b State Key Laboratory of Medical Genetics & School of Life Sciences, Central South University, Changsha 410013, China
Tetracycline (TC) is a group of broad-spectrum antibacterial agents, which is widely used in the treatment of human diseases and animal breeding. Owing to its various advantages such as effective antimicrobial properties, low price and low side effects, it can also be used as a drug additive [1]. Nowadays, the pollution of TC in surface and ground water has been proved to be a serious environmental concern due to the improper use and the relatively high solubility of TC in water [2, 3]. Besides, the extensive use of tetracyclines leads to excessive residues in daily food products, such as meat, milk, honey, fish and eggs [4-7]. The residual tetracycline could be transmitted to human body through the food chain. With the accumulation of the tetracycline increases in the human body, there will be a series of toxic and side effects, for instance, generating the drug resistance of bacteria and weaken the function of the body's immune system [8]. To safeguard human health, many countries and groups have set maximum residue limit for tetracycline, for example, the European Union has set maximum residue limits for tetracycline at 0.3 mg/kg in liver, 0.1 mg/kg in milk or muscle tissues, and 0.01 mg/kg in honey [9]. Therefore, developing a sensitive and effective analytical method that can fast detect the content of tetracycline is particularly important.
Commonly, traditional approaches for the detection of tetracycline are as follows: High-performance liquid chromatography (HPLC) [10, 11], capillary electrophoresis (CE) [12, 13], gas chromatography-mass spectrometry (GC-MS) [14, 15], enzyme-linked immunosorbent assay (ELISA) [16, 17], and microorganism inhibition [18]. The mentioned instrumental analysises are the most popular and sensitive methods used in antibiotic detection. However, they all require time consuming pretreatment steps and expensive instruments. Besides, the use of some organic solvents is harmful to health and the environment. Immunoassay methods have the characteristics of simplicity, high specificity and sensitivity, but the preparation of the antibodies via animal immunization is time-consuming, and the immunization may obtain false-positive test results because of its potential cross-reactivity of the substance with similar chemical structures [19, 20]. In order to overcome the aforementioned limitations of traditional approaches, some aptamer-based assays have been developed for the detection of tetracycline. Aptamers are single-stranded DNA or RNA molecules that can bind to their target molecules with high affinity and specificity by folding into distinct secondary and tertiary structures [21]. In comparison with antibodies, aptamers have many advantages such as small size, ability to sustain reversible denaturation, easy and controllable modification, slow degradation kinetics, nontoxicity and lack of immunogenicity. These excellent biochemical properties make aptamers ideal candidates as recognition units in developing sensing systems for bioanalytical applications [22]. Recently, lots of efforts have been made towards the development of simple, cheap, easy and selective aptamer-aptasensor for the determination of tetracycline. For example, Wang et al. developed a direct competitive assay-based aptasensor and an indirect competitive assay-based aptasensor for sensitive determination of tetracycline residue in honey, which was based on a modified direct competitive enzyme-linked aptamer assay scheme and an indirect competitive enzyme-linked aptamer assay, utilizing a 76-mer single-stranded DNA aptamer selected by Systematic Evolution of Ligands by Exponential Enrichment (SELEX). The limit of detection was 9.6×10-3 ng/mL with a linear working range from 0.01 to 100 ng/mL toward TCs in honey, and a mean recovery rate of 93.23% in tetracycline-spiked honey was obtained [23, 24]. Que et al. designed a sensitive electrochemical immunoassay of tetracycline residue by using platinum catalyzed hydrogen evolution reaction on an anti-tetracycline antibody modified immune sensor, which has high sensitivity and specificity. The detection limit is 6pg/mL, however, this immune sensor is complex and hard to apply to the real sample analysis [25]. Luo et al. devised a novel colorimetric aptasensor using cysteamine-stabilized gold nanoparticles (CS-AuNPs) as probes for rapid and specific detection of tetracycline in raw milk. The absorbance changes of CS-AuNPs were linearly proportional with the concentration of tetracycline in the range of 0.2-2.0 mg/mL with the detection limit of 0.039 mg/mL [26]. Zhou et al. developed a simple electrochemical tetracycline aptasensor with multi-walled carbon nanotubes (MWCNTs) modification. MWCNTs were dropped on the glassy carbon electrode to immobilize the anti-tetracycline aptamer to construct the aptasensor. This electrochemical aptasensor has a detection limit of 5×10-9 mol/L [27]. However, those assays mentioned above suffer from one or more following disadvantages, such as low sensitivity, high cost, complexity and time-consuming.
Herein, a label-free and simple aptamer-based fluorescent sensing platform which was based on triple-helix molecular switch (THMS) and G-quadruplex was developed for the first time for the detection of tetracycline. Two arm segments of the aptamer bind to the sequence of STP through Watson-Crick and Hoogsteen base pairings, leading to formation of THMS. Compared with double-helix DNA molecular switches and molecular beacon-based signaling aptamers, the THMS presents distinct advantages, including sensitivity, high stability, preserved selectivity and original aptamer affinity [28]. THMS system consists of a label-free target specific aptamer sequence with two arm segments and a dual-labeled oligonucleotide as a signal transduction probe (STP) [28, 29]. Instead of taking labeled oligonucleotide as a STP, we used a G-rich oligonucleotide, which can specifically bind to thioflavin T (ThT) and generated a high fluorescence enhancement [30, 31]. A shortened 8-mer ssDNA with high affinity to different tetracyclines was chosen as target specific aptamer sequence [32]. Our results indicated that this method was sensitive, selective and fast for the detection of tetracycline.
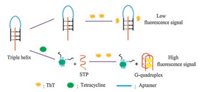
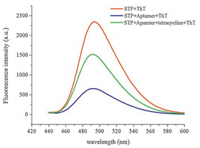
2. Results and discussion 2.1. Strategy for tetracycline detectionThe designed strategy for the detection of tetracycline is demonstrated in Scheme 1. The work employed THMS as a signal trigger which was composed of tetracycline's aptamer and G-rich STP by Watson-Crick and Hoogsteen base pairings [33, 34]. As we mentioned above, ThTcan specifically bind to a DNA G-quadruplex and generated a relatively high fluorescence enhancement. In the absence of tetracycline, the structure of triple-helix molecular switch remains stable, interaction between ThT and triple-helix molecular switch (THMS) is weak, which implies that the background fluorescence of the THMS structure will be low. In the presence of tetracycline, the aptamer-target binding results in the formation of a structured aptamer-target complex, which disassembles the THMS and releases the G-rich sequence (STP). The released STP can fold into a compact G-quadruplex structure, which can specifically bind to ThT and results in an enhanced fluorescence. Therefore, quantitative analysis of tetracycline can be achieved by monitoring the changes in fluorescence intensity of ThT. As shown in Fig. 1, the binding buffer contained STP only, ThT was added to the buffer solution after 15 min incubation, and the fluorescence intensity of the system was very high. When tetracycline aptamer and STP were incubated in binding buffer for the same time, and the fluorescence intensity became low with the addition of ThT. Then, tetracycline was added to the system, after 30 min incubation, the fluorescence intensity significantly increased. The results strongly indicated that our proposed strategy could be used to detect tetracycline.
|
Download:
|
| Scheme 1. Schematic drawing for the detectionof tetracycline: (a) in the absence of tetracycline; (b) in the presence of tetracycline. | |
|
Download:
|
| Fig. 1. Flouorescence spectra of free STP +ThT (red), STP+aptamer+ThT (blue) and STP+aptamer+tetracycline +ThT (green) in Tris-HCl buffer (20 mmol/L, containing 100 mmol/L NaCl, 20 mmol/L KCl, 2 mmol/L MgCl2, pH 7.5). Excitation: 425 nm; Emission: 492 nm. | |
2.2. Optimization of the experimental conditions
For the purpose of maximizing the performance of tetracycline assay, we investigated the effect of various parameters on the fluorescence response of the system to tetracycline. We first optimized the concentration of aptame. Fig. 2A shows that in the presence of 200 nmol/L STP, the fluorescence intensity was higher than others when the concentration of aptame was 200 nmol/L. Besides, higher concentration could lead to the decrease of the fluorescence intensity due to the function between target and excrescent aptamer, so we chose 200 nmol/L aptame in the next experiment.

|
Download:
|
| Fig. 2. (A) Fluorescence intensity of THMS in the presence of 200 nmol/L STP, 100 nmol/L tetracycline and various concentrations of tetracycline-aptamer (from top to bottom 200, 100, 400 and 600 nmol/L); (B) Relative fluorescence intensity ratio of THMS in Tris-HCl buffer with different pH values (6.0-8.0). F0 and F represent the fluorescence intensities at 492 nm before and after the addition of tetracycline, respectively. | |
The pH is a crucial parameter, which is vital to the stability of the Hoogsteen base pairing to form a stable THMS structure [28]. Hence, the influences of Tris-HCl buffer solution with different pH values on the formation of THMS were carefully investigated. Fig. 2B displays the fluorescence intensity ratio of the sensing platform (F-F0/F0, F0 and F represent the fluorescence intensities at 492 nm before and after the addition of tetracycline, respectively) in differentpH situations. It was observed that the fluorescentratio rose gradually with the increase of pH and then reached a peak at 7.5. Thus, the optimal pH was chosen to be 7.5.
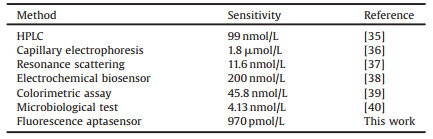
2.3. Sensitivity of the proposed assay for tetracyclineUnder optimal conditions, we incubated THMS sensing platform with various concentrations of tetracycline (0-100 nmol/L). As shown in Fig. 3A, the fluorescence intensity increased with the increasing concentration of tetracycline. It can be observed in Fig. 3B that the fluorescence enhancement of the sensing platform was proportional to the concentration of tetracycline. A linear correlation existed between the fluorescence intensity ratio (F-F0/F0) and the concentration of tetracycline in the range of 0.2 nmol/L to 20.0 nmol/L. The regression equation was y = 0.034x + 0.110 with a correlation coefficient of 0.9929, the limit of detection (LOD) was 970.0 pmol/L based on three times the signal-to-noise level, which was relatively low comparable to the recently reported assays (Table 1).

|
Download:
|
| Fig. 3. (A) Fluorescence responses in the presence of different concentrations of tetracycline. From bottom totop: 0, 0.2, 1.0, 2.0, 5.0, 10.0, 20.0, 50.0, 100.0 nmol/L tetracycline. (B) Relative fluorescence intensity ratio of the THMS in the presence of different concentrations of tetracycline. The inset presents a linear relationship in the concentrations of tetracycline ranging from 0.2 nmol/L to 20 nmol/L. | |
|
|
Table 1 Comparison of analytical methods for the detection of tetracycline. |
2.4. Specificity of the proposed assay for tetracycline
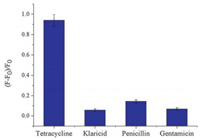
To demonstrate the selectivity of proposed assay, a series of antibiotics including tetracycline, klaricid, penicillin and gentamicin were investigated by this method. As shown in Fig. 4, the fluorescence intensity ratio of tetracycline was significantly higher than other antibiotics. The result indicated excellent selectivity of the presented sensing platform for detection of tetracycline.
