b Department of Biotechnology, Thiruvalluvar University, Vellore, 632 115, India;
c Department of Zoology, Bharathiar University, Coimbatore, 641 046, India;
d Department of Agriculture, Food and Environment, University of Pisa, Pisa 56124, Italy;
e TheBioRobotics Institute, Sant'Anna School of Advanced Studies, Pontedera 56025, Italy
Mercury is one of the persistent contaminants in the environment and has serious adverse effects on human health, mostly through drinking water, and the environment [1-4]. The contamination of drinking water by water-soluble Hg2+ is still the most commonly occurring incident. Therefore, it is crucial to monitor Hg2+ levels with sensitivity and selectivity in aqueous systems [5] the conventional instrumental methods employed for the detection of Hg2+ have disadvantages of labor-intensive, time-consuming, and high cost of the equipment [6-12]. Recently, a variety of detection methods have been developed, exploiting perturbed signal resulting from the interaction of the nanoparticle/organic/ biomolecule with a specific heavy metal such as fluorescent dyes [13-15], luminescent metal complexes [16, 17], DNAzymes and DNA aptamers [18], electrochemically active compounds [11, 19, 20], quantum dots [21], ion selective sensors [22, 23] and gold nanoparticles [24-34]. Among them, optical sensors are powerful alternative to conventional analytical techniques due to the advantages they have such as high sensitivity, specificity, quick response and cost effectiveness, has been widely employed in biosensing, and medical diagnostics [35-40]. Recently, Lu et al. [41] reported the green synthesis of fluorescent carbon nanoparticles and its selective applications in the detection of mercury (Ⅱ) ions. Similarly, Bhatt et al. [42] developed a turn-on fluorescence probe for selective detection of Hg (Ⅱ) based on calixpyrrole hydrazide reduced silver nanoparticle. Xi et al. [43] have reported the usage of CdSe/CdS quantum dots functionalized by thiourea for mercury ion detection. Moreover, Saikia et al. [44] reported the PET sensor based on CdTe/ZnS core/shell quantum dot capped with Mercaptosuccinic acid (MSA) for the detection of mercury with the detection limits of 1×10-12 mol/L.
Fluorescence sensor using dye immobilized solid particles with significant improvement in sensitivity has been reported [45]. However, a common limitation in dye based optical sensor is the low quantum efficiency of dyes in water. One of the available methods to amplify the emission efficiency of organic dye is metalenhanced fluorescence (MEF) [46-51] in dye-metal hybrids that occur when a fluorophore is brought in close proximity to a metal surface [49, 52-56]. In our previous work, we have utilized MEF to obtain enhanced light emission of rhodamine B and silver nanoparticles confined in a mesoporous host [57]. The rhodamine B doped silica has been studied as fluorescent probes for bioanalysis because mesoporous channels of silica serves as a versatile substrate for the immobilization of dyes, biomolecules, drugs and nanoparticles in addition to its optically transparency. The chosen Rhodamine B dye has excellent photostability compared to other commercial dyes [58] and has been applied as fluorescent probe for the detection of mercury [59]. Furthermore, many nanoparticles, such as gold, copper, silver and zinc, exhibit metal-enhanced fluorescence (MEF) [60]. Comparing with other metals, silver has received much attention due to its excellent MEF properties [61]. Among the noble metals, Au and Ag can support nanoparticle Plasmon resonance in the ultra violet, visible and near-infrared regions of the spectrum that can be modified by varying the nanoparticle size and shape. Moreover, silver has several advantages over gold, such as higher extinction coefficients, sharper extinction bands, higher ratio of scattering to extinction, and extremely high field enhancements. In the present research, we developed a detection assay for Hg2+ employing fluorescence quenching of Hg2+ which eliminates MEF through binding with Rhodamine B.
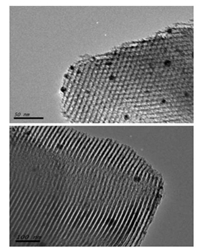
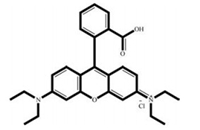
2. Results and discussion 2.1. Morphological characterization of Ag-SBA15The mesoporous silica thin film containing Rhodamine B dye and silver nanoparticle was characterized by HRTEM, XRD, TGA and FTIR analysis. Fig. 1 depicts the HRTEM of Ag-SBA15, spherical dark particles with diameter ranging from 5 to 20 nm can be observed on the images confirms the presence of silver nanoparticles assembled on the silica mesoporous channels. The amount of dye adsorbed on mesoporous nanostructure was calculated from thermogravimetric analysis. The TGA curves of RB-Ag-SBA15 and SBA15 are given in Supporting information (Fig. S2 in Supporting information). According to thermogram, there is a continuous weight loss of the SBA15 in the temperature range of 150-650 ℃ which corresponds to the decomposition of organic molecules adsorbed on SBA15 surface. From the thermogram, we estimated content of the APTMS and RB grafted to the SBA15 is about 10.70 wt%. The chemical structure of Rhodamine B is given in Fig. 2.
