductivity-based method. Generally, an AC voltage is applied to an actuator electrode, and an AC voltage is capacitively coupled into the electrolyte and measured at the pick-up electrode [25, 26]. The magnitude of the detected signal is proportional to the concentration and mobility of the ionic charge carriers within the solution [27]. Like other electrochemical techniques, C4D offers instrumental simplicity, low cost, rapid response, no transparent solution requirement and easy miniaturization [26-28]; in addition, it also has an outstanding advantage in comparison with contact electrochemical methods, i.e. a freerisk of contamination because the electrodes are separated from the solution measured. Nowadays many efforts have been made to not only improve the performance of devices [26, 29, 30], but also widenthe field of applications[26-28, 31]. Previously we have used C4D successfully to monitor the process of DNA amplification in real time [32].
We were prompted by the need to develop a simple and costeffective method for the determination of some commonly used drugs, such as ciprofloxacin hydrochloride (CIPHCl). In this paper, for the first time the C4D was introduced to develop a microtitration system. And the determination of CIPHCl in tablet was performed further. Though the theoretical basis and applications have been described in many works, the determination of CIPHCl via mictro-titration based on the C4D in this work was worthy of special consideration, as it had a series of significant advantages in comparison with that described in previous literatures.
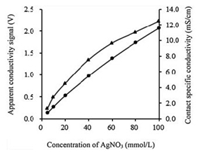
2. Results and discussion 2.1. Characterization of the micro-titration systemThe property of the monitoring system, i.e. the C4D, was characterized using a series of AgNO3 solutions at different concentration as probes. It can be seen that the apparent conductivity increases as a function of concentration over the range of 0.5-100.0mmol/L (Fig. 1). As expected, the curve shows a slight nonlinearity because the presence of a very small capacitive coupling between the two electrodes [33]. While, over the same range the contact conductivity also increases as a function of concentration with an approximate linear relationship. The nonlinear relationship does not bring negative effect on the determination (the reason would be described in the following sections), suggesting that the C4D can be used to monitor the change of solution conductivity during the process of titration, just like that by employing contact conductometers [9].
|
Download:
|
| Fig. 1. Apparent conductivity obtained with the C4D at an excitation amplitude of 16V (▲) and contact conductivity obtained with the contact conductometer at an excitation frequency of 2.0MHz (●) of 5.0, 10.0, 20.0, 40.0, 60.0, 80.0 and 100.0mmol/L AgNO3 solutions at room temperature. | |
The performance of delivery plays a key role in any titration system [1, 5]. In our experiments, the titrant volume per pulse was programmed tobe 10 μL. To facilitatethe formation of a dropat the end of delivery, a paraffin film was assembled onto the tip of the glass capillary. A small coefficient of variation was found (0.41%; n=11), which suggested satisfactory precision.
2.2. Titrating CIPHCl with AgNO3In many chemical reactions, the formation of a precipitate or precipitates alters the number of ions presentin the solution and in turn changes the conductivity [9, 34]. After the endpoint, the addition of excess titrant increases the number of ions, causing an increase of the conductivity. In a classic reaction, Cl- can be measured with Ag+ [9]. HereCIPHCl was quantified by determining the amount of Cl- in it, using Ag+ as a recognizing indicator.
The apparent conductivity of CIPHCl solution at different concentrations was measured with the C4D. It was found that over the range of 0.5-12.0mmol/L the apparent conductivity increases as a function of concentration with the optimum collection parameters [32]. Commonly, in order to minimize the dilution effect on the titration, the titrant concentration must not be less than ten times that of the titrand solution [24]. Thus from 50.0mmol/L to 140.0mmol/L, a series of AgNO3 titrant was used to titrate 5.0mmol/L CIPHCl. The results showed that the higher titrant concentration was used, the higher titration efficiency was. However, concentrations more than 100.0mmol/L led to unstable outcomes because of the frequent formation of agglomerate precipitates, which could block the mass transfer seriously. Thus, in all the other titrations 100.0mmol/L AgNO3 was used as titrant.
In traditional titration, mass transfer occurs fast due to adequate stirring [5, 7, 9]. Consequently, equal amounts of titrand and titrant react with each other at the endpoint [9, 11]. The amount of titrant consumed can be calculated from elapsed time and velocity. We confirmed these by employing the contact conductometer according to the method reported by Hail et al. [9]. However, it was found that the endpoint is far behind the theoretical one by employing the C4D to monitor the titration process (without stirring) due to the slow mass transfer. And the elapsed time depends on the selected delivery interval time. Further experiments show that the elapsed time increases nonlinearly with the increase of interval time; and that the volume of AgNO3 required is closer and closer to the theoretical value with longer interval times (Fig. S1 in Supporting information). When a short delivery internal time, such as 5s, was selected for titrating 5.0mmol/L CIPHCl solutionwith 100.0mmol/L AgNO3, the apparent conductivity increased constantly even beyond the endpoint; and the response curve was in undesired fluctuation, making it difficult to locate the endpoint accurately. The cause is the fluctuation of temperature, which was resulted from the heat of chemical reaction. As it well known, temperature is a key impact factor on the measurement of conductivity [32]. Thus, the suitable longer delivery internal time allows the reaction heat to release into the surrounding, reducing the negative effect of temperature fluctuation. Taking the analysis efficiency intoaccount, we selected a 60s delivery interval time in all other experiments.
When CIPHCl solutions at different concentration were titrated with 100.0mmol/L AgNO3, white precipitates were all formed that was attributed to ion pair formation between Ag+ and Cl- ion. Typical graphs of apparent conductivity versus titration time were shown in Fig. 2(a). Similar V-shaped curves were obtained; and endpoints could be identified easily from the point of inflection at the V-shaped titration curve as using a contact conductometer [9, 34]. As expected, the curves are nonlinear because of the dilution effects and the slow mass transfer. However, this doesn't affect the determination of endpoint, which is defined as the inflection point on the titration curve. Across the range of concentrations measured (1.0-10.0mmol/L), the initial apparent conductivity (i.e. the apparent conductivity obtained before the first delivery of titrant) increases as a function of concentration (Fig. S2 in Supporting information); the apparent conductivity obtained at the endpoint (inflection point) also increases with increasing concentrations (Fig. S3 in Supporting information). More meaningfully, as shown Fig. 2(b), the elapsed time increases as a function of the titrand concentration; over the range of 2.0-8.0mmol/L, there is a linear relationship, suggesting that a method for quantitative determination can be developed. By contrast, the linearity is a little wider than that obtained with HPLC Method [15], and much wider than that obtained with traditional conductometric titration [24]. The slope of the trend line is 185.5s/mmol/L, suggesting a rather high sensitivity. To verify the precision, 2.0mmol/L standard CIPHCl solution was titrated five times with the same parameters (Fig. S4 in Supporting information). Results show that the relative standard deviation (RSD) is 1.9%, confirming that the method is sufficiently precise.

|
Download:
|
| Fig. 2. (a): Titration curves of CIPHCl solutions at different concentration titrated with 100.0mmol/L AgNO3, monitored by the C4D in real time. (b): Plot of the elapsed time versus the initial concentration of CIPHCl. | |
In comparison with the micro-titration by the C4D, traditional titration by the contact conductometer was carried out, referring to Ayad et al. [35]. To make sure the working electrode works well, at least 10mL titrand should be loaded in the reaction cell. Using 100.0mmol/L AgNO3 as titrant, over the range of 0.4-15.0mmol/L, CIPHCl solutions could be determined by calculating from the consumption volumes of titrant. By contrast, the required titrant for each measurement was far more than that by the microtitration. Meanwhile, to minimize the memory effect resulting from physical and/or chemical foiling [36], steps of renewing working electrode were required between the measurements. This indicated that the operation of contact conductivity method was more complicated than that of contactless one. 2.0mmol/L standard CIPHCl solution was titrated five times with the contact conductometer. Results show that the RSD is 3.7%, suggesting that even with the renewal step the precision of the contact method is lower than that of the contactless one. Results of further experiments show that the precision is worse without the renewal step of working electrode.
2.3. Titrating CIPHCl with NH4Fe(SO4)2Based on the fast complexation reaction between Fe3+ and ciprofloxacin [12, 19, 24], the other mode for recognizing CIPHCl quantitatively was investigated with the proposed micro-titration. According to the procedure described in Section 2.2, the concentration of titrant, delivery interval time and other factors were all optimized.
When CIPHCl solution was titrated with 10.0mmol/L NH4Fe(SO4)2, bright orange complexation of Fe(Ⅲ)-ciprofloxacin formed immediately at room temperature and remained stable for at least 2h, causing a decrease of total conductivity. Typical graphs of apparent conductivity versus titration timewere shownin Fig. 3(a) when CIPHCl solutions at different concentration were measured. Similar "V" shaped titration curves were obtained; and endpoints can be identified easily from the point of inflection at the titration curve. Across the range of 0.5-3.0mmol/L, the apparent conductivity obtained at the endpoint increases with increasing concentrations (Fig. S5 in Supporting information). As shown Fig. 3(b), the elapsed time increases as a function of CIPHCl concentration; over the range of 1.0-2.5mmol/L, there is a linear relationship, suggesting that a method for quantitative determination can be developed. By contrast, the linearity is wider than that obtained with a visible-spectrophotometric method [19]. The slope of the trend line is 64s/mmol/L, suggesting a rather high sensitivity. To verify the precision, 1.0mmol/L standard CIPHCl solution was titrated five times (Fig. S6 in Supporting information). Results show that the RSD is 2.2%, confirming that the method is also sufficiently precise. By contrast, the sensitivity of this mode is lower than that of the other mode; the linearity range is narrower than that of the other mode; the accuracy of this mode is a little lower than that of the other mode. However, the usage of Fe3+ as the recognizer, the interference from some possible ions, such as Br-, etc. can be eliminated easily. That is to say, this mode can be a meaningful alternative in some specific cases.

|
Download:
|
| Fig. 3. (a): Titration curves of CIPHCl solutions at different concentration titrated with 10.0mmol/L NH4Fe(SO4)2, monitored by the C4D in real time. (b): Plot of the elapsed time versus the initial concentration of CIPHCl. | |
2.4. Application
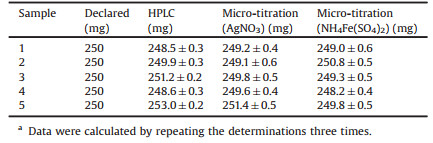
In order to evaluate its potential application in the real sample analysis, the proposed method above was applied to determine the content in commercial CIPHCl tablet in comparison with official method. Commonly, ten [12] or twenty [16, 24] tablets are needed to prepare testing solution in some methods reported previously. In the micro-titration, one commercial tablet (declared content: 250mg) is enough for the preparation of stock testing solution. Apart from the dissolution with water and the filtration with ordinary filter-paper, no more auxiliary chemicals and pretreatment operations were involved. Under optimum conditions, the titration curves of the properly diluted sample solutions were monitored with the C4D, using 100.0mmol/L AgNO3 and 10.0mmol/L NH4Fe(SO4)2 as titrant, respectively. Then the content was calculated according to the linear relationship obtained above. The results and the results obtained from HPLC method were shown in Table 1. It is clear that the data obtained with the two micro-titration modes are in good agreement. And they are also in good agreement with that obtained with HPLC as well as the declared content. The results obtained also indicate that the excipients present in the drug formulations have no effect on the determination [12].
