The chirality transfer from biomolecules to synthetic materials, including small molecules, polymer chains, nanostructures, and inorganic materials, has attracted much attention. Many biomolecules and their assemblies, such as amino acids [1-3], peptides [4-6], and DNAs [7, 8], have been used as templates to prepare chiral nanoarchitectures with endowed function in solutions. Depending on the chirality of the biomolecules, chiral nanoarchitectures such as chiral twists and helices with controlled handedness have been reported, and they have played an important role in the development of new materials for chiral sensing [9-11], chiral catalysis [12-14], chiral electronics [15-17], and photonics [18-21]. However, until now, the design and fabrication of chiral bulk materials with device-friendly properties are still challenging.
Block copolymers (BCPs), generally containing dissimilar chain segments of the polymers through covalent bonding, have been studied extensively owing to their easy formation of diverse morphologies such as spherical, cylindrical, lamellar, and gyroid morphologies. The construction of helical nanoarchitectures based on BCP assembly is an effective method for carrying out the three-dimensional package of nanohelices in bulk systems [22-31]. Jinnai research group investigated the formation of helical structures using an achiral BCP, poly(styrene)-b-polybutadiene-b-poly(methyl methacrylate) [22-25]. However, the handedness of the formed helical structure could not be controlled. Ho research group first reported helical nanostructures using a chiral BCP, poly (styrene)-b-poly(lactide) with clear handedness in both solution and bulk film [26-30]. The chiral entities D- and L-lactide were important driving factors in controlling the handedness of the helices. According to the vibration circular dichroism (VCD) of the block copolymer, the chirality was not transferred to the PS segments because of the absence of chirality even though a helical structure was formed. Previously, we reported nanohelix formation in an achiral BCP bulk using the additive-driven self-assembly strategy [31]. A helical structure with expected handedness was formed depending on the chirality of small additive molecules. However, although the helical-phase structures were clearly observed, the chirality transfer on a molecular scale was not still understood, especially to the segments which do not interact with the chiral additives. On the other hand, it was also important for the functionalization of this type of hybrid material to understand the relationship between the chiral transfer and formation of helical structure.
In this study, we investigated the chirality transfer from a chiral additive to a BCP using VCD, small-angle X-ray scattering (SAXS), and transmission electron microscopy (TEM). D- and L-Tartaric acid (D- and L-TA) were selected as the additives for introduction of chirality into di-BCP polystyrene-b-poly(ethylene oxide) (PS-b-PEO). To verify the effect of the supracomplex formation of PS-b-PEO and TA molecules on the chirality transfer, we investigated the three stages of the BCP/TA complex formation: (ⅰ) in the solution, (ⅱ) in the film without the thermal annealing treatment, and (ⅲ) in the film after the thermal annealing treatment. Depending on the loading and chirality of TA molecules, a lamellar tetragonal structure with clear handedness was obtained. The CD and VCD results show that the chirality was transferred not only to the PEO segments which directly interacted with TA through hydrogen bonding, but also to the polystyrene segments. The results obtained in this study may be helpful to design and functionalize helical BCPs and their hybrid materials.
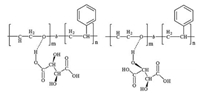
2. Results and discussion 2.1. Microstructure of chiral TA molecule-doped PS-b-PEO filmTA is a chiral acid with two stereocenters in its backbone that contains two carboxylic acid groups and two hydroxyl groups, as shown in Fig. 1. Upon blending TA into PS-b-PEO, both the carboxylic acid and hydroxyl groups can interact with the PEO segments and formed two types of hydrogen bonds depending on the bonding site: one is between the carboxyl group and PEO segments, and the other is between the hydroxyl group connected to the chiral centers and PEO segments. The incorporation of TA into PS-b-PEO will promote the microphase separation and cause a change in the microstructure of PS-b-PEO [32-34]. The effect of a chiral additive on the BCP microstructure was first investigated using SAXS. Two scattering peaks were observed at q of 0.21 and 0.34 nm-1 for the neat PS-b-PEO, close to the ratio of 1:√3, indicating that a hexagonal phase structure existed in the BCP (Fig. 2). The d-spacing of the hexagonal structure was about 29 nm. The introduction of TA molecules into the PS-b-PEO increased the phase separation as verified by the appearance of high-order scattering peaks in the SAXS profiles. Depending on the amount of chiral TA molecules, the TA/BCP hybrid films showed a group of clear scattering peaks at q = 0.20, 0.29, 0.41, and 0.60 nm-1 for 12 wt% TA loading (relative to BCP) and q = 0.18, 0.26, 0.34, and 0.54 nm-1 for 22 wt% TA loading. The relative ratio of q was ~1:√2:2:3. When the TA loading was up to 32 wt%, two peaks appeared at q = 0.17 and 0.35 nm-1. The relative ratio of q was 1:2, indicating the formation of a lamellar structure in the hybrid film. With increasing TA loading, the d spacing increased from 29 to 31.4, 35.7, and 37.4 nm for the TA content of 12 wt%, 22 wt%, and 32 wt%, respectively.
|
Download:
|
| Fig. 1. Two modes of hydrogen bonding between PS-b-PEO and TA molecules. | |

|
Download:
|
| Fig. 2. SAXS integrations of PS-b-PEO/TA with different loadings after the annealing. (a) D-TA; (b) L-TA. The samples were casting films obtained from their solutions in THF. | |
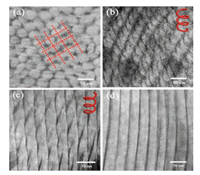
TEM was further used to confirm the microstructure of the hybrid. According to the composition of PS-b-PEO, the minor-phase PEO segments were dispersed in the major-phase PS in the cylindrical form for a neat block copolymer (Fig. S1 in Supporting information). No helical structure was observed for the solution samples and films without thermal annealing, irrespective of the loading of TA. For the film after the thermal annealing, the samples with 22 wt% and 32 wt% TA loading were used. For 22 wt% D-TA loading, the hybrid clearly showed a tetragonal phase structure as shown in Fig. 3a. Helical structures with clear left handedness were observed as shown in Fig. 3b. For the film with a TA loading of 32 wt%, the hybrid BCP/TA showed a lamellar structure, and no helical microstructure was observed (Fig. 3c). These results are consistent with the SAXS profiles.
|
Download:
|
| Fig. 3. TEM image of BCP/TA hybrid film with different TA loadings. (a) Crosssection of BCP film with 22 wt% D-TA loading; (b) helical structure of BCP/TA film with 22 wt% D-TA loading; (c) helical structure of BCP/TA film 22 wt% L-TA loading; (d) BCP film with 32 wt% D-TA loading. | |
2.2. Chirality transfer from chiral additives to the BCP
For the helical BCPs polymerized from chiral units those can form a helical structure in both the solution and bulk film, the chiral nature of the monomer units plays a key role in the formation of helical structure [26-30]. For the chiral complex obtained by the additive-driven self-assembly approach, the helical structure was only formed in the bulk film as verified above. To verify the relationship between the small chiral additives and final microstructure, both the CD and VCD spectra were used to characterize the chiral transfer during the formation of chiral complexes. Here, CD was first used to characterize the chirality of the chiral complex. The samples in solution and bulk film were used to verify the chirality transfer. Fig. 4 shows the CD spectra of PS-b-PEO/TA in THF solution and bulk film. For neat TA molecules, one mirror peak appeared at 228 nm, corresponding to the UV adsorption of the carboxyl group of TA. After blending with BCP, the CD signal clearly shows a blue shift to 223 nm in solution. In the complex film, the CD signals showed a red shift to 233 nm. These changes in CD signals can be attributed to the formation and cleavage of hydrogen bonds accompanied with the formation of complex: dissolution in THF cleaved the intermolecular hydrogen bonds among the TA molecules, and no hydrogen bond was formed between TA and BCP in the solution. Therefore, the CD signals showed a slightly blue shift. In the bulk film, the TA molecules were homogenously dispersed in the PEO phase, and new hydrogen bonds were formed between the TA and PEO segments, causing the red-shift of CD signals. The difference in the CD signal of PS-PEO/TA hybrid in the solution and bulk film also proved the formation of PS-PEO/TA supracomplex in the film state. However, because no clear CD signal can be attributed to the PS-b-PEO appeared, it is difficult to confirm the chirality transfer from the TA molecules to the polymer main chain.

|
Download:
|
| Fig. 4. CD spectra of BCP/TA in (a) THF solution and (b) film. | |
The chirality transfer was further confirmed from the VCD results of the PS-b-PEO/TA system. Compared to the CD spectra, VCD can be used to verify the handedness of the helical conformation of polymers with main-chain chirality. Thus, the effect of chiral TA molecules on the PS-b-PEO main chains can be determined. Fig. 5 shows the VCD spectra of PS-b-PEO/TA in a diluted THF solution. For enantiopure TA (Fig. 5a), the VCD spectra of all the samples in solution showed two groups of characteristic split-peaks at (1731 and 1738 cm-1) and (1750 and 1757 cm-1), corresponding to the absorption of C=O group of TA at 1734 and 1754 cm-1. For l-TA molecule, the two groups of VCD peaks showed (negative, positive) and (negative, positive) signals; For d-TA molecules, the two groups of VCD peaks showed mirror signals, indicating converse cotton effect. For the PS-b-PEO/TA in THF solution (Fig. 5b and c), the VCD spectra showed no characteristic peaks, even when the TA loading up to 42 wt%. The peak at 1458 cm-1 is related to solvent THF. The concentration of PS-b-PEO/TA further increased to the saturation state, and only weak signals appeared at these positions. These results indicate that there is no hydrogen bonding formed between TA and PS-b-PEO chains in the solution.

|
Download:
|
| Fig. 5. VCD spectra of (a) TA molecules, (b) BCP/D-TA and (c) BCP/L-TA in THF. | |
For the bulk film, two types of films were used to characterize the chirality transfer of BCP/TA blends: one is film without thermal annealing treatment and the other is subjected to thermal annealing treatment at 60 ℃ for 72h before the VCD measurement. Thermal annealing at elevated temperatures can enhance the mobility of BCP molecules and produce an ordered nanostructure. For the first film, a series of signals appeared in the range 1700-1800 cm-1, corresponding to the C=O group in TA molecules (Fig. S2 in Supporting information). However, these signals showed no regularity, and no signal attributed to PS-b-PEO appeared. After the thermal annealing, the VCD signals showed regular chiral signals depending on the TA loading (Fig. 6). For low TA loading, i.e., 12wt%, no chiral signal appeared in the VCD spectrum. When the TA loading was 22wt%, regular mirror signals corresponding to the C=O group appeared at 1742 cm-1, and the intensity was much stronger than that in the corresponding solution. When the TA loadings were 32wt% and 42wt%, these chiral signals further increased with the increase in TA loading. This amplification of chiral signals can be attributed to the formation of PS-b-PEO/TA supramolecules [35, 36, 2]. One notable phenomenon is the appearance of a newchiral signal at 1600cm-1, corresponding to the vibration absorption of aromatic ring (Fig. 6). The chirality of PS-b-PEO shows great dependence on the TA loading. Although a helical structure was formed, the VCD spectra of 22wt% BCP samples did notshowa chiralsignal. However, the base line significantly deviated in the range from 1500 to 1680 cm-1. For the 32wt% and 42wt% loading films, a new mirror signal appeared at 1600 cm-1 as shown in Fig. 6c and d. The benzene ring showed a telescopic vibration peak at 1600 cm-1 (Figs. S3 and S4 in Supporting information). These results indicate that the PS segments also had a chiral confirmation, even no direct interaction with TA molecules.