|
Download:
|
| Fig. 1. TEM images of Ag-SBA15. | |
|
Download:
|
| Fig. 2. Rhodamine B dye. | |
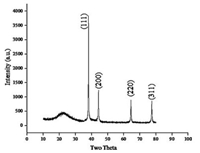
Fig. 3 shows the XRD pattern of silver nanoparticles dispersed in SBA15. The presence of peaks at 2θ values of 38.1°, 44.09°, 64.36°, and 77.29° corresponds to (111), (200), (220), (311) planes of silver, respectively. Thus, the XRD spectrum confirmed the crystalline structure of silver nanoparticles. No peaks for other impurity crystalline phases have been detected. The XRD patterns thus clearly illustrates that the Ag NPs synthesized by the present method are crystalline in nature. All the peaks in XRD pattern can be readily indexed to a face centered cubic structure of silver as evidenced from available literature (JCPDS, File No. 4-0783). Further, the presence of a mesoporous silica was also confirmed from the existence of a broad peak at 2θ = 22.
|
Download:
|
| Fig. 3. XRD of Ag-SBA15. | |
The FTIR spectrum of APTMS anchored to SBA15 (Supporting information Fig. S3a) shows a band in the range 2900-3000 cm-1, which are attributed to the C—H stretching modes of —CH2 in APTMS. The weak absorption band corresponding to of Si—OH was appeared in the range of 3000-3400 cm-1indicating successful immobilizations of APTMS on SBA15. Asymmetric stretching Si—O and Si—O—Si vibrations observed at 1045 cm-1, O—Si—O symmetric stretching appear in 954 and 792 cm-1 [62]. The FTIR spectrum of Rhodamine B dye anchored Ag-SBA15 shown in Fig. S3b have carbonyl stretching vibration band of acid functional group at 1693 cm-1 and OH stretching vibration was observed at 3300 cm-1. The peak at 1479 cm-1 and 2900 cm-1 assigned to the aromatic C—H stretching present in the RB molecule.
2.2. Optical characterization of RB-Ag-SBA15 coated quartz glass for mercury detectionThe detection of mercury ions in aqueous solution at room temperature with high sensitivity and selectivity by using a combination of dye emission and SPR of Ag which give high quantum yield in optical emission. The scheme combines the advantages of specific binding interactions between Hg2+ and RB molecule and optical emission properties of RB. This method adopts simple procedure, label-free and all of the materials are commercially available. The detection analysis was carried out by simply immersing sensing platform, consists of RB-Ag-SBA15 on Quartz glass, in aqueous solution of heavy metals. The UV-vis and Photoluminescence spectra were recorded immediately.
The UV-vis spectrum of RB-Ag-SBA15 coated Quartz glass shows a broad absorption peak in the range of 450-650 nm. After immersing in Hg2+solution a remarkable increase in the absorbance is observed in the range of 450-650 nm, which rises with the increasing concentration of Hg2+(Fig. 4) and the band position is also significantly blue shifted by about 50 nm.

|
Download:
|
| Fig. 4. UV-vis absorption spectra of RB-Ag-SBA15 with varying Hg2+ concentration. | |
MEF of Rhodamine B is well discussed in our previous report [57]. Herein we demonstrate influence of Hg2+ on the MEF in silica host as a tool to detect mercury in aqueous solution. Figs. 5 and 6 show detailed photoluminescence spectra of gradual titration of Hg2+ions. The increasing concentration of Hg2+ions quenched the fluorescent signal of RB proportional to its concentration; the color of slide was changed from pink to yellow, which could be easily detected by the naked eye. The interaction of Hg2+ ions to the substrate resulted in a gradual decrease in photoluminescence band at 575 as well as at 400 nm, suggesting the formation of ringopened rhodamine species. This rapid response to mercury is very important in practical real-time detection. Huang et al. studied the fluorescent sensor using rhodamine dye immobilized gold nanoparticle for detection of Hg2+ ions [64]. In their work, emission intensity of RB dye has been quenched by Au NPs as a result of FRET, however, in the presence of Hg2+ ions, the RB molecules are detached from the surface of Au NPs, which restores the fluorescence of RB. In our case, the detachment of RB molecules from Ag nanoparticle surface by Hg2+ ions diminishes the MEF and hence fluorescence intensity decreases (Scheme 1 in Supporting information).

|
Download:
|
| Fig. 5. Photoluminescence studies on RB-Ag-SBA15 with varying Hg2+ concentration. | |

|
Download:
|
| Fig. 6. Photoluminescence studies on RB-Ag-SBA15 with varying Hg2+ concentration (0.0002 μmol/L-1 μmol/L). | |
We varied the concentration of Hg2+ ions solution from 1 μmol/L to 0.0002 μmol/L, the quenching of fluorescence was observed even for the very low concentration of 0.0002 μmol/L. The fluorescence intensity changes with the concentrations, and an obvious blue shift was observed at higher Hg2+ concentration (Fig. 6) because the surface plasmon absorption in the visible wavelength region is very sensitive to both particle size and shape and to the properties of the surrounding medium. However, when the film thickness becomes sufficiently large, scattering becomes significant, resulting in a strong increase in the absorbance at shorter wavelength. This effect promotes a blue shift of the surface plasmon band and a weakening in the apparent intensity of the Plasmon band.
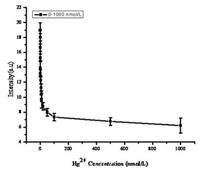
The overall standard graph by plotting the intensity ratio versus the concentrations of Hg2+ ions as illustrated in Fig. 7. Further, a good linear relationship is obtained for the Hg2+ ions concentration ranging from 0.001 μmol/L to 0.01 μmol/L and their respective coefficient of determination was found to be 0.99703 (Fig. 8). Based on this, the detection limit, defined as three times of the standard deviation of background, was calculated to be 10.54 nmol/L of Hg2+ ions.
|
Download:
|
| Fig. 7. Overall standard graph using RB-Ag-SBA15. The concentrations of Hg2+ ions were varied from 0.0002 μmol/L-1 μmol/L. | |

|
Download:
|
| Fig. 8. Linear region of standard graph using RB-Ag-SBA15. The Concentrations of Hg2+ ions were varied from 0.0002 μmol/L-1 μmol/L. | |
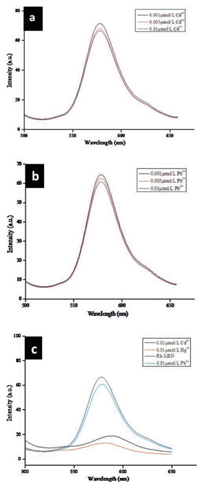
The fluorescence responses of RB-Ag-SBA15 to interfering metal ions are shown in Fig. 9(a-c). The emission spectra shown in Fig. 9a and b is corresponds to the various concentration of Cd2+ and Pb2+ solutions, the RB-Ag-SBA15 sensing material shows very little response as compared to that of for Hg2+, as observed from Fig. 9c the extent of quenching from original emission was found to be higher for Hg2+ when compared to Cd2+ and Pb2+. These data demonstrate that RB-Ag-SBA15 is a highly selective turn-off fluorescent sensor for Hg2+. The inspiring results suggest that the use of a mesoporous silica as a support for grafting fluorescent dye and Ag nanoparticle which provided new opportunities to fabricate an efficient optical sensing glass slides for heavy metals in water without buffer solution (Fig. 10).
|
Download:
|
| Fig. 9. Photoluminescence studies on RB-Ag-SBA15 with varying concentration of interfering cations a) Pb2+ solution b) Cd2+ solution c) comparison of emission spectra of RB-Ag-SBA15 for Hg2+, Cd2+, Pb2+ solutions having 0.01 μmol/L concentration. | |

|
Download:
|
| Fig. 10. Selectivity of the sensing system of Hg2+ Ions using RB-Ag-SBA15 to other competing metal ions. | |
Similarly, the performance of a sensing system was evaluated with a variety of environmentally relevant metal ions including Hg2+ interfering metal ion Pb2+ and Cd2+. As illustrated in Fig. 10, it exhibits a maximum response only in the presence of Hg2+ ions. Thus, the present system offers a higher selectivity over interfering cations such as Cd2+ and Pb2+.
Furthermore, the feasibility of the sensing system was evaluated with real environmental water samples to demonstrate its practical application. The tap water and lake water samples were used as a environmental water samples in this study and they were analysed using RB-Ag-SBA15 and AAS (Atomic Absorbance Spectrometer). Various concentrations of Hg2+ ions, such as 0, 1 and 10 nmol/L were spiked with these water samples.The water samples were first filtered and then centrifuged for 15 min at 10, 000 rpm. The concentrations of total Hg2+ ions in tap water and lake water samples were measured to be 0.048 nmol/L and 0.062 nmol/L by AAS. The results are summarized in Table 1, which shows good agreement with the values determined by AAS. The results demonstrated that the developed mercury sensor is suitable for the detection of Hg2+ ions in the environmental water samples.
|
|
Table 1 Determination of Hg2+ ions in environmental water samples using the proposed method and AAS. |
3. Conclusion
Overall, we have developed an optical assay having a wellordered mesoporous SBA15 containing Ag-RB for selective detection of Hg2+ in aqueous solution. The scheme combines the advantages of specific binding interactions between Hg2+ and RB molecule and optical emission properties of RB. The sensor displays a distinct change in color and fluorescence upon the alteration of Hg2+ levels in solution. The sensitivity of detection was improved by employing photoemission of RB coupled with the surface Plasmon resonance energy transfer of silver nanoparticle. Furthermore, the RB-Ag-SBA15 based fluorescent mercury sensor also efficiently detected the presence of Hg2+ ions in real time water samples, which was comparably detected by the conventional AAS. The metal enhanced fluorescence intensity of RB was selectively quenched by Hg2+ and the limit of detection was found to be 10.54 nmol/L. The method is suitable for a single-shot and irreversible analytical assay in a quartz glass/microtiter plate.
4. Experimental 4.1. Materials and methodsPluronic P123 (ethylene oxide (EO)-propylene oxide (PO)) triblock copolymer, with number average molecular weight of about 5300, Tetraethylorthosilicate (TEOS), rhodamine B base and silver nitrate were purchased from Aldrich. Analytical grade hydrochloric acid, absolute anhydrous ethanol (99.7%) and Metal salts such as HgSO4 and AgNO3, were purchased from SRL India Ltd. Milli-Q water was used in all experiments.
Fourier Transform-Infra Red Spectroscopy (FT-IR): FT-IR spectra were recorded on a JASCO FTIR instrument. The optical-grade KBr and substance were ground in a mortar with a pestle, to make 1 wt % mixture of making KBr pellets. After the sample was loaded, a minimum of 16 scans was collected for each sample at a resolution of ± 4 cm-1. UV-vis Spectroscopy: The absorption of RB-Ag-SBA15 with Hg2+ in various concentrations was recorded on a Shimadzu UV-2500 spectrophotometer in distilled water. X-ray Diffraction (XRD) Analysis: Wide-angle X-ray spectra obtained using a Powder X-ray diffract meter with powder sample (with Cu Kα radiation). The spectral window ranged from 2θ = 10 to 70. JEOL JEM-3010 analytical transmission electron microscopes, operating at 300 kV with a measured point-to-point resolution of 0.23 nm were used to record TEM images. Photoluminescence analysis was carried out in a JASCO fluorescence spectrometer; the spectra were recorded in the wavelength range of 360-900 nm with an excitation wavelength of 350 nm.
4.2. Synthesis of rhodamine B/Ag nanoparticle anchored SBA15RB-Ag-SBA15 was synthesized according to literature [62, 63]. The amount of Rhodamine B dye loading can be controlled by varying the concentration of dye solution used for the impregnation on Ag-SBA15 coated quartz glass. The detailed scheme is given in Supporting information.
4.3. Optical detection of mercury by rhodamine B/Ag nanoparticle anchored SBA15The quartz glass plate of dimension 2× 0.5 cm was cleaned thoroughly in an ultra-sonication process by using water followed by ethanol. The cleaned glass plates were then immersed in ethanol dispersion of RB-Ag-SBA15 solution for 30 min and dried. The dried glass plates were then dip coated with an aqueous solution containing different concentrations of mercury (Hg2+ salt dissolved in Hot NaCl) and dried in air. The modified quartz glass plates were analyzed with UV-vis and fluorescence spectrometer.
AcknowledgmentK. Dinakaran kindly acknowledges the financial support from the Department of Science and Technology, Govt of India through Fast Track Young Scientist Scheme (No. SR/FT/CS-103/2009).