|
|
Table 1 Comparison of the results obtained with micro-titration and HPLC for the determination of CIPHCl tablet samples.a |
In order to verify the applicability of the present micro-titration method in clinical applications, studies of recovery of CIPHCl were evaluated in triplicate at three concentrations. The percentage of recoveries were calculated and listed in Table 2. The recovery is between 98.4%-102.4%, indicating a satisfactory accuracy and repeatability. These outcomes suggest that this micro-titration method can be used for the determination of pharmaceutical dosage forms.
|
|
Table 2 The results of accuracy (n = 3). |
3. Conclusion
Based on the developed C4D, a new electrical micro-titration system and a new relevant analytical method (two modes) were constructed. The practicability of this method for the determination of CIPHCl in tablet sample was evaluated successfully. In comparison with some existing analysis methods, including titrations and HPLC, the new approach has some distinct features, and exhibits a unique nature.
(1) Byemploying the C4D to real time monitor the titration, there is totally risk-free for the head stage contamination due to the separation of testing solution, just like employing optical instrumentation [17-19]. Moreover, by contrast, the addition of color indicators and the requirement of sample pretreatment (to ensure transparency) [37] were both eliminated. In addition, unlike optical titration technique [5], the C4D-based is free of sophisticated optical-electrical signal transfer components [32] that reduce the complexity in miniaturizing instrumentation and hence manufacturing costs. While, the new approach is superior to contact electrochemical titration methods [1, 2, 7, 34] in fields 1 no requirement for detector exchange/renewal between different tests; 2 non-invasive successive monitoring; and 3 free of probes, indicators or labels.
(2) To locate the endpoint using the precipitate reaction is easier than using complexation reaction. And the sensitivity of determination using precipitate titration is higher than that using complexation titration.
(3) Apart from standard CIPHCl and AgNO3 (or NH4Fe(SO4)2), no chemical is involved for thedetermination.Therefore, it iscosteffective and environmental-friendly.
(4) In traditional titration methods, the total sample volume is often more than 10mL [9-11]. The proposed method here uses only 500 mL titrand. Thus this micro-titration is especially meaningful for limit amount of samples [6].
(5) Traditionally, the equivalence point is often used as an endpoint to quantify the concentration of titrand. Though we can obtain an equivalence point by prolonging the delivery interval time, it is time-consuming for the analysis. Therefore, an alternative mode for quantitative determination was established based on that the elapsed time changes linearly with initial titrand concentrations over a certain range. No requirement to calculate the consumption value of titrant is a distinct feature of our approach, which also simplifies the measurement process.
In summary, the present micro-titration based on the C4D is simple, cost-effective and accurate for the determination of CIPHCl in real drug formulations. It matches well the three dominant qualities for a good analytical method [18]. Of course, at present the titration efficiency and accuracy are not satisfactory enough. However, this shortcoming can be resolved by developing a multichannel platform, and that is our next aim.
4. Experimental 4.1. Reagents and chemicalsCIPHCl standard (C17H18ClFN3O3, CAS: 86393-32-0) was kindly supplied by National Standard Material Research Center (Beijing, China). The tablet sample of CIPHCl was purchased from a local pharmacy and was freshly prepared just before the analysis. Silver nitrate was purchased from Aladdin Chemical Reagent Co., Ltd. (Shanghai, China). Ammonium ferric sulfate dodecyl hydrate and other chemicals were all purchased from Shanghai Chemical Reagent Co. (Shanghai, China) and were all of analytical grade. All the chemicals were used as received without further treatment.
4.2. Preparation of solutionsA standard stock solution of 10.0 mmol/L CIPHCl was prepared in water and protected from light in a refrigerator (4 ℃). Working standard solutions were prepared by appropriate dilutions of this stock solution. A 500.0 mmol/L AgNO3 solution was prepared by dissolving the accurate weight in water. Before used as titrant, it was standardized against NaCl [38]. Working titrants were prepared by appropriate dilutions of this stock solution. A 100.0 mmol/L NH4Fe(SO4)2 solution was prepared in water by dissolving the accurate weight of dried Ammonium ferric sulfate dodecyl hydrate in a 100 mL calibrated volumetric flask and used after an ultrasonic (30 min) to ensure complete dissolution. This solution was standardized against potassium permanganate after complete reduction with hydroxylamine [12]. Before being used as titrant, it was diluted to desired concentration with water.
The tablet solution of CIPHCl was prepared referring to the approach described by Abulkibash et al. [12]. In brief, one tablet (Henan Zhongjie Pharmaceutical Co., Ltd, Xinxiang, China) containing 250 mg CIP was dissolved in 20 mL water under ultrasonic (for 10 min). Then it was filtered through an ordinary filter-paper, washed with water for three times. At last the filtrate plus washings were diluted to the mark in a 100 mL calibrated volumetric flask. Testing solutions were prepared by appropriate dilutions of this stock solution. Solutions were all prepared with ultrapure water (Resistivity: 18.2 MΩ/cm) from Poseidon-R70 water purification system (Research Scientific Instruments Co., Ltd, Xiamen, China).
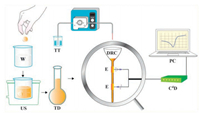
4.3. Micro-titrationAs sketched in Fig. 4, the proposed titration system contains a portable computer, a C4D (ER225 System, eDAQ Pty Ltd., Sydney, Australia), a custom-made working head stage (including two electrodes -an excitation electrode and a pick-up electrode, as showed under a magnifier) and an automatic peristaltic pump (Lab-2015, Baoding Shenchen Precision Pump Co., Ltd., China). The inner and outer diameters for the bottom part of the disposable reaction cell (DRC) are 2.2 mm and 3.0 mm, respectively. In the proposed titration, small volume titrand was involved each time ( < 1.0 microliter). Thus we called it micro-titration.
|
Download:
|
| Fig. 4. Schematic demonstration of the determination of CIPHCl in tablet using a micro-titration system based on a capacitively coupled contactless conductivity detector (C4D). W = water, US = ultrasonic, TD = titrand, TT = titrant, PP = peristaltic pump, E = electrode, DRC = disposable reaction cell, PC = personal computer. | |
Typically, the titrant is delivered into the titrand with 10 μL per pulse time at a desired time interval. Meanwhile, the apparent conductivity (output in voltage) of the solution in the DRC is monitored in real time with optimized parameters, i.e. an excitation frequency of 2.0 MHz and excitation amplitude of 16 V [32]. Data collection started at the moment titration began with a fixed time interval of 1 s. Unless stated otherwise: 1) The delivery interval time was 60 s; 2) The experiments were carried out at room temperature (25±1 ℃); 3) The tip of the glass capillary was not immersed into the titrand solutions; and 4) The initial volume of titrand was 500 μL. For sensitivity and resolution, the concentrations of the titrand and titrant solutions were optimized according to the C4D's conductivity values (between 100 mV and 2400 mV). The elapsed time was defined as the length from the beginning of the titration to the time when the endpoint was obtained, and the graphs of apparent conductivity versus the titration time were constructed with the Chart software provided by eDAQ Pty Ltd.
4.4. Contact conductivity titration measurementsThe control experiments were carried out by employing a commercial conductometer (Seven Excellence S470-K, MettlerToledo) to perform the contact conductivity titration according to Ayad et al. [35]. In brief, the conductivity head stage was immersed in the 10 mL titrand. The titrant is delivered into the titrand with 100 μL per pulse time at an interval time of 6 min by the automatic peristaltic pump. And the conductivity was collected subsequent to each addition of the titrent, after thorough stirring for 2 min. Between the measurements the contact working electrode was washed carefully by water with the help of ultrasonics for 2 min. A graph of corrected conductivity versus the volume of added titrant was constructed and the end point was determined from the inflection point. Then the amount of titrend was calculated according to the consumption of titrent volume and its concentration.