|
Download:
|
| Fig. 4. Efficiency of fluorescence intensity ratio in the presence of various antibiotics. F0 and F represent the fluorescence intensities at 492 nm before and after the addition of tetracycline, respectively. | |
2.5. Tetracycline assay in biological samples
In order to evaluate the applicability of the sensing system in biological samples, spiked-recovery experiments with different concentrations of tetracycline were performed in 100-fold-diluted human serum. As shown in Table 2, the recovery rates were 117% for 5 nmol/L, 103% for 10 nmol/L, and 104% for 20 nmol/L spiked tetracycline (Table 2). The relative standard deviation was in the range of 1.05%-3.16%. These results revealed that the proposed assay can potentially be applied to the detection of tetracycline in real biological samples.
|
|
Table 2 Recovery experiments of tetracycline in diluted human serum by this method. |
3. Conclusion
Summarily, in order to detect tetracycline, a label-free and aptamer-based fluorescence assay depending on THMS and G-quadruplex has been developed. The high affinity and selectivity of the TC aptamer play a significant role in conjugating to the tetracycline whereas the stability and sensitivity of the THMS help to measure the tetracycline in a short time. Besides, the generation of the fluorescent signal by G-quadruplex binding to ThT makes the sensing platform label-free. The assay showed a good selectivity to tetracycline with a detection limit of 970 pmol/L. Instead of requiring complex experimental techniques, our method is sensitive and selective. Moreover, this sensing platform could well detect tetracycline in real biological sample. It is expected that this THMS sensing platform could be generalized for the detection and control of other toxic and harmful substances in food.
4. Experimental 4.1. Materials and measurementsThioflavin T, tetracycline, klaricid, penicillin and gentamicin were purchased from Sigma-Aldrich. Tris-HCl buffer (20 mmol/L, containing 100 mmol/L NaCl, 20 mmol/L KCl, 2 mmol/L MgCl2, pH 7.5) was used for fluorescence detection of tetracycline. The oligonucleotides used in this work were synthesized and HPLC-purified by Sangon Biotech. Co., Ltd. (Shanghai, China). STP sequence is 5'-ATGGGAAGGGAGGGATGGGT-3', and tetracycline-binding aptamer sequence is 5'-TCCCTTCCGGTGGTGCTTCCCT-3' (The italic letters are the aptamer and the underlined sequences indicate the bases that form a triplex). All other reagents were of analytical reagent grade. Ultrapure water (18.2 MΩ/cm) obtained from a Milli-Qwater purification system (Millipore Corp, Bedford, MA, USA) was used throughout the experiments. Human serum samples were collected from Xiangya Hospital, Central South University. All fluorescence measurements were carried out on an F-2700 fluorescence spectrophotometer (Hitachi, Japan) with excitation at 425 nm and emission at 492 nm for ThT.
The study was approved by the Ethics Committee of Central South University, Changsha, China. All people had given informed consent. We declare that the experiments performed in this study comply with the current laws of China.
4.2. Preparations for triple-helix aptamer and signal probeThe THMS was obtained by incubating the STP (200 nmol/L final concentration) and the aptamer (200 nmol/L final concentration) in binding buffer (20 mmol/L Tris-HCl, containing 100 mmol/L NaCl, 20 mmol/L KCl, 2 mmol/L MgCl2, pH 7.5) for 15 min at room temperature.
4.3. Optimization of tetracycline-binding aptamer concentrationIncreasing concentrations of tetracycline-aptamer (100-600 nmol/L) were added to a constant concentration of STP (200 nmol/L) in the Tris-HCl buffer (pH 7.5). The mixture was incubated at room temperature for 15 min. Then, ThT was added to a final concentration of 10μmol/L to each well and the fluorescence spectrum was recorded at room temperature after 30 min.
4.4. Effect of pH on the fluorescence of THMSThe 200 nmol/L tetracycline-binding aptamer was added to 200 nmol/L signal transduction probe in Tris-HCl buffer at different pH values (6.0-8.0) and incubated for 15 min at room temperature. The fluorescence intensities were measured as previous description.