Appendix A. Supplementary dataSupplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.cclet.2017.01.018.
| [1] | L. Campbell, D.G. Dixon, R.E. Hecky. A review of mercury in lake Victoria, East Africa: implications for human and ecosystem health. J. Toxicol. Environ. Health B 6 (2003) 325–356. DOI:10.1080/10937400306474 |
| [2] | P.D. Selid, H.Y. Xu, E.M. Collins, M.S.F. Collins, J.X. Zhao. Sensing mercury for biomedical and environmental monitoring. Sensors 9 (2009) 5446–5459. DOI:10.3390/s90705446 |
| [3] | I. Onyido, A.R. Norris, E. Buncel. Biomolecule-mercury interactions: modalities of DNA base-mercury binding mechanisms. Remediation strategies. Chem. Rev. 104 (2004) 5911–5930. DOI:10.1021/cr030443w |
| [4] | E.M. Nolan, S.J. Lippard. Tools and tactics for the optical detection of mercuric ion. Chem. Rev. 108 (2008) 3443–3480. DOI:10.1021/cr068000q |
| [5] | L. Jarup. Hazards of heavy metal contamination. Br. Med. Bull. 68 (2003) 167–182. DOI:10.1093/bmb/ldg032 |
| [6] | M.V.B. Krishna, J. Castro, T.M. Brewer, R.K. Marcus. Online mercury speciation through liquid chromatography with particle beam/electron ionization mass spectrometry detection. J. Anal. Atom. Spectrum. 22 (2007) 283–291. DOI:10.1039/B609362K |
| [7] | M. Leermakers, W. Baeyens, P. Quevauviller, M. Horvat. Mercury in environmental samples: speciation, artifacts and validation. Trends Anal. Chem. 24 (2005) 383–393. DOI:10.1016/j.trac.2004.01.001 |
| [8] | M.B. Gumpu, S. Sethuraman, U. Maheswari Krishnan, J.B. Balaguru Rayappan. A review on detection of heavy metal ions in water-an electrochemical approach. Sens. Actuators B 213 (2015) 515–533. DOI:10.1016/j.snb.2015.02.122 |
| [9] | N. Ratner, D. Mandler. Electrochemical detection of low concentrations of mercury in water using gold nanoparticles. Anal. Chem. 87 (2015) 5148–5155. DOI:10.1021/ac504584f |
| [10] | J.M. Gong, T. Zhou, D.D. Song, L.Z. Zhang, X.L. Hu. Stripping voltammetric detection of Mercury(Ⅱ) based on a bimetallic Au-Pt inorganic-organic hybrid nanocomposite modified glassy carbon electrode. Anal. Chem. 82 (2010) 567–573. DOI:10.1021/ac901846a |
| [11] | G. Mor-Piperberg, R. Tel-Vered, J. Elbaz, I. Willner. Nanoengineered electrically contacted enzymes on DNA scaffolds: functional assemblies for the selective analysis of Hg2+ ions. J. Am. Chem. Soc. 132 (2010) 6878–6879. DOI:10.1021/ja1006355 |
| [12] | I.H. Hsu, T.C. Hsu, Y.C. Sun. Gold-nanoparticle-based graphite furnace atomic absorption spectrometry amplification and magnetic separation method for sensitive detection of mercuric ions. Biosens. Bioelectron. 26 (2011) 4605–4609. DOI:10.1016/j.bios.2011.04.048 |
| [13] | Y.K. Che, X.M. Yang, L. Zang. Ultraselective fluorescent sensing of Hg2+ through metal coordination-induced molecular aggregation. Chem. Commun. (2008) 1413–1415. |
| [14] | J. Wang, B. Liu. Highly sensitive and selective detection of Hg2+ in aqueous solution with mercury-specific DNA and Sybr Green I. Chem. Commun. (2008) 4759–4761. |
| [15] | Z.D. Wang, J.H. Lee, Y. Lu. Highly sensitive turn-on fluorescent sensor for Hg2+ in aqueous solution based on structure-switching DNA. Chem. Commun. (2008) 6005–6007. |
| [16] | X.R. Zhang, Y. Li, H.R. Su, S.S. Zhang. Highly sensitive and selective detection of Hg2+ using mismatched DNA and a molecular light switch complex in aqueous solution. Biosens. Bioelectron. 25 (2010) 1338–1343. DOI:10.1016/j.bios.2009.10.023 |
| [17] | X. Zhu, L.F. Chen, Z.Y. Lin, B. Qiu, G.N. Chen. A highly sensitive and selective signal-on electrochemiluminescent biosensor for mercury. Chem. Commun. (2010) 3149–3151. |
| [18] | J.W. Liu, Y. Lu. Rational design of turn-on allosteric DNAzyme catalytic beacons for aqueous mercury ions with ultrahigh sensitivity and selectivity. Angew. Chem. Int. Ed. 46 (2007) 7587–7590. DOI:10.1002/(ISSN)1521-3773 |
| [19] | D.H. Wu, Q. Zhang, X. Chu, et al., Ultrasensitive electrochemical sensor for mercury (Ⅱ) based on target-induced structure-switching DNA. Biosens. Bioelectron. 25 (2010) 1025–1031. DOI:10.1016/j.bios.2009.09.017 |
| [20] | S.J. Liu, H.G. Nie, J.H. Jiang, G.L. Shen, R.Q. Yu. Electrochemical sensor for mercury(Ⅱ) based on conformational switch mediated by interstrand cooperative coordination. Anal. Chem. 81 (2009) 5724–5730. DOI:10.1021/ac900527f |
| [21] | R. Freeman, T. Finder, I. Willner. Multiplexed analysis of Hg2+ and Ag+ ions by nucleic acid functionalized CdSe/ZnS quantum dots and their use for logic gate operations. Angew. Chem. Int. Ed. 48 (2009) 7818–7821. DOI:10.1002/anie.v48:42 |
| [22] | S.J. Lee, J.H. Jung, J. Seo, et al., A chromogenic macrocycle exhibiting cationselective and anion-controlled color change: an approach to understanding structure-color relationships. Org. Lett. 8 (2006) 1641–1643. DOI:10.1021/ol0602405 |
| [23] | R. Métivier, I. Leray, B. Lebeau, B. Valeur. A mesoporous silica functionalized by a covalently bound calixarene-based fluoroionophore for selective optical sensing of mercury(Ⅱ) in water. J. Mater. Chem. 15 (2005) 2965–2973. DOI:10.1039/b501897h |
| [24] | D. Li, A. Wieckowska, I. Willner. Optical analysis of Hg2+ ions by oligonucleotide-gold-nanoparticle hybrids and DNA-based machines. Angew. Chem. Int. Ed. 47 (2008) 3927–3931. DOI:10.1002/anie.v47:21 |
| [25] | T. Li, B.Q. Li, E.K. Wang, S.J. Dong. G-quadruplex-based DNAzyme for sensitive mercury detection with the naked eye. Chem. Commun. (2009) 3551–3553. |
| [26] | Y. Xiang, Z.D. Wang, H. Xing, N.Y. Wong, Y. Lu. Label-free fluorescent functional DNA sensors using unmodified DNA: a vacant site approach. Anal. Chem. 82 (2010) 4122–4129. DOI:10.1021/ac100244h |
| [27] | J.S. Lee, M.S. Han, C.A. Mirkin. Colorimetric detection of mercuric ion (Hg2+) in aqueous media using DNA-functionalized gold nanoparticles. Angew. Chem. Int. Ed. 46 (2007) 4093–4096. DOI:10.1002/(ISSN)1521-3773 |
| [28] | J.J. Du, Z.K. Wang, J.L. Fan, X.J. Peng. Gold nanoparticle-based colorimetric detection of mercury ion via coordination chemistry. Sens. Actuators B 212 (2015) 481–486. DOI:10.1016/j.snb.2015.01.110 |
| [29] | B.C. Ye, B.C. Yin. Highly sensitive detection of mercury(Ⅱ) ions by fluorescence polarization enhanced by gold nanoparticles. Angew. Chem. Int. Ed. 47 (2008) 8386–8389. DOI:10.1002/anie.v47:44 |
| [30] | C.W. Liu, T.H. Hsieh, C.C. Huang, Z.H. Lin, H.T. Chang. Detection of mercury(Ⅱ) based on Hg2+-DNA complexes inducing the aggregation of gold nanoparticles. Chem. Commun. (2008) 2242–2244. |
| [31] | S.J. He, D. Li, C.F. Zhu, et al., Design of a gold nanoprobe for rapid and portable mercury detection with the naked eye. Chem. Commun. (2008) 4885–4887. |
| [32] | G.H. Chen, Y.C. Wei, Y.C. Yen, et al., Detection of mercury(Ⅱ) ions using colorimetric gold nanoparticles on paper-based analytical devices. Anal. Chem. 86 (2014) 6843–6849. DOI:10.1021/ac5008688 |