In cased where it needed, the conductivities of some solutions were also measured with the contact conductometer at room temperature. Between the measurements the working electrode was also washed carefully with 0.1 mol/L HCl and water, respectively, for 2 min.
4.5. Chromatographic measurementsThe chromatographic determination of CIPHCl was performed with the method reported by Aksoy et al. [15]. A system consisted of an Agilent Technologies (Waldbronn, Germany) 1100 series instrument equipped with a quaternary solvent delivery system and an Agilent series 1100 UV detector was used. An Agilent G1329A ALS auto-sampler with a 100 μL sample loop was used for the injection of analytes. Chromatographic data were collected and processed using Agilent Chemstation software. The separation was performed at room temperature on a reversed phase Inertsil ODS3 C8 column.
AcknowledgmentsThe authors gratefully acknowledge the financial support from Key R&D of Shandong Province (No. 2016GSF120008) and Qingdao National Laboratory for Marine Science and Technology (No. 2015ASKJ02-05).
Appendix A. Supplementary dataSupplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.cclet.2017.03.019.
| [1] | C.M.N.V. Almeida, A.S.L. Rui, J.L.F.C. Lima, et al., Precipitation titrations using an automatic titrator based on a multicommutated unsegmented flow system. Analyst 125 (2000) 333–340. DOI:10.1039/a907917c |
| [2] | M.D. DeGrandpre, T.R. Martz, R.D. Hart, et al., Universal tracer monitored titrations. Anal. Chem. 83 (2011) 9217–9220. DOI:10.1021/ac2025656 |
| [3] | M. Archer, M. Brits, D. Prevoo-Franzsen, L. Quinn. High concentration aqueous sodium fluoride certified reference materials for forensic use certified by complexometric titration. Anal. Bioanal. Chem. 407 (2015) 3205–3209. DOI:10.1007/s00216-014-8229-2 |
| [4] | S. Karita, T. Kaneta. Acid-base titrations using microfluidic paper-based analytical devices. Anal. Chem. 86 (2014) 12108–12114. DOI:10.1021/ac5039384 |
| [5] | E.N. Gaião, R.S. Honorato, S.R.B. Santos, M.C.U. Araújo. An automated flowinjection titrator for spectrophotometric determinations of total acidity in wines, using a single standard solution and gradient calibration. Analyst 124 (1999) 1727–1730. DOI:10.1039/a905188k |
| [6] | R.A. Lima, L.F. Almeida, W.S. Lyra, et al., Digital movie-based on automatic titrations. Talanta 147 (2016) 226–232. DOI:10.1016/j.talanta.2015.09.053 |
| [7] | Z. Stanić, J. Stepanović. Natural metal sulfides as electrochemical sensors for redox titrations in c-butyrolactone and propylene carbonate. Monatsh. Chem. 141 (2010) 137–142. DOI:10.1007/s00706-009-0246-z |
| [8] | S. Peper, A. Ceresa, E. Bakker, E. Pretsch. Improved detection limits and sensitivities of potentiometric titrations. Anal. Chem. 73 (2001) 3768–3775. DOI:10.1021/ac001475b |
| [9] | M.E. Hail, F.J. Holler. High precision automated conductometric titrations with the bipolar pulse technique. Microchim. Acta 90 (1986) 295–308. DOI:10.1007/BF01199272 |
| [10] | W.G. Santos, E.'T.G. Cavalheiro. Assembling and using an LED-based detector to monitor absorbance changes during acid-base titrations. J. Chem. Educ. 92 (2015) 1709–1715. DOI:10.1021/ed500931p |
| [11] | B.C. Janegitz, W.T. Suarez, O. Fatibello-Filho, L.H. Marcolino Junior. Conductometric determination of N-acetylcysteine in pharmaceutical formulations using copper(Ⅱ) sulphate as titrant. Anal. Lett. 41 (2008) 3264–3271. DOI:10.1080/00032710802507554 |
| [12] | A.M. Abulkibash, S.M. Sultan, A.M. Al-Olyan, S.M. Al-Ghannam. Differential electrolytic potentiometric titration method for the determination of ciprofloxacin in drug formulations. Talanta 61 (2003) 239–244. DOI:10.1016/S0039-9140(03)00246-7 |
| [13] | X. Zhang, Y. Wei, Y. Ding. Electrocatalytic oxidation and voltammetric determination of ciprofloxacin employing poly(alizarin red)/graphene composite film in the presence of ascorbic acid, uric acid and dopamine. Anal. Chim. Acta 835 (2014) 29–36. DOI:10.1016/j.aca.2014.05.020 |
| [14] | S. Wu, C. Chein, Y. Wen. Analysis of ciprofloxacin by a simple high-performance liquid chromatography method. J. Chromatogr. Sci. 46 (2008) 490–495. DOI:10.1093/chromsci/46.6.490 |
| [15] | B. Aksoy, İ. Küçükgüzel, S. Rollas. Development and validation of a stability-_ indicating HPLC method for determination of ciprofloxacin hydrochloride and its related compounds in film-coated tablets. Chromatographia 66 (2007) 57–63. DOI:10.1365/s10337-007-0287-6 |
| [16] | K. Basavaiah, P. Nagegowda. Titrimetric and spectrophotometric assay methods for ciprofloxacin in pharmaceuticals based on neutralization reaction. Nati. Acad. Sci. Lett. 29 (2006) 189–194. |
| [17] | M.I. Pascual-Reguera, G.P. Parras, A.M. Diaz. Solid-phase UV spectrophotometric method for determination of ciprofloxacin. Microchem. J. 77 (2004) 79–84. DOI:10.1016/j.microc.2004.01.003 |
| [18] | Z. Guo, L. Chen, H. Lv, Z. Yu, B. Zhao. Magnetic imprinted surface enhanced Raman scattering (MI-SERS) based ultrasensitive detection of ciprofloxacin from a mixed sample. Anal. Methods 6 (2014) 1627–1632. DOI:10.1039/C3AY40866C |
| [19] | E.C.L. Cazedey, R. Bonfilio, M.B. Araújo, H.R.N. Salgado. A first-derivative spectrophotometric method for the determination of ciprofloxacin hydrochloride in ophthalmic solution. Phys. Chem. 2 (2013) 116–122. DOI:10.5923/j.pc.20120206.06 |
| [20] | S.M. Sultan, F.E.O. Suliman. Flow injection spectrophotometric determination of the antibiotic ciprofloxacin in drug formulations. Analyst 117 (1992) 1523–1526. DOI:10.1039/an9921701523 |
| [21] | Y. Ni, Y. Wang, S. Kokot. Simultaneous determination of three fluoroquinolones by linear sweep stripping voltammetry with the aid of chemometrics. Talanta 69 (2006) 216–225. DOI:10.1016/j.talanta.2005.09.032 |
| [22] | Š. Komorsky-Lovrić, B. Nigović. Identification of 5-aminosalicylic acid, ciprofloxacin and azithromycin by abrasive stripping voltammetry. J. Pharm. Biomed. 36 (2004) 81–89. DOI:10.1016/j.jpba.2004.05.008 |
| [23] | F. Lida, M. Alahyari. Electrochemical behavior and analytical application of ciprofloxacin using a multi-walled nanotube composite film-glassy carbon electrode. Colloid Surf. B 81 (2010) 110–114. DOI:10.1016/j.colsurfb.2010.06.030 |
| [24] | Y.M. Issa, W.F. El-Hawary, A.F. Ahmed, Ion-pair formation in pharmaceutical analysis. conductimetric determination of promazine chlorpromazine. promethazine, imipramine and ciprofloxacin hydrochlorides in pure form, drug formulations and urine. Microchim. Acta 134 (2000) 9–14. DOI:10.1007/s006040070046 |
| [25] | J.G.A. Brito-Neto, J.A.F. da Silva, L. Blanes, C.L. do Lago. Understanding capacitively coupled contactless conductivity detection in capillary and microchip electrophoresis. Part 1. Fundamentals. Electroanalysis 17 (2005) 1198–1206. DOI:10.1002/(ISSN)1521-4109 |
| [26] | K.A. Mahabadi, I. Rodriguez, C.Y. Lim, et al., Capacitively coupled contactless conductivity detection with dual top-bottom cell configuration for microchip electrophoresis. Electrophoresis 31 (2010) 1063–1070. |
| [27] | D.A. Collins, E.P. Nesterenko, D. Brabazon, B. Paull. In-process phase growth measurement technique in the fabrication of monolithic porous layer open tubular (monoPLOT) columns using capacitively coupled contactless conductivity. Analyst 138 (2013) 2540–2545. DOI:10.1039/c3an00133d |
| [28] | T.G. Drummond, M.G. Hill, J.K. Barton. Electrochemical DNA sensors. Nat. Biotechnol. 21 (2003) 1192–1199. DOI:10.1038/nbt873 |
| [29] | H. Zheng, M. Li, J. Dai, et al., Double input capacitively coupled contactless conductivity detector with phase shift. Anal. Chem. 86 (2014) 10065–10070. DOI:10.1021/ac501199e |
| [30] | T.D. Mai, T.T.T. Pham, H.V. Pham, et al., Portable capillary electrophoresis instrument with automated injector and contactless conductivity detection. Anal. Chem. 85 (2013) 2333–2339. DOI:10.1021/ac303328g |
| [31] | J.M. Cabot, E. Duffy, S. Currivan, et al., Characterisation of graphene fibres and graphene coated fibres using capacitively coupled contactless conductivity detector. Analyst 141 (2016) 2774–2782. DOI:10.1039/C5AN02534F |
| [32] | X. Zhang, Q. Li, X. Jin, et al., Quantitative determination of target gene with electrical sensor. Sci. Rep.-UK 5 (2015) 12539. DOI:10.1038/srep12539 |
| [33] | P. Kubáň, P.C. Hauser. Fundamental aspects of contactless conductivity detection for capillary electrophoresis. Part Ⅰ: frequency behavior and cell geometry. Electrophoresis 25 (2004) 3387–3397. DOI:10.1002/elps.v25:20 |
| [34] | S. Beck, M. Me'thot, J. Bouchard. General procedure for determining cellulose nanocrystal sulfate half-ester content by conductometric titration. Cellulose 22 (2015) 101–116. DOI:10.1007/s10570-014-0513-y |
| [35] | M.M. Ayad, H.E. Abdellatef, M.M. Hosny, Y.A. Sharaf. Conductometric titration method for determination of naftidrofuryl oxalate, propafenone HCl and sotalol HCl using silver nitrate. Eur. J. Chem. 3 (2012) 332–336. DOI:10.5155/eurjchem.3.3.332-336.637 |
| [36] | Z. Stanić, T. Dimić. Natural mineral pyrite and analytical application thereof in precipitation titrations in non-aqueous solvents. New J. Chem. 37 (2013) 3612–3619. DOI:10.1039/c3nj00577a |
| [37] | A.P.S. Paim, B.F. Reis. An automatic spectrophotometric titration procedure for ascorbic acid determination in fruit juices and soft drinks based on volumetric fraction variation. Anal. Sci. 16 (2000) 487–491. DOI:10.2116/analsci.16.487 |
| [38] | J. Bassett, R. C. Denney, G. H. Jeffery, J. Mendham, fourth ed. , Vogel's Textbook of Quantitative Inorganic Analysis, Longman Group Limited, UK, 1978. |
 2017, Vol. 28
2017, Vol. 28