4.5. Sensitivity and SelectivityIncreasing concentrations of tetracycline (0 to 100 nmol/L) were added to the assay solution (final volume 100 μL) containing 200 nmol/L signal transduction probe and 200 nmol/L tetracyclinebinding aptamer in Tris-HCl buffer (pH 7.5). The mixtures were incubated for 15 min at room temperature. The fluorescence intensities were recorded as previous description. The selectivity of triple-helix molecular switch was assessed in the presence of 100 nmol/L tetracycline, klaricid, penicillin and gentamicin.
AcknowledgmentsThis work was supported by National Natural Science Foundation of China (Nos. 21205142, 31370104), The Research Innovation Program for Graduates of Central South University (No. 2016zzts580).
| [1] | A.N. Sapadin, R. Fleischmajer. Tetracyclines: nonantibiotic properties and their clinical implications. J. Am. Acad. Dermatol. 54 (2006) 258–265. DOI:10.1016/j.jaad.2005.10.004 |
| [2] | L.C. Chen, S. Lei, M.Z. Wang, J. Yang, X.W. Ge. Fabrication of macroporous polystyrene/graphene oxide composite monolith and its adsorption property for tetracycline. Chin. Chem. Lett. 27 (2016) 511–517. DOI:10.1016/j.cclet.2016.01.057 |
| [3] | Z.Y. Guo, P.P. Gai, J. Duan, H.N. Zhang, S. Wang. Tetracycline selective electrode based on molecularly imprinted polymer particles. Chin. Chem. Lett. 21 (2010) 1235–1238. DOI:10.1016/j.cclet.2010.04.007 |
| [4] | F.K. Muriuki, W.O. Ogara, F.M. Njeruh, E.S. Mitema. Tetracycline residue levels in cattle meat from Nairobi salughter house in Kenya. J. Vet. Sci. 2 (2001) 97–101. |
| [5] | Y.D. Zhang, N. Zheng, R.W. Han, et al., Occurrence of tetracyclines sulfonamides, sulfamethazine and quinolones in pasteurized milk and UHT milk in China's market. Food Control 36 (2014) 238–242. DOI:10.1016/j.foodcont.2013.08.012 |
| [6] | G.T. Peres, S. Rath, F.G.R. Reyes. A HPLC with fluorescence detection method for the determination of tetracyclines residues and evaluation of their stability in honey. Food Control 21 (2010) 620–625. DOI:10.1016/j.foodcont.2009.09.006 |
| [7] | H. Oka, Y. Ito, H. Matsumoto. Chromatographic analysis of tetracycline antibiotics in foods. J. Chromatogr. A 882 (2000) 109–133. DOI:10.1016/S0021-9673(99)01316-3 |
| [8] | J.L. Martinez. Environmental pollution by antibiotics and by antibiotic resistance determinants. Environ. Pollut. 157 (2009) 2893–2902. DOI:10.1016/j.envpol.2009.05.051 |
| [9] | Z.Y. Guo, P.P. Gai. Development of an ultrasensitive electrochemiluminescence inhibition method for the determination of tetracyclines. Anal. Chim. Acta 688 (2011) 197–202. DOI:10.1016/j.aca.2010.12.043 |
| [10] | W.A. Moats. Determination of tetracycline antibiotics in beef and pork tissues using ion-paired liquid chromatography. J. Agric. Food Chem. 48 (2000) 2244–2248. DOI:10.1021/jf990649r |
| [11] | L. Monser, F. Darghouth. Rapid liquid chromatographic method for simultaneous determination of tetracyclines antibiotics and 6-Epidoxycycline in pharmaceutical products using porous graphitic carbon column. J. Pharm. Biomed. Anal. 23 (2000) 353–362. DOI:10.1016/S0731-7085(00)00329-0 |
| [12] | P. Kowalski. Capillary electrophoretic method for the simultaneous determination of tetracycline residues in fish samples. J. Pharm. Biomed. Anal. 47 (2008) 487–493. DOI:10.1016/j.jpba.2008.01.036 |
| [13] | K. Ng, S.W. Linder. HPLC separation of tetracycline analogues: comparison study of laser-based polarimetric detection with UV detection. J. Chromatogr. Sci. 41 (2003) 460–466. DOI:10.1093/chromsci/41.9.460 |
| [14] | T.A. Ternes, R. Hirsch, J. Mueller, K. Haberer, et al., Methods for the determination of neutral drugs as well as betablockers and b2-sympathomimetics in aqueous matrices using GC/MS and LC/MS/MS. Anal. Bioanal. Chem 362 (1998) 329–340. |
| [15] | T.A. Ternes. Analytical methods for the determination of pharmaceuticals in aqueous environmental samples. Trends. Anal. Chem 20 (2001) 419–434. DOI:10.1016/S0165-9936(01)00078-4 |
| [16] | Y.L. Zhang, S.X. Lu, W. Liu, C.B. Zhao, R.M. Xi. Preparation of anti-tetracycline antibodies and development of an indirect heterologous competitive enzymelinked immunosorbent assay to detect residues of tetracycline in milk. J. Agric. Food Chem. 55 (2007) 211–218. DOI:10.1021/jf062627s |
| [17] | S.Q. Han, E.B. Liu, H. Li. Determination of tetracycline, chlortetracycline and oxytetracycline by flow injection with inhibitory chemiluminescence detection using copper(Ⅱ) as a probe ion. Luminescence 21 (2006) 106–111. DOI:10.1002/(ISSN)1522-7243 |
| [18] | L. Okerman, S. Croubels, S.D. Baere, et al., Inhibition tests for detection and presumptive identification of tetracyclines, (-lactam antibiotics and quinolones in poultry meat. Food Addit. Contam. 18 (2001) 385–393. DOI:10.1080/02652030120410 |
| [19] | X.M. Chen, L.M. Zhao, X.T. Tian, et al., A novel electrochemiluminescence tetracyclines sensor based on a Ru(bpy)32+-doped silica nanoparticles/Nafion film modified electrode. Talanta 129 (2014) 26–31. DOI:10.1016/j.talanta.2014.04.054 |
| [20] | H.L. Tan, C.J. Ma, Y.H. Song, et al., Determination of tetracycline in milk by using nucleotide/lanthanide coordination polymer-based ternary complex. Biosens. Bioelectron. 50 (2013) 447–452. DOI:10.1016/j.bios.2013.07.011 |
| [21] | H.M. Meng, H. Liu, H.L. Kuai, et al., Aptamer-integrated DNA nanostructures for biosensing, bioimaging and cancer therapy. Chem. Soc. Rev. 45 (2016) 2583–2602. DOI:10.1039/C5CS00645G |
| [22] | W.H. Tan, M.J. Donovan, J.H. Jiang. Aptamers from cell-based selection for bioanalytical applications. Chem. Rev. 113 (2013) 2842–2862. DOI:10.1021/cr300468w |
| [23] | S. Wang, J.H. Liu, W. Yong. A direct competitive assay-based aptasensor for sensitive determination of tetracycline residue in Honey. Talanta 131 (2015) 562–569. DOI:10.1016/j.talanta.2014.08.028 |
| [24] | S. Wang, W. Yong, J.H. Liu, et al., Development of an indirect competitive assaybased aptasensor for highly sensitive detection of tetracycline residue in honey. Biosens. Bioelectron. 57 (2014) 192–198. DOI:10.1016/j.bios.2014.02.032 |
| [25] | X.H. Que, X. Chen, L.B. Fu, et al., Platinum-catalyzed hydrogen evolution reaction for sensitive electrochemical immunoassay of tetracycline residues. J. Electroanal. Chem. 704 (2013) 111–117. DOI:10.1016/j.jelechem.2013.06.023 |
| [26] | Y. Luo, J.Y. Xu, Y. Li. A novel colorimetric aptasensor using cysteaminestabilized gold nanoparticles as probe for rapid and specific detection of tetracycline in raw milk. Food Control 54 (2015) 7–15. DOI:10.1016/j.foodcont.2015.01.005 |
| [27] | L. Zhou, D.J. Li, L. Gai, J.P. Wang, Y.B. Li. Electrochemical aptasensor for the detection of tetracycline with multi-walled carbon nanotubes amplification. Sens. Actuat. B Chem. 162 (2012) 201–208. DOI:10.1016/j.snb.2011.12.067 |
| [28] | J. Zheng, J.S. Li, Y. Jiang. Design of aptamer-based sensing platform using triplehelix molecular switch. Anal. Chem. 83 (2011) 6586–6592. DOI:10.1021/ac201314y |
| [29] | A. Verdian-Doghaei, M.R. Housaindokht, K. Abnous. A fluorescent aptasensor for potassium ion detection-based triple-helix molecular switch. Anal. Biochem. 466 (2014) 72–75. DOI:10.1016/j.ab.2014.08.014 |
| [30] | J. Mohanty, N. Barooah, V. Dhamodharan, et al., Thioflavin T as an efficient inducer and selective fluorescent sensor for the human telomeric Gquadruplex DNA. J. Am. Chem. Soc. 135 (2013) 367–376. DOI:10.1021/ja309588h |
| [31] | A. R. de la Faverie, A. Guédin, A. Bedrat, L. A. Yatsunyk, J. L. Mergny, Thioflavin T as a fluorescence light-up probe for G4 formation, Nucleic Acids Res. 42(2014) e65. |
| [32] | Y.S. Kwon, N.H. Ahmad Raston, M.B. Gu. An ultra-sensitive colorimetric detection of tetracyclines using the shortest aptamer with highly enhanced affinity. Chem. Commun. 50 (2014) 40–42. DOI:10.1039/C3CC47108J |
| [33] | K. Hoogsteen. The structure of crystals containing a hydrogen-bonded complex of 1-methylthymine and 9-methyladenine. Acta Crystallogr. 12 (1959) 822–823. DOI:10.1107/S0365110X59002389 |
| [34] | K.M. Vasquez, P.M. Glazer. Triplex-forming oligonucleotides: principles and Applications. Q. Rev. Biophys. 35 (2002) 89–107. |
| [35] | J.W. Fritz, Y.G. Zuo. Simultaneous determination of tetracycline, oxytetracycline, and 4-epitetracycline in milk by high-performance liquid chromatography. Food Chem. 105 (2007) 1297–1301. DOI:10.1016/j.foodchem.2007.03.047 |
| [36] | S.M. Santos, M. Henriques, A.C. Duarte, V.I. Esteves. Development and application of a capillary electrophoresis based method for the simultaneous screening of six antibiotics in spiked milk samples. Talanta 71 (2007) 731–737. DOI:10.1016/j.talanta.2006.05.049 |
| [37] | Y.F. Luo, L. He, S.S. Zhan, et al., Ultrasensitive resonance scattering (RS) spectral detection for trace tetracycline in milk using aptamer-coated nanogold (ACNG) as a catalyst. J. Agric. Food Chem. 62 (2014) 1032–1037. DOI:10.1021/jf403566e |
| [38] | H.T. Wang, H.M. Zhao, X. Quan. Gold modified microelectrode for direct tetracycline detection. Front. Environ. Sci. Eng. 6 (2012) 313–319. DOI:10.1007/s11783-011-0323-5 |
| [39] | L. He, Y.F. Luo, W.T. Zhi, P. Zhou. Colorimetric sensing of tetracyclines in milk based on the assembly of cationic conjugated polymer-aggregated gold nanoparticles. Food Anal. Methods 6 (2013) 1704–1711. DOI:10.1007/s12161-013-9577-9 |
| [40] | J. Kurittu, S. Lönnberg, M. Virta, M. Karp. A group-specific microbiological test for the detection of tetracycline residues in raw milk. J. Agric. Food Chem. 48 (2000) 3372–3377. DOI:10.1021/jf9911794 |
 2017, Vol. 28
2017, Vol. 28