| [33] | X.W. Xu, J. Wang, K. Jiao, X.R. Yang. Colorimetric detection of mercury ion (Hg2+) based on DNA oligonucleotides and unmodified gold nanoparticles sensing system with a tunable detection range. Biosens. Bioelectron. 24 (2009) 3153–3158. DOI:10.1016/j.bios.2009.03.025 |
| [34] | M. Liu, Z.Y. Wang, S.F. Zong, et al., SERS detection and removal of mercury(Ⅱ)/silver(Ⅰ) using oligonucleotide-functionalized core/shell magnetic silica sphere@Au nanoparticles. ACS Appl. Mater Interfaces 6 (2014) 7371–7379. DOI:10.1021/am5006282 |
| [35] | Y. Sato, J. Tian, T. Ichihashi, et al., Enhancement in fluorescence response by a quencher for amiloride upon binding to thymine opposite an abasic site in a DNA duplex. Anal. Chim. Acta 675 (2010) 49–52. DOI:10.1016/j.aca.2010.06.042 |
| [36] | A. Balamurugan, H.I. Lee. Water-soluble polymeric probes for the selective sensing of mercury ion: pH-driven controllable detection sensitivity and time. Macromolecules 48 (2015) 1048–1054. DOI:10.1021/ma502350p |
| [37] | Z.P. Liu, W.J. He, Z.J. Guo. Metal coordination in photoluminescent sensing. Chem. Soc. Rev. 42 (2013) 1568–1600. DOI:10.1039/c2cs35363f |
| [38] | Y.M. Yang, Q. Zhao, W. Feng, F.Y. Li. Luminescent chemodosimeters for bioimaging. Chem. Rev. 113 (2012) 192–270. |
| [39] | M. Santra, D. Ryu, A. Chatterjee, et al., A chemodosimeter approach to fluorescent sensing and imaging of inorganic and methylmercury species. Chem. Commun. (2009) 2115–2117. |
| [40] | M.H. Lee, S.J. Lee, J.H. Jung, H. Lim, J.S. Kim. Luminophore-immobilized mesoporous silica for selective Hg2+ sensing. Tetrahedron 63 (2007) 12087–12092. DOI:10.1016/j.tet.2007.08.113 |
| [41] | W.B. Lu, X.Y. Qin, S. Liu, et al., Economical, green synthesis of fluorescent carbon nanoparticles and their use as probes for sensitive and selective detection of mercury (Ⅱ) ions. Anal. Chem. 84 (2012) 5351–5357. DOI:10.1021/ac3007939 |
| [42] | K.D. Bhatt, D.J. Vyas, B.A. Makwana, et al., Turn-on fluorescence probe for selective detection of Hg(Ⅱ) by calixpyrrole hydrazide reduced silver nanoparticle: application to real water sample. Chin. Chem. Lett. 27 (2016) 731–737. DOI:10.1016/j.cclet.2016.01.012 |
| [43] | L.L. Xi, H.B. Ma, G.H. Tao. Thiourea functionalized CdSe/CdS quantum dots as a fluorescent sensor for mercury ion detection. Chin. Chem. Lett. 27 (2016) 1531–1536. DOI:10.1016/j.cclet.2016.03.002 |
| [44] | D. Saikia, P. Dutta, N.S. Sarma, N.C. Adhikary. CdTe/ZnS core/shell quantum dotbased ultrasensitive PET sensor for selective detection of Hg(Ⅱ) in aqueous media. Sens. Actuators B 230 (2016) 149–156. DOI:10.1016/j.snb.2016.02.035 |
| [45] | P. Zhou, Q.T. Meng, G.J. He, et al., Highly sensitive fluorescence probe based on functional SBA-15 for selective detection of Hg2+ in aqueous media. J. Environ. Monit. 11 (2009) 648–653. DOI:10.1039/B815287J |
| [46] | J.R. Lakowicz. Plasmonics in biology and plasmon-controlled fluorescence. Plasmonics 1 (2006) 5–33. DOI:10.1007/s11468-005-9002-3 |
| [47] | J.R. Lakowicz, Y. Fu. Modification of single molecule fluorescence near metallic nanostructures. Laser. Photon. Rev. 3 (2009) 221–232. DOI:10.1002/lpor.v3:1/2 |
| [48] | O.G. Tovmachenko, C. Graf, D.J. van den Heuvel, A. van Blaaderen, H.C. Gerritsen. Fluorescence enhancement by metal-core/silica-shell nanoparticles. Adv. Mater. 18 (2006) 91–95. DOI:10.1002/(ISSN)1521-4095 |
| [49] | J.P. Yang, F. Zhang, Y.R. Chen, et al., Core-shell Ag@SiO2@mSiO2 mesoporous nanocarriers for metal-enhanced fluorescence. Chem. Commun. 47 (2011) 11618–11620. DOI:10.1039/c1cc15304h |
| [50] | G.B. Zhang, et al., Fabrication of Ag@SiO2@Y2 O3:Er nanostructures for bioimaging: tuning of the upconversion fluorescence with silver nanoparticles. J. Am. Chem. Soc. 132 (2010) 2850–2851. DOI:10.1021/ja909108x |
| [51] | S.Y. Cho, H.J. Jeon, H.W. Yoo, et al., Highly enhanced fluorescence signals of quantum dot-polymer composite arrays formed by hybridization of ultrathin plasmonic Au nanowalls. Nano Lett. 15 (2015) 7273–7280. DOI:10.1021/acs.nanolett.5b02355 |
| [52] | C.D. Geddes, J.R. Lakowicz. Editorial: metal-enhanced fluorescence. J. Fluoresc. 12 (2002) 121–129. DOI:10.1023/A:1016875709579 |
| [53] | J. Zhang, Y. Fu, M.H. Chowdhury, J.R. Lakowicz. Metal-enhanced singlemolecule fluorescence on silver particle monomer and dimer: coupling effect between metal particles. Nano Lett. 7 (2007) 2101–2107. DOI:10.1021/nl071084d |
| [54] | W. Deng, D.Y. Jin, K. Drozdowicz-Tomsia, et al., Ultrabright Eu-doped plasmonic Ag@SiO2 nanostructures: time-gated bioprobes with single particle sensitivity and negligible background. Adv. Mater. 23 (2011) 4649–4654. DOI:10.1002/adma.201102027 |
| [55] | K. Ray, R. Badugu, H. Szmacinski, J.R. Lakowicz. Several hundred-fold enhanced fluorescence from single fluorophores assembled on silver nanoparticledielectric-metal substrate. Chem. Commun. 51 (2015) 15023–15026. DOI:10.1039/C5CC03581C |
| [56] | K. Aslan, M. Wu, J.R. Lakowicz, C.D. Geddes. Fluorescent core-shell Ag@SiO2 nanocomposites for metal-enhanced fluorescence and single nanoparticle sensing platforms. J. Am. Chem. Soc. 129 (2007) 1524–1525. DOI:10.1021/ja0680820 |
| [57] | K. Dinakaran, I. Chandramohan, M.R. Venkatesan, et al., Surface plasmon enhanced photoluminescence of Rhodamine B confined in SBA15. Bull. Korean Chem. Soc. 32 (2011) 3861–3864. DOI:10.5012/bkcs.2011.32.11.3861 |
| [58] | A. Dubois, M. Canva, A. Brun, F. Chaput, J.P. Boilot. Photostability of dye molecules trapped in solid matrices. Appl. Opt. 35 (1996) 3193–3199. DOI:10.1364/AO.35.003193 |
| [59] | Y. Liu, E.B. Yang, R. Han, et al., A new rhodamine-based fluorescent chemosensor for mercury in aqueous media. Chin. Chem. Lett. 25 (2014) 1065–1068. DOI:10.1016/j.cclet.2014.04.033 |
| [60] | M.M. Ahamed Karns, M. Goodson, et al., DNA damage response to different surface chemistry of silver nanoparticles in mammalian cells. Toxicol. Appl. Pharmacol. 233 (2008) 404–410. DOI:10.1016/j.taap.2008.09.015 |
| [61] | M.H. Chowdhury, K. Aslan, S.N. Malyn, J.R. Lakowicz, C.D. Geddes. Metalenhanced chemiluminescence: radiating plasmons generated from chemically induced electronic excited states. Appl. Phys. Lett. 88 (2006) 173104. DOI:10.1063/1.2195776 |
| [62] | S.L. Pan, Z.J. Wang, L.J. Rothberg. Enhancement of adsorbed dye monolayer fluorescence by a silver nanoparticle overlayer. J. Phys. Chem.. 110 (2006) 17383–17387. DOI:10.1021/jp063191m |
| [63] | J.T. Li, L.M. Guo, J.L. Shi. Stepwise in situ synthesis and characterization of metallophthalocyanines@mesoporous matrix SBA-15 composites. Phys. Chem. Chem. Phys. 12 (2010) 5109–5114. DOI:10.1039/b925431e |
| [64] | C.C. Huang, H.T. Chang. Selective gold-nanoparticle-based turn-on fluorescent sensors for detection of mercury(Ⅱ) in aqueous solution. Anal. Chem. 78 (2006) 8332–8338. DOI:10.1021/ac061487i |
 2017, Vol. 28
2017, Vol. 28 



