b Department of Biology, Science and Art University, Yazd, Iran
In recent years, interest in green chemistry [1-3] has developed, and a major challenge to organic chemists is to identify facile, efficient, and nonpolluting synthetic procedures that reduce the use of organic solvents and toxic reagents. In this area, use of natural materials as promising catalysts in organic reactions has received a considerable amount of attention due to their green credentials [4, 5] and also ionic liquids (ILs) have become increasingly popular over the last few years in the field of green organic synthesis [6] owing to several advantages such as their catalytic role, ability to dissolve a wide range of materials and mild reaction conditions, non-inflammability, high isolation and purification yields, reusability and high thermal stability [7, 8].
In order to achieve economic savings and pollution prevention, multi-component reactions [9-12] (MCRs) have considerable ecological interest as a powerful strategy in the synthesis of complex heterocyclic molecules, drug design and drug discovery, arising from minimization of time, waste, energy, and cost.
Functionalized nitrogen-and oxygen-containing heterocyclic molecules play key roles in medicinal chemistry [13]. Among them, oxazines and their derivatives have been recognized as an important class of heterocyclic compounds due to a diversity of biological functions [14-16]. Both natural and synthetic oxazine compounds exhibit a wide range of biological activities, including anticoagulant [17], fungicidal [18], AMPA receptors modulation [19], analgesic, antispasmodic [20], antidiabetic and hypolipidaemic activities [21].
Also, phenazine-based compounds are nitrogen-containing heterocycles that have been illustrated to possess numerous biological functions including antimicrobial [22], antimycobacterial [23], antifungal [24] and antitumour [25] activities. For example, pyridophenazinediones and pyridazinophenazinedione derivatives are antitumor agents [26, 27].
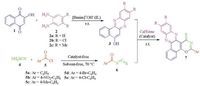
Considering the significance of oxazine and phenazine derivatives and as part of our continuing interest in the development of new synthetic methods for heterocyclic compounds [28-32] herein, we report a green and efficient method for the synthesis of novel benzo[a][1, 3]oxazino[6, 5-c]phenazine derivatives through a sequential, one-pot, four-component condensation reaction between 2-hydroxy-1, 4-naphthoquinone 1, 1, 2-diamines 2, ammonium thiocyanate 4 and acid chlorides 5 catalyzed by caffeine as a green and natural catalyst in 1-butyl-3-methylimidazolium hydroxide (ionic liquid), which acts as reaction medium as well as a basic catalyst and is easily prepared (Scheme 1).
|
Download:
|
| Scheme1. One-pot, four-component synthesis of novel benzo[a][1, 3]oxazino[6, 5-c]phenazine derivatives in the presence of caffeine as a natural and solid base catalyst. | |
2. Results and discussion
Benzo[a][1, 3]oxazino[6, 5-c]phenazine derivatives can be synthesized via a three-step procedure: in the first step, 2-hydroxy-1, 4-naphthoquinone 1 (1 mmol) and 1, 2-diamines 2 (1 mmol) were mixed at room temperature until benzo[a]phenazines 3 was formed ( < 30 min) in [bmim]+OH- (ionic liquid). Then, ammonium thiocyanate 4 (1 mmol) and acid chlorides 5 (1 mmol) were mixed at 70 ℃ (solvent-free) to produce a solid of aroyl isothiocyanate derivatives 6. The final step involves the reaction between the products of the first step 3 with aroyl isothiocyanate derivatives 6 in the presence of caffeine in [bmim]+OH- to form the corresponding products 7.
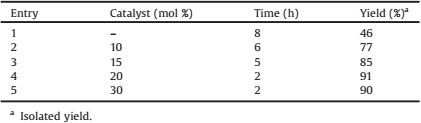
To further appraise the activity of the catalyst, the one-pot fourcomponent reaction of 2-hydroxy-1, 4-naphthoquinone 1 (1 mmol), benzene-1, 2-diamine 2a (1 mmol), ammonium thiocyanate 4 (1 mmol) and benzoyl chloride 5a (1 mmol) in [bmim]+OH- (IL) was selected as a model reaction and the yield and reaction time were monitored in different molar ratios of caffeine. The obtained results have been summarized in Table 1. As shown in Table 1, higher yield and shorter reaction time were obtained when the reaction was carried out in the presence of 20 mol% of the catalyst (Table 1, entry 4).
|
|
Table 1 Condensation reaction between 1 (1 mmol), 2a (1 mmol), 4 (1 mmol), and 5a (1 mmol) in the presence of different amounts of caffeine in [bmim]+OH- (IL) at room temperature. |
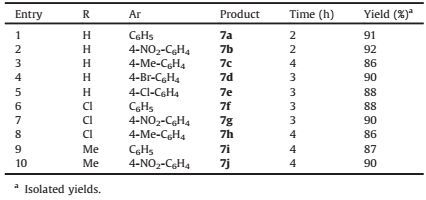
With the optimized conditions in hand, the scope and efficiency of the reaction were explored for the synthesis of benzo[a][1, 3] oxazino[6, 5-c]phenazine derivatives. Thus, 2-hydroxy-1, 4-naphthoquinone 1 was condensed with various aromatic 1, 2-diamines 2, ammonium thiocyanate 4, and different acid chlorides 5 in the presence of caffeine (20 mol%) in ionic liquids at room temperature. The results are shown in Table 2.
In the first step of this one-pot sequential reaction, different aromatic 1, 2-diamines containing benzene-1, 2-diamine, 4, 5-dichlorobenzene-1, 2-diamine and 4, 5-dimethylbenzene-1, 2-diamine were condensed with 2-hydroxy-1, 4-naphthoquinone to form the corresponding benzo[a]phenazines. Using benzene-1, 2-diamine, higher yields of the products were obtained in shorter reaction times in comparison with 4, 5-dichlorobenzene-1, 2-diamine and 4, 5-dimethylbenzene-1, 2-diamine (Table 2, entries 1, 6, and 9).
|
|
Table 2 Synthesis of novel benzo[a][1, 3]oxazino[6, 5-c]phenazine derivatives 7 from the reaction of 1, 2, 4 and 5 in the presence of caffeine (20 mol%) as catalyst in [bmim]+OH- (IL) at room temperature. |
In the next step of this sequential protocol, various acid chlorides containing electron-withdrawing groups and electrondonating groups were used. In all cases the products were obtained in good to high yields (Table 2, entries 1-10).
The structures of all the newly synthesized compounds were characterized by IR, 1H NMR, and 13C NMR spectroscopy and by elemental analysis. The mass spectra of these compounds displayed molecular ion peaks with the appropriate m/z values.
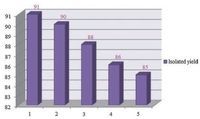
We also studied the recycling of the ionic liquid using a selected model reaction of 2-hydroxy-1, 4-naphthoquinone, benzene-1, 2-diamine, ammonium thiocyanate and benzoyl chloride in the presence of caffeine (20 mol%) in [bmim]+OH- (0.5 mL) at room temperature (Table 2, entry 1). After the completion of the reaction, 5 mL of water was added and the precipitate was filtered off for the separation of crude products. After washing the solid products with water completely, the water containing ionic liquid (ionic liquid is soluble in water) was evaporated under reduced pressure and ionic liquid was recovered and reused. As shown in Fig. 1, the reaction media could be successfully recycled for up to five runs with limited loss of activity (the yield decreased from 91% to 85% after 5 runs, Fig. 1).
|
Download:
|
| Fig. 1. The recycling of [bmim]+OH- using a model reaction of 1, 2, 4 and 5 in the presence of caffeine at room temperature. | |
Although we have not established the mechanism of the reaction experimentally, an acceptable reaction scenario for the sequential cyclocondensation is outlined in Scheme 2. Initially, 2-hydroxy-1, 4-naphthoquinone 1 tautomerizes to intermediate 8. The condensation of 4-hydroxy-1, 2-naphthoquinone 8 with benzene-1, 2-diamine 2 produces benzo[a]phenazin-5-ol 3. Then, the formation of aroyl isothiocyanate 6, followed by formation of the 1:1 adduct 9 and its subsequent protonation by benzo[a] phenazin-5-ol 3 produces 10. The positively charged ion 10 is attacked by the anion of benzo[a]phenazin-5-ol 11. Finally, intermediate 12 undergoes a cyclization reaction and elimination of water to produce 7 (Scheme 2).

|
Download:
|
| Scheme2. The proposed mechanism for the synthesis of novel benzo[a][1, 3]oxazino[6, 5-c]phenazine derivatives in the presence of caffeine. | |
3. Conclusion
In summary, we have developed a green procedure for the facile synthesis of various potentially biologically active polyfunctionalized benzo[a][1, 3]oxazino[6, 5-c]phenazines, using novel fourcomponent sequential reactions in ionic liquid at room temperature. This one-pot condensation reaction was carried out in the presence of caffeine as a natural, inexpensive, non-toxic and solid catalyst. This protocol has the advantages of mild reaction conditions, cost effectiveness, environmental friendliness, simple workup, high yields and usage in synthesis of complex molecules.
4. Experimental 4.1. General informationAll melting points were determined on an Electrothermal 9100 apparatus and are uncorrected. IR spectra were recorded on a Shimadzu IR-470 spectrometer. The 1H NMR and 13C NMR spectra were recorded on Bruker DRX-400 Avance instruments using dimethyl sulfoxide (DMSO) as a solvent. Elemental analyses were performed using a Costech ECS 4010 CHNS-O analyser at analytical laboratory of Islamic Azad University Yazd branch. Mass spectra were recorded on an Agilent Technology (HP) spectrometer operating at an ionization potential of 70 eV. Thin-layer chromatography (TLC) was performed on silica-gel Polygram SILG/UV 254 plates. All reagents were purchased from Merck and Aldrich and used without further purification. The weak basic ionic liquid (1-butyl-3-methylimidazolium hydroxide) was prepared according to the reported procedure [33].
4.2. General procedure for the synthesis of novel benzo[a][1, 3]oxazino [6, 5-c]phenazine derivatives (7a-j)At first, 2-hydroxynaphthalene-1, 4-dione 1 (1 mmol) and benzene-1, 2-diamine 2 (1 mmol) were taken in a 10-mL roundbottomed flask containing 0.5 mL of [bmim]+OH-. The mixture was stirred at room temperature in less than 30 min and benzo[a] phenazine 3 was formed. Then, ammonium thiocyanate 4 (1 mmol) and acid chlorides 5 (1 mmol) were mixed at 70 ℃ (solvent-free) in less than 5 min to produce a solid of aroyl isothiocyanate derivatives 6. Finally, the obtained aroyl isothiocyanate 6 and caffeine (20 mol%) were added into the initial reaction system and the reaction mixture was stirred at room temperature for the appropriate time (Table 2). Upon the completion of the reaction, monitored by TLC analysis, 5 mL of water was added to the mixture. The ionic liquid was dissolved in water and the crude product was collected by filtration. The separated product was washed twice with water (2 × 5 mL). The solid crude product was recrystallized from hot EtOH to give the pure product 7. After isolation of the insoluble products, the IL was recovered by evaporation of the water, washing the remaining viscous liquid with CH2Cl2 (5 mL) and drying under reduced pressure. The spectral and analytical data are presented below:
3-Phenyl-1H-benzo[a][1, 3]oxazino[6, 5-c]phenazine-1-thione (7a): Yellow solid; yield 0.356 g (91%); m.p. 291-294 ℃; IR (KBr, cm-1) : νmax 1656, 1580, 1442, 1378, 1265, 1195, 760; 1H NMR (400 MHz, DMSO-d6) : δ 7.07-7.11 (m, 1H, Ar-H), 7.36-7.41 (m, 4H, Ar-H), 7.89-7.93 (m, 2H, Ar-H), 7.94-8.00 (m, 2H, Ar-H), 8.10-8.13 (m, 1H, Ar-H), 8.24-8.27 (m, 1H, Ar-H), 8.41 (d, 1H, J = 8.0 Hz, Ar-H), 9.15 (d, 1H, J = 8.0 Hz, Ar-H); 13C NMR (100 MHz, DMSO-d6) : δ 121.2, 122.4, 124.5, 125.8, 126.5, 127.8, 128.4, 128.9, 129.2, 129.6, 130.1, 130.4, 130.5, 130.7, 138.5, 140.3, 140.8, 141.5, 144.6, 145.8, 159.3, 198.1; Anal. Calcd. for C24H13N3OS: C, 73.64; H, 3.35; N, 10.73; S, 8.19%. Found: C, 73.89; H, 3.47; N, 10.58; S, 8.41%. MS (m/z, %): 391 (M+, 5).
3-(4-Nitrophenyl)-1H-benzo[a][1, 3]oxazino[6, 5-c]phenazine-1-thione (7b): Yellow solid; yield 0.401 g (92%); m.p. 280-282 ℃; IR (KBr, cm-1) : νmax 1659, 1591, 1508, 1386, 1351, 1226, 769; 1H NMR (400 MHz, DMSO-d6) : δ 7.60 (d, 2H, J = 8.4 Hz, Ar-H), 7.68 (d, 2H, J = 8.4 Hz, Ar-H), 7.92-7.96 (m, 2H, Ar-H), 8.10 (d, 2H, J = 8.4 Hz, Ar-H), 8.14-8.16 (m, 1H, Ar-H), 8.27-8.29 (m, 1H, Ar-H), 8.48 (d, 1H, J = 8.0 Hz, Ar-H), 9.24 (d, 1H, J = 8.0 Hz, Ar-H); 13C NMR (100 MHz, DMSO-d6) : δ 120.0, 122.3, 124.9, 125.4, 125.8, 128.4, 128.7, 129.4, 129.6, 130.0, 130.2, 130.5, 130.8, 130.9, 131.0, 139.5, 140.9, 144.3, 152.4, 159.7, 197.6; Anal. Calcd. for C24H12N4O3S: C, 66.05; H, 2.77; N, 12.84; S, 7.35%. Found: C, 66.27; H, 2.89; N, 12.71; S, 7.57%. MS (m/z, %): 436 (M+, 8).
3-(p-Tolyl)-1H-benzo[a][1, 3]oxazino[6, 5-c]phenazine-1-thione (7c): Yellow solid; yield 0.348 g (86%); m.p. 288-289 ℃; IR (KBr, cm-1) : νmax 1668, 1592, 1390, 1265, 1219, 1167, 761; 1H NMR (400 MHz, DMSO-d6) : δ 2.36 (s, 3H, CH3), 7.39 (d, 2H, J = 8.0 Hz, ArH), 7.42 (d, 2H, J = 8.0 Hz, Ar-H), 7.91-8.02 (m, 4H, Ar-H), 8.14-8.17 (m, 1H, Ar-H), 8.25-8.28 (m, 1H, Ar-H), 8.43-8.44 (m, 1H, Ar-H), 9.21 (d, 1H, J = 8.0 Hz, Ar-H); 13C NMR (100 MHz, DMSO-d6) : δ 21.2, 121.6, 122.1, 123.5, 125.8, 127.4, 128.5, 129.2, 129.5, 130.1, 130.5, 130.6, 131.0, 135.6, 140.2, 141.4, 146.7, 150.4, 159.8, 163.5, 167.9, 198.4; Anal. Calcd. for C25H15N3OS: C, 74.05; H, 3.73; N, 10.36; S, 7.91%. Found: C, 73.84; H, 3.96; N, 10.51; S, 7.76%. MS (m/z, %): 405 (M+, 4).
3-(4-Bromophenyl)-1H-benzo[a][1, 3]oxazino[6, 5-c]phenazine-1-thione (7d): Yellow solid; yield 0.421 g (90%); m.p. 277-279 ℃; IR (KBr, cm-1) : νmax 1662, 1575, 1391, 1246, 1193, 1150, 758; 1H NMR (400 MHz, DMSO-d6) : δ 7.47-7.51 (m, 4H, Ar-H), 7.93-8.06 (m, 4H, Ar-H), 8.17-8.22 (m, 1H, Ar-H), 8.29-8.32 (m, 1H, Ar-H), 8.45-8.47 (m, 1H, Ar-H), 9.24-9.28 (m, 1H, Ar-H); 13C NMR (100 MHz, DMSO-d6) : δ 118.7, 119.5, 122.3, 124.6, 124.8, 125.6, 128.2, 128.5, 129.2, 129.3, 129.6, 129.7, 130.2, 130.3, 130.6, 130.8, 130.9, 140.2, 140.3, 141.4, 147.2, 161.2, 199.6; Anal. Calcd. for C24H12BrN3OS: C, 61.29; H, 2.57; N, 8.93; S, 6.82%. Found: C, 61.47; H, 2.66; N, 9.18; S, 7.03%. MS (m/z, %): 468 (M+, 6).
3-(4-Chlorophenyl)-1H-benzo[a][1, 3]oxazino[6, 5-c]phenazine-1-thione (7e): Yellow solid; yield 0.374 g (88%); m.p. 286-289 ℃; IR (KBr, cm-1) : νmax 1595, 1573, 1398, 1254, 1230, 1191, 759; 1H NMR (400 MHz, DMSO-d6) : δ 7.43-7.45 (m, 4H, Ar-H), 7.89-8.01 (m, 4H, Ar-H), 8.07-8.10 (m, 1H, Ar-H), 8.24-8.27 (m, 1H, Ar-H), 8.41-8.43 (m, 1H, Ar-H), 9.19-9.22 (m, 1H, Ar-H); 13C NMR (100 MHz, DMSOd6) : δ 120.2, 122.5, 123.1, 124.7, 125.6, 128.4, 128.7 129.6, 129.7, 130.4, 130.5, 130.8, 130.9, 131.0, 138.5, 141.0, 144.5, 153.2, 159.5, 199.3; Anal. Calcd. for C24H12ClN3OS: C, 67.68; H, 2.84; N, 9.87; S, 7.53%. Found: C, 67.54; H, 2.61; N, 10.11; S, 7.68%. MS (m/z, %): 425 (M+, 3).
11, 12-Dichloro-3-phenyl-1H-benzo[a][1, 3]oxazino[6, 5-c]phenazine-1-thione (7f): Yellow solid; yield 0.404 g (88%); m.p. >300 ℃; IR (KBr, cm-1) : νmax 1658, 1576, 1440, 1381, 1266, 1195, 763; 1H NMR (400 MHz, DMSO-d6) : δ 7.11-7.16 (m, 1H, Ar-H), 7.40-7.47 (m, 4H, Ar-H), 7.93-7.99 (m, 2H, Ar-H), 8.17-8.19 (m, 1H, Ar-H), 8.51 (s, 1H, Ar-H), 8.58 (s, 1H, Ar-H), 9.17 (d, 1H, J = 8.0 Hz, Ar-H); 13C NMR (100 MHz, DMSO-d6) : δ 120.3, 123.1, 124.7, 125.2, 125.1, 126.7, 128.6, 128.8, 129.4, 129.6, 130.2, 130.6, 130.9, 131.3, 138.5, 141.4, 142.1, 143.5, 146.7, 145.1, 158.4, 198.7; Anal. Calcd. for C24H11Cl2N3OS: C, 62.62; H, 2.41; N, 9.13; S, 6.97%. Found: C, 62.80; H, 2.33; N, 9.37; S, 7.16%. MS (m/z, %): 459 (M+, 9).
11, 12-Dichloro-3-(4-nitrophenyl)-1H-benzo[a][1, 3]oxazino [6, 5-c]phenazine-1-thione (7g): Yellow solid; yield 0.453 g (90%); m.p. >300 ℃; IR (KBr, cm-1) : νmax 1658, 1591, 1515, 1392, 1345, 1270, 751; 1H NMR (400 MHz, DMSO-d6) : δ 7.62 (s, 1H, Ar-H), 7.95-8.11 (m, 4H, Ar-H), 8.42-8.49 (m, 4H, Ar-H), 9.12-915 (m, 1H, Ar-H); 13C NMR (100 MHz, DMSO-d6) : δ 120.7, 121.4, 123.5, 125.6, 126.4, 128.6, 128.3, 129.6, 129.9, 130.4, 130.5, 130.7, 130.9, 131.2, 131.5, 138.2, 142.1, 144.0, 153.3, 160.1, 198.2; Anal. Calcd. for C24H10Cl2N4O3S: C, 57.04; H, 1.99; N, 11.09; S, 6.35%. Found: C, 56.79; H, 2.16; N, 11.21; S, 6.59%. MS (m/z, %): 503 (M+, 6).
3-(p-Tolyl)-1H-benzo[a][1, 3]oxazino[6, 5-c]phenazine-1-thione (7h): Yellow solid; yield 0.407 g (86%); m.p. 293-295 ℃; IR (KBr, cm-1) : νmax 1664, 1597, 1396, 1292, 1222, 1171, 765; 1H NMR (400 MHz, DMSO-d6) : δ 2.42 (s, 3H, CH3), 7.32-7.37 (m, 4H, Ar-H), 7.93-7.97 (m, 1H, Ar-H), 8.00-8.04 (m, 1H, Ar-H), 8.35-8.37 (m, 1H, Ar-H), 8.50 (s, 1H, Ar-H), 8.55 (s, 1H, Ar-H), 9.18 (d, 1H, J = 8.0 Hz, ArH); 13C NMR (100 MHz, DMSO-d6) : δ 21.8, 122.0, 122.4, 123.1, 124.5, 126.2, 128.1, 128.5, 129.3, 129.4, 129.9, 130.2, 130.8, 131.4, 133.6, 141.3, 142.0, 146.1, 152.1, 156.9, 162.1, 165.6, 198.8; Anal. Calcd. for C25H13Cl2N3OS: C, 63.30; H, 2.76; N, 8.86; S, 6.76%. Found: C, 63.12; H, 2.78; N, 9.01; S, 6.69%. MS (m/z, %): 473 (M+, 9).
11, 12-Dimethyl-3-phenyl-1H-benzo[a][1, 3]oxazino[6, 5-c] phenazine-1-thione (7i): Yellow solid; yield 0.365 g (87%); m.p.297-299 ℃; IR (KBr, cm-1) : νmax 1662, 1594, 1490, 1383, 1265, 1201, 766; 1H NMR (400 MHz, DMSO-d6) : δ 2.44 (s, 6H, 2CH3), 7.09 (t, 1H, J = 7.6 Hz, Ar-H), 7.34-7.38 (m, 4H, Ar-H), 7.63 (s, 1H, Ar-H), 7.84-7.96 (m, 3H, Ar-H), 8.40 (d, 1H, J = 8.0 Hz, Ar-H), 9.14 (d, 1H, J = 8.0 Hz, Ar-H); 13C NMR (100 MHz, DMSO-d6) : δ 20.3, 20.4, 122.1, 123.5, 126.1, 127.2, 127.5, 128.2, 129.1, 129.2, 129.5, 129.9, 130.2, 130.3, 130.8, 131.2, 135.5, 138.8, 140.5, 141.2, 145.1, 145.9, 157.3, 198.4; Anal. Calcd. for C26H17N3OS: C, 74.44; H, 4.08; N, 10.02; S, 7.64%. Found: C, 74.48; H, 4.19; N, 10.33; S, 7.50%. MS (m/z, %): 419 (M+, 5).
11, 12-Dimethyl-3-(4-nitrophenyl)-1H-benzo[a][1, 3]oxazino [6, 5-c]phenazine-1-thione (7j): Yellow solid; yield 0.418 g (90%); m.p. >300 ℃; IR (KBr, cm-1) : νmax 1659, 1590, 1510, 1388, 1346, 1214, 762; 1H NMR (400 MHz, DMSO-d6) : δ 2.44 (s, 3H, CH3), 2.46 (s, 3H, CH3), 7.57-7.61 (m, 3H, Ar-H), 7.83 (s, 1H, Ar-H), 7.98-8.07 (m, 2H, Ar-H), 8.05-8.09 (m, 2H, Ar-H), 8.43 (dd, 1H, J1 = 8.0 Hz, J2 = 1.6 Hz, Ar-H), 9.12 (dd, 1H, J1 = 8.0 Hz, J2 = 1.2 Hz, Ar-H); 13C NMR (100MHz, DMSO-d6) : δ 20.3, 20.5, 121.2, 123.3, 124.2, 126.1, 126.5, 127.3, 127.9, 129.1, 129.5, 130.1, 130.2, 130.4, 130.6, 130.9, 133.2, 138.1, 141.5, 144.5, 151.8, 158.1, 197.9; Anal. Calcd. for C26H16N4O3S: C, 67.23; H, 3.47; N, 12.06; S, 6.90%. Found: C, 67.01; H, 3.29; N, 12.28; S, 7.11%. MS (m/z, %): 464 (M+, 4).
AcknowledgmentWe gratefullyacknowledge financial support from the Research Council of Young Researchers and Elite Club, Yazd Branch, Islamic Azad University, Yazd, Iran and University of Science and Arts of Yazd, Iran.
| [1] | P. Anastas, N. Eghbali. Green chemistry:principles and practice. Chem. Soc. Rev. 39(2010)301–312. DOI:10.1039/B918763B |
| [2] | P. T. Anastas, J. C. Warner, Green Chemistry: Theory and Practice, Oxford University Press, New York, 1998, pp. 30. |
| [3] | W. Leitner. Green solvents-progress in science and application. Green Chem. 11(2009)603. DOI:10.1039/b907013n |
| [4] | M.R. Mousavi, N. Hazeri, M.T. Maghsoodlou, et al., Entirely green protocol for the synthesis of β-aminoketones using saccharose as a homogenous catalyst. Chin. Chem. Lett. 24(2013)411–414. DOI:10.1016/j.cclet.2013.03.022 |
| [5] | M.T. Maghsoodlou, N. Hazeri, M. Lashkari, et al., Saccharose as a new, natural, and highly efficient catalyst for the one-pot synthesis of 4, 5-dihydropyrano3, 2-cchromenes, 2-amino-3-cyano-4 H-chromenes, 1, 8-dioxodecahydroacridine, and 2-substituted benzimidazole derivatives. Res. Chem. Intermed. 41(2015)6985–6997. DOI:10.1007/s11164-014-1793-4 |
| [6] | P. Wasserscheid, T. Welton, Ionic Liquids in Synthesis, Wiley-VCH, Weinheim, Germany, 2008. |
| [7] | P. Wasserscheid, W. Keim. Ionic liquids-new solutions for transition metal catalysis. Angew. Chem. Int. Ed. 39(2000)3772–3789. DOI:10.1002/(ISSN)1521-3773 |
| [8] | J. Dupont, R.F. Souza, P.A.Z. Suarez. Ionic liquid (Molten salt) phase organometallic catalysis. Chem. Rev. 102(2002)3667–3692. DOI:10.1021/cr010338r |
| [9] | J. Zhu, H. Bienayme, Multicomponent Reactions, Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim, 2005. |
| [10] | B.B. Toure, D.G. Hall. Natural product synthesis using multicomponent reaction strategies. Chem. Rev. 109(2009)4439–4486. DOI:10.1021/cr800296p |
| [11] | J.D. Sunderhaus, S.F. Martin. Applications of multicomponent reactions to the synthesis of diverse heterocyclic scaffolds. Chem. Eur. J. 15(2009)1300–1308. DOI:10.1002/chem.v15:6 |
| [12] | B. Ganem. Strategies for innovation in multicomponent reaction design. Acc. Chem. Res. 42(2009)463–472. DOI:10.1021/ar800214s |
| [13] | V.A. Chebanov, E.A. Muravyova, S.M. Desenko, et al., Microwave-assistedthreecomponent synthesis of 7-aryl-2-alkylthio-4, 7-dihydro-1, 2, 4-triazolo1, 5-apyrimidine-6-carboxamides and their selective reduction. J. Comb. Chem. 8(2006)427–434. DOI:10.1021/cc060021a |
| [14] | A. Krantz, W.R. Spencer, F.T. Tam, et al., Design and synthesis of 4 H-3, 1-benzoxazin-4-ones as potent alternate substrate inhibitors of human leukocyte elastase. J. Med. Chem. 33(1990)464–479. DOI:10.1021/jm00164a002 |
| [15] | W.P. Hsieh, R.F. Chang, H.C. Chang, et al., 2-Substituted benzoxazinone analogues as anti-human coronavirus (anti-HCoV) and ICAM-1 expression inhibition agents. Bioorg. Med. Chem. Lett. 14(2004)4751–4754. DOI:10.1016/j.bmcl.2004.06.083 |
| [16] | M. Gutschow, U. Neumann. Inhibition of cathepsin G by 4H-3, 1-benzoxazin-4-ones. Bioorg. Med. Chem. 5(1997)1935–1942. DOI:10.1016/S0968-0896(97)00128-4 |
| [17] | R.L. Sawant, M.S. Mhaske, J.B. Wadekar. Anticoagulant potential of schiff bases of 1, 3-oxazines. Int. J. Pharm. Pharm. Sci. 4(2012)320–323. |
| [18] | Z. Tang, W. Chen, Z. Zhu, H. Liu. Synthesis of 2, 3-diaryl-3, 4-dihydro-2H-1, 3-benzoxazines and their fungicidal activities. J. Heterocycl. Chem. 48(2011)255–260. DOI:10.1002/jhet.533 |
| [19] | R. Mueller, Y.X. Li, A. Hampson, et al., Benzoxazinones as potent positive allosteric AMPA receptormodulators:part Ⅰ. Bioorg. Med. Chem. Lett. 21(2011)3923–3926. DOI:10.1016/j.bmcl.2011.05.026 |
| [20] | H.S. Mosher, M.B. Frankel, M. Gregory. Heterocyclic diphenylmethane derivatives. J. Am. Chem. Soc. 75(1953)5326–5328. DOI:10.1021/ja01117a054 |
| [21] | G.R. Madhavan, R. Chakrabarti, K.A. Reddy, et al., Dual PPAR-a and -g activators derived from novel benzoxazinone containing thiazolidinediones having antidiabetic and hypolipidemic potential. Bioorg. Med. Chem. 14(2006)584–591. DOI:10.1016/j.bmc.2005.08.043 |
| [22] | H. Hussain, S. Specht, S.R. Sarite, et al., A new class of phenazines with activity against a chloroquine resistant plasmodium falciparum strain and antimicrobial activity. J. Med. Chem. 54(2011)4913–4917. DOI:10.1021/jm200302d |
| [23] | S.G. Franzblaul, F. J.. O'sullivan, Structure-activity relationships of selected phenazines against Mycobacterium leprae in vitro. Antimicrob. Agents Chemother. 32(1988)1583–1585. DOI:10.1128/AAC.32.10.1583 |
| [24] | Y. Ge, D. Pei, Y. Zhao, et al., Correlation between antifungal agent phenazine-1-carboxylic acid and pyoluteorin biosynthesis in Pseudomonassp. M18. Curr. Microbiol. 54(2007)277–281. DOI:10.1007/s00284-006-0317-x |
| [25] | G.W. Rewcastle, W.A. Denny, B.C. Baguley. Potential antitumor agents. 51. Synthesis and antitumor activity of substituted phenazine-1-carboxamides. J. Med. Chem. 30(1987)843–851. DOI:10.1021/jm00388a017 |
| [26] | H.J. Lee, J.S. Kim, S.Y. Park, et al., Synthesis and cytotoxicity evaluation of 6, 11-dihydro-pyridazo-and 6, 11-dihydro-pyrido2, 3-bphenazine-6, 11-diones. Bioorg. Med. Chem. 12(2004)1623–1628. DOI:10.1016/j.bmc.2004.01.029 |
| [27] | J.S. Kim, H.K. Rhee, H.J. Park, et al., Synthesis of 6-chloroisoquinoline-5, 8-diones and pyrido3, 4-bphenazine-5, 12-diones and evaluation of their cytotoxicity and DNA topoisomerase Ⅱ inhibitory activity. Bioorg. Med. Chem. 15(2007)451–457. DOI:10.1016/j.bmc.2006.09.040 |
| [28] | Yazdani Elah Abadi A., M.T. Maghsoodlou, R. Heydari, R. Mohebat. An efficient four-component domino protocol for the rapid and green synthesis of functionalized benzoapyrano2, 3-cphenazine derivatives using caffeine as a homogeneous catalyst. Res. Chem. Intermed. 42(2016)1227–1235. DOI:10.1007/s11164-015-2083-5 |
| [29] | R. Mohebat, