Rotaxane is a type of mechanically interlocked supramolecular system formed by a ring molecule on a dumbbell-shaped linear molecule [1-4]. While polyrotaxanes [5-8] can be consided as a plurality of cyclic molecules on a dumbbell-shaped molecule, which shows not only the unique structural features but also wide potential applications in biology, optoelectronic material and molecular machines. It is worth mentioning that the design and synthesis of molecular machines based on rotaxanes and catenanes was awarded the 2016 Nobel Prize in Chemistry [9]. During the past two decades, scientists have paid more and more attentions to prepare functional polyrotaxanes using various macrocyclic molecules, such as cyclodextrins [10, 11], crown ethers [12-16], cucurbiturils [17-19], and so on. Among them, crown ether have attracted increasing interest for their diverse binding selectivities and easy to be prepared. By introducing of host-guest interactions between crown ethers and ammonium salts to prepare polyrotaxane, the "threading-followed-by-stoppering" and template-directed "clipping" approach [20, 21] have been developed. Previously, we reported [22] an efficient and facile method to construct high order polyrotaxane by connecting the "pseudosuitane" complex, which was formed by triptycenederived bis(crown ether) host and a functionalized bis secondary dialkylammonium salts containing an anthracenyl core through host-guest interactions. Recently, Goldup and coworks [23] reported a simple iterative coupling strategy for the synthesis of oligomeric homo-and hetero[n]rotaxanes with precise control over the position of each macrocycle. Although significant efforts have been made, the reports about construction of well-organized AABB-type heteropolyrotaxanes comprising alternative different ring moieties are very limited.
Self-sorting strategy is a quite common and important process in biological systems, which has the unique capability of selective self-assembly in complex mixtures [24-29]. For example, the emergence of "life" efficently employs the principle of self-sorting to afford intricate and functional archetectures with increasing complexity. The process of self-sorting mainly relies on reversible convalent and non-convalent interactions including hydrogenbonding, hydrophobic interactions, π-π interactions, host-guest interactions and metal-ion coordination interactions [30-41]. In 2008, Schalley and coworks [42] reported an efficient integrative self-sorting strategy to construct of cascade-stoppered hetero[3] rotaxane by incorporating two kinds of polyether macrocycles into a single axle molecule with two kinds of secondary ammoniums. Qu [43] empolyed a self-sorting strategy to synthesis of a [c2] daisy-chain-containing hetero[4]rotaxane. Liu and coworks [44] descrided the preparation of a twin-axial hetero[7]rotaxane through the combination of self-assembly and covalent synthetic "click chemistry".
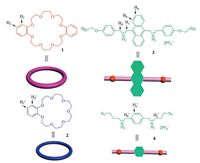
Different from the above reports, we present herein a facile onepot construction of heteropolyrotaxane from two kinds of [3] pseudorotaxanes by combination of self-sorting and cascade-stoppered strategy. In this system, a bisammonium scaffold based on a 9, 10-anthracenyl core guest 3 and a bisammonium scaffold based on a 1, 4-phenyl core guest 4 could complex with dibenzo[24] crown-8 (DB24C8, host 1) and benzo-21-crown-7 (B21C7, host 2) to form 1:2 complexes, respectively. Additionally, the synthesis of heterployrotaxane could be achieved by simply connecting the two formed [3] pseudorotaxanes (Fig. 1).
|
Download:
|
| Fig. 1. Graphical representation of structures and the proton designations of hosts 1, 2 and guests 3, 4. | |
2. Results and discussion
Firstly, the complexation of host 1 and guest 3, host 2 and guest 4 were investigated by 1H NMR spectra. As shown in Fig. 2c, the 1H NMR spectrum of host 1 and guest 3 at a molar ratio of 2:1 in CDCl3/CD3CN (2:3, v/v) solution showed a significantly difference from those of 1 and 3. The signals for the catechol ring protons (H1 and H2) in DB24C8 shifted upfield probably as a consequence of their stacking with the anthracene core of guest 3. It was also of interest to note that the methylene protons (Hc and Hd) adjacent to the NH2+ showed considerable downfield shift owing to the C-H·…O interactions between the benzylic methylene hydrogen atoms and the crown oxygen atoms of the host 1. These results were consistent with the complexation between DB24C8 and bisammonium scaffold based on a 9, 10-anthracenyl core reported by Stoddart and coworks [45], which suggested the formation of [3] pseudorotaxane 12·3 between host 1 and guest 3. Similarly, the formation of [3]pseudorotaxane between host 2 and guest 4 were also studied by 1H NMR spectra. For instance, when 2 and 4 were mixed at a 2:1 molar ratio in CDCl3/CD3CN (2:3, v/v), it was found that the signals from proton Ha' of 4 shifted upfield and the signal from proton Hb' and Hc' adjacent to the NH2+ showed dramatical downfield shift (Fig. 2e), which might be attributed to its hydrogen bonding interactions with crown oxygen atoms of host 2. Moreover, the signals for catechol ring protons (H1' and H2') in B21C7 were also showed considerable shift. These results were consistent with the complexation between host 2 and dibutylammonium salt reported before [46], which indicated the formation of [3] pseudorotaxane 22·4 between host 2 and guest 4.

|
Download:
|
| Fig. 2. Partial 1H NMR spectra (400 MHz, CD3CN:CDCl3 = 2:3, 295 K) of (a) free host 1, (b)free guest 3, (c) guest 3 and 2.0 equiv of host 1, (d) 1, 2, 3 and 4 at a ratio of 2:2:1:1, (e) guest 4 and 2.0 equiv of host 2, (f) free host 2, and (g) free guest 4. [3]0 = [4]0 = 4.0 mmol/L. | |
On the basis of these results, we further investigated the selfsorting processes of compounds 1-4 by 1H NMR spectra. Two sets of sharp signals, consistent with two discrete [3] pseudorotaxane were observed in 1H NMR spectra. As shown in Fig. 2d, when compounds 1, 2, 3 and 4 at a molar ratio of 2:2:1:1 were mixed in CD3CN:CDCl3 (2:3, v/v), the 1H NMR spectrum featured two sets of sharp signals that correlated well to [3]pseudorotaxanes 12·3 and 22·4 were observed, which suggested that host 1 only forms the complex with 3, while host 2 only complexes with 4. Thus, the selfsorting process in the mixture of four components 1-4 did occure, and two kinds of [3]pseudorotaxane were formed.
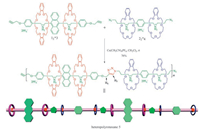
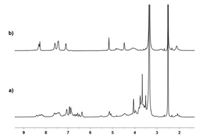
The self-sorting behavior between 1, 2, 3 and 4 provided us an opportunity to investigate the construction of heteropolyrotaxane in a one-pot reaction. Since the formed [3] pseudorotaxane 12·3 and 22·4 have two terminal propargyl and azide groups, respectively, we then tried to synthesize the linear heteropolyrotaxane by the high efficient CuAAC 'click' reaction. As shown in Scheme 1, the mixture of 1, 2, 3 and 4 at a molar ratio of 2:2:1:1 in dry CH2Cl2 was stirred at room temperature for 4 hours under nitrogen atmosphere, and it was stirred for another 24 h after the addition of catalytic amount of Cu(CH3CN)4PF6. During the reaction process, the precipite formed, which was filtered and washed with CH2Cl2, CH3OH, H2O, and Et2O sequentially. After drying in vacuo, heteropolyrotaxane 5 was obtained in 76% yield. A control polymer 6 was also prepared by the CuAAC 'click' reaction of 3 and 4 in the absence of hosts 1 and 2. In the 1H NMR spectrum of 5, the signals for the protons of crown ethers emerged at 4.06-3.52 ppm, and the signals corresponding to the protons of 1, 2, 3-triazole could also be found at 8.41 (H3) and 5.08 (H4) ppm, respectively. These results indicated that the heteropolyrotaxane was successfully obtained by the effective CuAAC 'click' reaction between [3]pseudorotaxane 12·3 and 22·4. However, the 1H NMR spectrum of 6 was significantly different from that of 5, which only exhibits the proton signals of monomers and the triazole moieties, suggesting again the formation of heteropolyrotaxane 5 (Fig. 3). This was also confirmed by the results from FT-IR spectrum of 5, in which the peaks at 1102, 2102 and 3446 cm-1 could be found, corresponding to the triazole and azido and unreacted alkynyl groups, respectively. Furthermore, the number-average molecular weight (Mn) and polydispersity index of polymer 5 were determined by gel permeation chromatography as well using polystyrene (PS) as a standard and dimethylformamide (DMF) as the eluent. It was found that the Mn of the polyrotaxane is 8.4 kDa with the polydispersity index (PDI) value of 1.24 (Fig. S7 in Supporting information).
|
Download:
|
| Scheme 1. Synthesis of heteropolyrotaxane 5. | |
|
Download:
|
| Fig. 3. Partial 1H NMR spectra (400 MHz, DMSO-d6, 295 K) of (a) polymer 5, and (b) polymer 6. | |
3. Conclusion
In conclusion, the self-sorting principle afforded a highly efficient strategy for the synthesis of well-organized heteropolyrotaxane. Four components could selectively selfassemble into two novel [3]pseudorotaxanes driven by host-guest interactions as well as size and steric-effect controlled selfsorting processes. Host 1 was selectively complex with guest 3 and Host 2 was selectively bound with guest 4 to form of [3] pseudorotaxanes 12·3 and 22·4, respectively. By the highly efficient CuAAC reaction, a linear main-chain heteropolyrotaxane 5 could be conveniently synthesized in excellent yield. By employing this strategy, the construction of complex multicomponent interlocked molecules, particularly when encoded with multiple molecular information, has turned out to be feasible and practicable. Further studies on functionalized polyrotaxanes directed by multiple self-sorting processes are under investigation in our laboratory.
4. Experimental1H NMR spectra was recorded on a Bruker DMX400 NMR spectrometer. FT-IR spectrum was determined by Nicolet Is50 FTIR Spectrometer. The Mn and polydispersity index of polymer 5 were determined by gel permeation chromatography (GPC) (Waters Co.) using polystyrene (PS) as standard and dimethylformamide (DMF) as eluent.
4.1. Synthesis of polymer 5A mixture of 1 (89.8 mg, 0.2 mmol), 2 (71.3 mg, 0.2 mmol), 3 (81.7 mg, 0.1 mmol) and 4 (59.5 mg, 0.1 mmol) in dichloromethane (20 mL) was stirred at room temperature for 4 h under nitrogen atmosphere, and then stirred for another 24 h after the addition of Cu(CH3CN)4PF6 (112.3 mg, 0.3 mmol). During the reaction process, the precipitation was formed, which was filtered and washed with CH2Cl2, CH3OH, H2O, and Et2O, respectively, and then dried in vacuo to give pale green powder (229.7 mg, 76%). FT-IR: ν≡C-H = 3446 cm-1, νN3 = 2102 cm-1, νtrizole = 1102 cm-1. 1H NMR (400 MHz, DMSO-d6, 295 K): δ 8.41 (br s, 3H), 8.30-8.18 (br m, 9H), 7.61-7.41 (br m, 19H), 7.06-6.38 (br m, 40H), 5.52 (br s, 3H), 5.15-5.08 (br m, 10H), 4.46 (br s, 13H), 4.06-3.52 (br m, 86H), 3.03(br s, 6H), 2.08-1.99(br m, 7H). GPC data: Mn = 8.4 KDa, PDI = 1.24.
4.2. Synthesis of polymer 6A mixture of 3 (70.0 mg, 0.086 mmol), 4 (51.0 mg, 0.086 mmol) and Cu(CH3CN)4PF6 (96.2 mg, 0.26 mmol) in dichloromethane (20 mL) was stirred at room temperature for 24 h under nitrogen atmosphere. During the reaction process, the precipitation was formed, which was filtered and washed with CH2Cl2, CH3OH, H2O, and Et2O, respectively, and then dried in vacuo to give yellow powder (102.5 mg, 78%). FT-IR: ν≡C-H = 3460 cm-1, νN3 = 2121 cm-1, νtrizole = 1103 cm-1. 1H NMR (400 MHz, DMSO-d6, 295 K): δ 8.31 (br s, 10H), 7.59-7.45 (br m, 21H), 7.11-7.09 (br m, 6H), 5.16 (br s, 6H), 4.83 (br s, 6H), 4.48 (br s, 7H), 4.09 (br s, 10H), 2.82 (br s, 4H), 2.12 (br s, 8H).
AcknowledgmentsWe thank the National Natural Science Foundation of China (No. 21602055) and Sci-Tech Innovation Teams in Universities of Hunan Province for financial support.
Appendix A. Supplementary dataSupplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.cclet.2017.03.009.
| [1] | P.L. Anelli, N. Spencer, J.F. Stoddart. A. molecular shuttle. J. Am. Chem. Soc. 113 (1991) 2679–2681. |
| [2] | T. Han, C.F. Chen. Efficient potassium-ion-templated synthesis and controlled destruction of [2] rotaxanes based on cascade complexes. J. Org. Chem. 73 (2008) 7735–7742. DOI:10.1021/jo801522f |
| [3] | Z. Meng, C.F. Chen. A molecular pulley based on a triply interlocked [2] rotaxane. Chem. Commun. 51 (2015) 8241–8244. DOI:10.1039/C5CC01301A |
| [4] | Z. Meng, J.F. Xiang, C.F. Chen. Tristable [n] rotaxanes: from molecular shuttle to molecular cable car. Chem. Sci. 5 (2014) 1520–1525. DOI:10.1039/c3sc53295j |
| [5] | J. Yang, J.F. Ma, S.R. Batten. Polyrotaxane metal-organic frameworks (PMOFs). Chem. Commun. 48 (2012) 7899–7912. DOI:10.1039/c2cc33060a |
| [6] | A. Harada, A. Hashidzume, H. Yamaguchi, et al., Polymeric rotaxanes. Chem. Rev. 109 (2009) 5974–6023. DOI:10.1021/cr9000622 |
| [7] | S.A. Nepogodiev, J.F. Stoddart. Cyclodextrin-based catenanes and rotaxanes. Chem. Rev. 98 (1998) 1959–1976. DOI:10.1021/cr970049w |
| [8] | M. Xue, Y. Yang, X. Chi, et al., Development of pseudorotaxanes and rotaxanes: from synthesis to stimuli-responsive motions to applications. Chem. Rev. 115 (2015) 7398–7501. DOI:10.1021/cr5005869 |
| [9] | The Nobel Prize in Chemistry 2016-Advanced Information. Nobelprize. org. Nobel Media AB 2014. Web. October 6, 2016, http://www.nobelprize.org/nobel_prizes/chemistry/laureates/2016/advanced.html |
| [10] | A. Harada, J. Li, M. Kamachi. The molecular necklace: a rotaxane containing many threaded α-cyclodextrins. Nature 356 (1992) 325–327. DOI:10.1038/356325a0 |
| [11] | A. Harada. Cyclodextrin-based molecular machines. Acc. Chem. Res. 34 (2001) 456–464. DOI:10.1021/ar000174l |
| [12] | E.N. Guidry, J. Li, J.F. Stoddart, et al., Bifunctional [c2]daisy-chains and their incorporation into mechanically interlocked polymers. J. Am. Chem. Soc. 129 (2007) 8944–8945. DOI:10.1021/ja0725100 |
| [13] | Y. Jiang, J.B. Guo, C.F. Chen. Bifunctionalized [3] rotaxane and its incorporation into mechanically interlocked polymer. Chem. Commun. 46 (2010) 5536–5538. DOI:10.1039/c0cc00999g |
| [14] | S.H. Lee, P.T. Engen, H.W. Gibson. Blocking group/initiators for the synthesis of polyrotaxanes via free radical polymerizations. Macromolecules 30 (1997) 337–343. DOI:10.1021/ma960653t |
| [15] | C. Gong, Q. Ji, C. Subramaniam, et al., Main chain polyrotaxanes by threading crown ethers onto a preformed polyurethane: preparation and properties. Macromolecules 31 (1998) 1814–1818. DOI:10.1021/ma9713116 |
| [16] | T. Oku, Y. Furusho, T. Takata. A concept for recyclable cross-linked polymers: topologically networked polyrotaxane capable of under going reversible assembly and disassembly. Angew. Chem. Int. Ed. 116 (2004) 984–987. DOI:10.1002/(ISSN)1521-3757 |
| [17] | D. Whang, Y.M. Jeon, J. Heo, et al., Self-assembly of a polyrotaxane containing a cyclic bead in every structural unit in the solid state: cucurbituril molecules threaded on a one-dimensional coordination polymer. J. Am. Chem. Soc. 118 (1996) 11333–11334. DOI:10.1021/ja961551l |
| [18] | K.M. Park, D. Whang, E. Lee, J. Heo, et al., Transition metal ion directed supramolecular assembly of one-and two-dimensional polyrotaxanes incorporating cucurbituril. Chem. Eur. J. 8 (2002) 498–508. DOI:10.1002/(ISSN)1521-3765 |
| [19] | L. Mei, Q.Y. Wu, C.M. Liu, et al., The first case of an actinide polyrotaxane incorporating cucurbituril: a unique 'dragon-like' twist induced by a specific coordination pattern of uranium. Chem. Commun. 50 (2014) 3612–3615. DOI:10.1039/C4CC00690A |
| [20] | W. Zhang, W.R. Dichtel, A.Z. Stieg, et al., Folding of a donor-acceptor polyrotaxane by using noncovalent bonding interactions. Proc. Natl. Acad. Sci. U. S. A. 105 (2008) 6514–6519. DOI:10.1073/pnas.0711072105 |
| [21] | J. Wu, K.C.F. Leung, J.F. Stoddart. Efficient production of [n]rotaxanes by using template-directed clipping reactions. Proc. Natl. Acad. Sci. U. S. A. 104 (2007) 1726617271. |
| [22] | F. Zeng, Z. Meng, Y. Han, C.F. Chen. Formation of a pseudosuitane-type complex between a triptycene-derived bis(crown ether) host and 10-(anthracene-9, 10-diyl)bis(N-benzylmethanaminium): a new method for the synthesis of linear polyrotaxanes. Chem. Commun. 50 (2014) 7611–7613. DOI:10.1039/C4CC02904F |
| [23] | J.E.M. Lewis, J. Winn, L. Cera, et al., Iterative synthesis of oligo[n]rotaxanes in excellent yield. J. Am. Chem. Soc. 138 (2016) 16329–16336. DOI:10.1021/jacs.6b08958 |
| [24] | M.M. Safont-Sempere, G. Fernández, F. Würthner. Self-sorting phenomena in complex supramolecular systems. Chem. Rev. 111 (2011) 5784–5814. DOI:10.1021/cr100357h |
| [25] | P.N. Taylor, H.L. Anderson. Cooperative self-assembly of double-strand conjugated porphyrin ladders. J. Am. Chem. Soc. 121 (1999) 11538–11545. DOI:10.1021/ja992821d |
| [26] | B.H. Northrop, Y.R. Zheng, K.W. Chi, et al., Self-organization in coordination-driven self-assembly. Acc. Chem. Res. 42 (2009) 1554–1563. DOI:10.1021/ar900077c |
| [27] | F. Wang, C. Han, C. He, et al., Self-sorting organization of two heteroditopic monomers to supramolecular alternating copolymers. J. Am. Chem. Soc. 130 (2008) 11254–11255. DOI:10.1021/ja8035465 |
| [28] | W. Wang, Y. Zhang, B. Sun, et al., The construction of complex multicomponent supramolecular systems via the combination of orthogonal self-assembly and the self-sorting approach. Chem. Sci. 5 (2014) 4554–4560. DOI:10.1039/C4SC01550A |
| [29] | D. Račkauskaitè, R. Gegevičcius, Y. Matsuo, et al., An enantiopure hydrogen-9 bonded octameric tube: self-sorting and guest-induced rearrangement. Angew. Chem. Int. Ed. 55 (2016) 208–212. DOI:10.1002/anie.201508362 |
| [30] | H. Gan, B.C. Gibb. Guest-controlled self-sorting in assemblies driven by the hydrophobic effect. Chem. Commun. 48 (2012) 1656–1658. DOI:10.1039/C2CC16603H |
| [31] | F. Zeng, Y. Han, C.F. Chen. Self-sorting behavior of a four-component host-guest system and its incorporation into a linear supramolecular alternating copolymer. Chem. Commun. 51 (2015) 3593–3595. DOI:10.1039/C5CC00035A |
| [32] | L. Isaacs. Stimuli responsive systems constructed using cucurbit[n]uril-type molecular containers. Acc. Chem. Res. 47 (2014) 2052–2062. DOI:10.1021/ar500075g |
| [33] | N. Song, D.X. Chen, M.C. Xia, et al., Supramolecular assembly-induced yellow emission of 9, 10-distyrylanthracene bridged bis(pillar [5] arene)s. Chem. Commun. 51 (2015) 5526–5529. DOI:10.1039/C4CC08205B |
| [34] | B. Zheng, F. Klautzsch, M. Xue, et al., Self-sorting of crown ether/secondary ammonium ion hetero-[c2]daisy chain pseudorotaxanes. Org. Chem. Front. 1 (2014) 532–540. DOI:10.1039/C4QO00064A |
| [35] | P. Wei, X. Yan, F. Huang. Supramolecular polymers constructed by orthogonal self-assembly based on host-guest and metal-ligand interactions. Chem. Soc. Rev. 44 (2015) 815–832. DOI:10.1039/C4CS00327F |
| [36] | L. Li, H.Y. Zhang, J. Zhao, et al., Self-sorting of four organic molecules into a heterowheel polypseudorotaxane. Chem. Eur. J. 19 (2013) 6498–6506. DOI:10.1002/chem.201204583 |
| [37] | Z. Huang, L. Yang, Y. Liu, et al., supramolecular polymerization promoted and controlled through self-sorting. Angew. Chem. Int. Ed. 53 (2014) 5351–5355. DOI:10.1002/anie.v53.21 |
| [38] | F. Wang, C. Han, C. He, et al., Self-sorting organization of two heteroditopic monomers to supramolecular alternating copolymers. J. Am. Chem. Soc. 130 (2008) 11254–11255. DOI:10.1021/ja8035465 |
| [39] | F. Wang, B. Zheng, K. Zhu, et al., Formation of linear main-chain polypseudorotaxanes with supramolecular polymer backbones via two selfsorting host-guest recognition motifs. Chem. Commun. 45 (2009) 4375–4377. |
| [40] | S. Dong, X. Yan, B. Zheng, et al., A supramolecular polymer blend containing two different supramolecular polymers through self-sorting organization of two heteroditopic monomers. Chem. Eur. J. 18 (2012) 4195–4199. DOI:10.1002/chem.v18.14 |
| [41] | S. Dong, B. Zheng, M. Zhang, et al., Preparation of a diblock supramolecular copolymer via self-sorting organization. Macromolecules 45 (2012) 9070–9075. DOI:10.1021/ma301642y |
| [42] | W. Jiang, H.D.F. Winkler, C.A. Schalley. Integrative self-sorting: construction of a cascade-stoppered hetero [3] rotaxane. J. Am. Chem. Soc. 130 (2008) 13852–13853. DOI:10.1021/ja806009d |
| [43] | X. Fu, Q. Zhang, S.J. Rao, et al., One-pot synthesis of a [c2]daisy-chain-containing hetero [4] rotaxane via a self-sorting strategy. Chem. Sci. 7 (2016) 1696–1701. DOI:10.1039/C5SC04844C |
| [44] | Z.J. Zhang, H.Y. Zhang, H. Wang, et al., A twin-axial hetero [7] rotaxane. Angew. Chem. Int. Ed. 50 (2011) 10834–10838. DOI:10.1002/anie.v50.46 |
| [45] | T. Chang, A.M. Heiss, S.J. Cantrill, et al., Toward interlocked molecules beyond catenanes and rotaxanes. Org. Lett. 2 (2000) 2943–2946. DOI:10.1021/ol006187g |
| [46] | C. Zhang, S. Li, J. Zhang, et al., Benzo-21-crown-7/secondary dialkylammonium salt [2] pseudorotaxane-and [2] rotaxane-type threaded structures. Org. Lett. 9 (2007) 5553–5556. DOI:10.1021/ol702510c |
 2017, Vol. 28
2017, Vol. 28 