|
Download:
|
| Fig. 6. VCD spectra of BCP/TA film with different TA loadings after the thermal annealing treatment. | |
2.3. Discussion
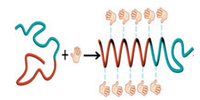
For a chiral BCP synthesized from chiral units such as PS-b-PLA, the formation of chiral helices was attributed to the shifting and twisting of chiral units at the microphase-separated interface. The natural chirality of LA enantiomers determines the chirality of microphase nanostructure. The chiral PS-b-PLLA can form a helical structure both in solution and bulk film [27]. In this study, no helical structure was formed in the neat PS-b-PEO and PS-b-PEO/TA solution. The VCD results also proved that no chiral signals can be attributed to PS-b-PEO. In the film state, the strong interaction between TA and PEO segments by hydrogen bonding leads to the formation of BCP/TA complex, as confirmed by the amplification of the chiral signals of TA molecules (Fig. 6). However, for the film without thermal annealing, only the chiral signals of TA molecules appeared irrespective of the TA loading, indicating that the chirality was still not transferred to the BCP main chains. Thermal annealing can enhance the mobility of BCP molecules as well as promote the chirality transfer from the PEO-TA segments to the PS segments as indicated by the appearance of the chiral signals of aromatic group, i.e., the achiral PS units also showed chiral conformation in the film. However, the chirality of PS-b-PEO strongly depended on the TA loading in the hybrid. In the film with 12wt% TA loading, the BCP formed a tetragonal phase structure with the minor-phase PEO segments dispersed in the major-phase PS domain with a cylindrical shape. The molar ratio between the EO units and OH group was ~1.8, which means that only half the EO units formed hydrogen bond with the TA molecules. At this level, the lowcontent of the complex could not assure the polymer chain in chiral conformation. For the 22wt% TA film, the molar ratio between the EO units and OH group was close to 1, and almost all the EO units formed hydrogen bonds with the TA molecules. After the thermal annealing treatment, the PEO/TA complex could be arranged in a twist model and then formed the helical phase structure as confirmed by the SAXS and TEM analyses. However, the chirality in the PS segments was still not clear, as shown in Fig. 6b. A further increase in the TA loading changed the microstructure from cylindrical to lamellar. In this case, all the EO units formed hydrogen bonds with the TA molecules. Although no helical structure was observed, the BCP chains still showed a strong chirality, particularly the PS segments (Fig. 6c and d). These results indicate that the chirality of PS units cannot be only attributed to the formation of a helical phase structure in the film. During the chirality transfer, the hydrogen bonding interactions between the EO and TA molecules first assembled the PEO segments in a chiral conformation, as shown in Fig. 7. After the thermal annealing treatment, the chirality was transferred to the PS segments via the cooperative motion, producing an ordered nanostructure depending on the BCP components of BCP/TA complex.
|
Download:
|
| Fig. 7. Illustration of the chirality transfer from TA enantiomer to BCP. | |
On the other hand, although no chiral signal of PS segments was observed when the BCP/TA complex formed a helical structure, we noticed that the baseline of the VCD spectrum showed a large deviation with a mirror image for the film with 22wt% TA loading. This deviation also occurred for the 32wt% and 42wt% films. This deviation probably arises from the weak chiral arrangement of PS segments at the interface between PEO and PS domains. Previously Ho reported that an achiral perylene group connected with chiral lacitde segments can also show chiral conformation due to the microphase separation and intermolecular interaction [28]. Therefore, the perylene group shows chiral conformation even in the solution state. For the PS-b-PEO/TA hybrid, a pure microphase separation could not cause the chiral conformation of PS segments. Considering the effect of thermal annealing on the polymer chain mobility, the chirality of PS was attributed to the cooperative motion of two segments during the thermal annealing. PEO/TA segments with a preferred chiral conformation are dispersed in the PS matrix, and the PS domains will follow the chirality of PEO domains at the interface of two domains through cooperative motion, thus causing the chiral arrangement of PS chains. Based on this assumption, the segments with a high mobility would show a strong chirality after the thermal annealing. To verify this inference, a block copolymer, PIP-b-PEO, was further used to investigate the chiral transfer to the PIP segments that do not interact with TA molecules. Compared to the PS segments, the PIP segments have a lower glass transmission temperature and higher mobility. As shown in Fig. 8, a split-type cotton effect with a positive VCD band at 1696 cm-1 and a negative VCD band at 1558 cm-1wereobservedforPIP-b-PEO/D-TA film, and a mirror image of the spectrum was observed for PIP-b-PEO/L-TA. The inflection point at 1648cm-1 corresponds to the characterization absorptionof the C=C stretching motionin Pip segments (Figs. S5 and S6 in Supporting information). These results prove that the PIP segments also exhibit chiral conformation in this chiral complex, and the intensity of the chiral signal increases with the increase in TA loading.

|
Download:
|
| Fig. 8. VCD spectra of PIP-b-PEO/TA film with different TA loading. | |
3. Conclusion
In this study, we investigated the chirality transfer from small chiral additives to BCP main chains using the additive-driven selfassembly strategy. Based on the VCD results of the three stages of the BCP/TA complex formation, it can be concluded that the chirality was transferred to not only the EO segments which directly interacted with the TA molecules through hydrogen bonding, but also the PS segments which did not interact with the TA molecules. The chiral transfer was independent on the formation of a helical structure in the hybrid film. The chirality transfer was different for both the segments: For the PEO segments, the chirality transfer was carried out directly through hydrogen bonding; however, for the segments with no directive interaction with chiral additives, the chirality transfer was carried out through the cooperative motion of the two segments. The results presented here may provide guidance for the design of chiral materials with a controlled nanostructure, especially for the chiral porous materials if we can selectively remove the chiral additives.
4. Experimental 4.1. MaterialsBCP, polystyrene-block-poly(ethylene oxide) (PS-b-PEO, PS, 19K, and PEO, 6.5K, PDI=1.08), poly(1, 4-isoprene)-b-poly(ethylene oxide) (PIP-b-PEO, PIP, 18.5K, and PEO, 5.5K, PDI=1.05) were purchased from Polymer Source Co. (Canada). Ruthenium tetroxide, 0.5% stabilized aqueous solution, was purchased from Tansoole Co. D- and L-TA were purchased from J & K Chemical Co. (China).
4.2. Polymer characterization 4.2.1. SAXS analysisThe SAXS measurements were performed using the SAXS instrumentation at the Shanghai Synchrotron Radiation Facility (SSRF). PS-b-PEO was blended with TA at a given mass ratio in tetrahydrofuran (THF) and then drop-cast on glass slides dried at 60 ℃. Then, the samples were subjected to thermal annealing depending on the experiment. After scraping from the glass slides, the dried bulk samples were placed in the center of metal washers, sandwiched using a Kapton film, and placed on a vertical holder.
4.2.2. TEM analysisA JOEL 2000FXelectron microscopeoperating atan accelerating voltage of 200kV was used for the TEM measurements. A Leica Ultracut microtome was used to directly cut the bulk sample using a diamond knife at low temperature (-120 ℃). Thin pieces of the samples were then detached from the diamond knife and transferred to copper grids coated with carbon films. The copper grids with the sample pieces were then stained with ruthenium tetroxide (0.5% stabilized aqueous solution) for ~15 min before the TEM measurements.
4.2.3. CD and VCD analysisThe CD spectra were acquired using a CD spectrometer (JASCOJ-815, Japan). The BCP blended with D- or L-TA was prepared in THF with a concentration of ~10 mg/mL. The path length of the quartz cuvettes was 1 mm. The bulk films of PS-PEO blended with D- or L-TA were prepared by drop-casting the THF solution on a quartz slide, followed by drying at 40 ℃ and thermal annealing at 60 ℃ for 72 h.
The VCD spectra were acquired using a VCD spectrometer (JASCO J-815, Japan). The solutions of the samples were prepared using the same method as for the CD measurement. For the bulk films, the THF solution of BCP blended with D- or L-TA was dropcasted on a BaF2 disk and dried at 40 ℃, followed by thermal annealing at 60 ℃ for 72 h. All the samples were measured directly using the VCD instrument in the rotating mode.
AcknowledgmentsThis work was supported by National Natural Science foundation of China (Nos. 21374060 and 21574081). The author thanks Dr Ruibin Wang for the help of vibrational circular dichroism measurement.
Appendix A. Supplementary dataSupplementary data associatedwith this article can be found, in the online version, at http://dx.doi.org/10.1016/j.cclet.2017.04.009.
| [1] | A. Cecconello, J.S. Kahn, C.H. Lu, et al., DNA scaffolds for the dictated assembly of left-/right-handed plasmonic Au NP helices with programmed chiro-optical properties. J. Am. Chem. Soc. 138 (2016) 9895–9901. DOI:10.1021/jacs.6b04096 |
| [2] | L. Zhang, T. Wang, Z. Shen, et al., Chiral nanoarchitectonics: towards the design, self-assembly, and function of nanoscale chiral twists and helices. Adv. Mater. 28 (2016) 1044–1059. DOI:10.1002/adma.201502590 |
| [3] | M. Liu, L. Zhang, T. Wang. Supramolecular chirality in self-assembled systems. Chem. Rev. 115 (2015) 7304–7397. DOI:10.1021/cr500671p |
| [4] | J.H. Jung, S.J. Moon, J. Ahn, et al., Controlled supramolecular assembly of helical silica nanotube-graphene hybrids for chiral transcription and separation. ACS Nano 7 (2013) 2595–2601. DOI:10.1021/nn306006s |
| [5] | C.J. Bueno-Alejo, L.A. Villaescusa, A.E. Garcia-Bennett. Supramolecular transcription of guanosine monophosphate into mesostructured silica. Angew. Chem. Int. Ed. 53 (2014) 12106–12110. DOI:10.1002/anie.201407005 |
| [6] | T.G. Barclay, K. Constantopoulos, J. Matisons. Nanotubes self-assembled from amphiphilic molecules via helical intermediates. Chem. Rev. 114 (2014) 10217–10291. DOI:10.1021/cr400085m |
| [7] | X.M. Fu, Z.J. Liu, S.X. Cai, et al., Electrochemical aptasensor for the detection of vascular endothelial growth factor (VEGF) based on DNA-templated Ag/Pt bimetallic nanoclusters. Chin. Chem. Lett. 27 (2016) 920–926. DOI:10.1016/j.cclet.2016.04.014 |
| [8] | J. Oelerich, G. Roelfes. DNA-based asymmetric organometallic catalysis in water. Chem. Sci. 4 (2013) 2013–2017. DOI:10.1039/c3sc00100h |
| [9] | E. Anger, H. Iida, T. Yamaguchi, et al., Synthesis and chiral recognition ability of helical polyacetylenes bearing helicene pendants. Polym. Chem. 5 (2014) 4909–4914. DOI:10.1039/C4PY00692E |
| [10] | T. Miyabe, H. Iida, M. Banno, et al., Synthesis and visualization of a core crosslinked star polymer carrying optically active rigid-rod helical polyisocyanide arms and its chiral recognition ability. Macromolecules 44 (2014) 8687–8692. |
| [11] | W. Zou, Y. Yan, J. Fang, et al., Biomimetic superhelical conducting microfibers with homochirality for enantioselective sensing. J. Am. Chem. Soc. 136 (2014) 578–581. DOI:10.1021/ja409796b |
| [12] | Y. Akai, L. Konnert, T. Yamamoto, et al., Asymmetric Suzuki-Miyaura crosscoupling of 1-bromo-2-naphthoates using the helically chiral polymer ligand PQXphos. Chem. Commun. 51 (2015) 7211–7214. DOI:10.1039/C5CC01074H |
| [13] | H. Iida, S. Iwahana, T. Mizoguchi, et al., Main-chain optically active riboflavin polymer for asymmetric catalysis and its vapochromic behavior. J. Am. Chem. Soc. 134 (2012) 15103–15113. DOI:10.1021/ja306159t |
| [14] | B.T. Jahromi, A.N. Kharat, S. Zamanian. Chiral electron deficient ruthenium helical coordination polymer as a catalyst for the epoxidation of substituted styrenes. Chin. Chem. Lett. 26 (2015) 137–140. DOI:10.1016/j.cclet.2014.10.013 |
| [15] | X. Feng, V. Marcon, W. Pisula, et al., Towards high charge-carrier mobilities by rational design of the shape and periphery of discotics. Nat. Mater. 8 (2009) 421–426. DOI:10.1038/nmat2427 |
| [16] | C. Réthoré, N. Avarvari, E. Canadell, et al., Chiral molecular metals: syntheses, structures, and properties of the AsF6-salts of racemic (±)-, (R)-, and (S)-tetrathiafulvalene-oxazoline derivatives. J. Am. Chem. Soc. 127 (2005) 5748–5749. DOI:10.1021/ja0503884 |
| [17] | Y. Yan, R. Wang, X. Qiu, et al., Hexagonal superlattice of chiral conducting polymers self-assembled by mimicking β-sheetproteins with anisotropic electrical transport. J. Am. Chem. Soc. 132 (2010) 12006–12012. DOI:10.1021/ja1036447 |
| [18] | H. Hayasaka, T. Miyashita, K. Tamura, et al., Helically π-stacked conjugated polymers bearing photoresponsive and chiral moieties in side chains: reversible photoisomerization-enforced switching between emission and quenching of circularly polarized fluorescence. Adv. Funct. Mater. 20 (2010) 1243–1250. DOI:10.1002/adfm.v20:8 |
| [19] | Y. Nagata, K. Takagi, M. Suginome. Solid polymer films exhibiting handedness-switchable, full-color-tunable selective reflection of circularly polarized light. J. Am. Chem. Soc. 136 (2014) 9858–9861. DOI:10.1021/ja504808r |
| [20] | H. Wu, Y. Zhou, L. Yin, et al., Helical self-assembly induced singlet-triplet emissive switching in a mechanically-sensitive system. J. Am. Chem. Soc. 139 (2017) 785–791. DOI:10.1021/jacs.6b10550 |
| [21] | L. Yin, H. Wu, M. Zhu, Q. Zou, Q. Yan, L. Zhu. Sequential block copolymer selfassemblies controlled by metal-ligand stoichiometry. Langmuir 32 (2016) 6429–6436. DOI:10.1021/acs.langmuir.6b01787 |
| [22] | T. Higuchi, H. Sugimori, X. Jiang, et al., Morphological control of helical structures of an ABC-type triblock terpolymer by distribution control of a blending homopolymer in a block copolymer microdomain. Macromolecules 46 (2013) 6991–6997. DOI:10.1021/ma401193u |
| [23] | S. Hong, T. Higuchi, H. Sugimori, et al., Highly oriented and ordered double-helical morphology in ABC triblock terpolymer films up to micrometer thickness by solvent evaporation. Polymer 44 (2012) 567–572. DOI:10.1038/pj.2012.69 |
| [24] | R. Ishige, T. Higuchi, X. Jiang, et al., Structural analysis of microphase separated interface in an ABC-type triblock terpolymer by combining methods of synchrotron-radiation grazing incidence small-angle X-ray scattering and electron microtomography. Macromolecules 48 (2015) 2697–2705. DOI:10.1021/ma502596a |
| [25] | H. Jinnai, T. Kaneko, K. Matsunaga, et al., A double helical structure formed from an amorphous, achiral ABC triblock terpolymer. Soft Matter 5 (2009) 2042–2046. DOI:10.1039/b901008d |
| [26] | C.K. Chen, H.Y. Hsueh, Y.W. Chiang, et al., Single helix to double gyroid in chiral block copolymers. Macromolecules 43 (2010) 8637–8644. DOI:10.1021/ma1009885 |
| [27] | R.M. Ho, Y.W. Chiang, C.K. Chen, et al., Block copolymers with a twist. J. Am. Chem. Soc. 131 (2009) 18533–18542. DOI:10.1021/ja9083804 |
| [28] | R.M. Ho, M.C. Li, S.C. Lin, et al., Transfer of chirality from molecule to phase in self-assembled chiral block copolymers. J. Am. Chem. Soc. 134 (2012) 10974–10986. DOI:10.1021/ja303513f |
| [29] | W.H. Tseng, C.K. Chen, Y.W. Chiang, et al., Helical nanocomposites from chiral block copolymer templates. J. Am. Chem. Soc. 131 (2009) 1356–1357. DOI:10.1021/ja808092v |
| [30] | H.F. Wang, L.H. Yu, X.B. Wang, et al., A facile method to fabricate double gyroid as a polymer template for nanohybrids. Macromolecules 47 (2014) 7993–8001. DOI:10.1021/ma501957b |
| [31] | L. Yao, X. Lu, S. Chen, et al., Formation of helical phases in achiral block copolymers by simple addition of small chiral additives. Macromolecules 47 (2014) 6547–6553. DOI:10.1021/ma501714g |
| [32] | Y. Lin, V.K. Daga, E.R. Anderson, et al., Nanoparticle-driven assembly of block copolymers: a simple route to ordered hybrid materials. J. Am. Chem. Soc. 133 (2011) 6513–6516. DOI:10.1021/ja2003632 |
| [33] | L. Yao, Y. Lin, J.J. Watkins. Ultrahigh loading of nanoparticles into ordered block copolymer composites. Macromolecules 47 (2014) 1844–1849. DOI:10.1021/ma500338p |
| [34] | L. Yao, J.J. Watkins. Photoinduced disorder in strongly segregated block copolymer composite films for hierarchical pattern formation. ACS Nano 7 (2013) 1513–1523. DOI:10.1021/nn3052956 |
| [35] | K. Maeda, S. Wakasone, K. Shimomura, et al., Chiral amplification in polymer brushes consisting of dynamic helical polymer chains through the long-range communication of stereochemical information. Macromolecules 47 (2014) 6540–6546. DOI:10.1021/ma501612e |
| [36] | E. Yashima, N. Ousaka, D. Taura, et al., Supramolecular helical systems: helical assemblies of small molecules, foldamers, and polymers with chiral amplification and their functions. Chem. Rev. 116 (2016) 13752–13990. DOI:10.1021/acs.chemrev.6b00354 |
 2017, Vol. 28
2017, Vol. 28 


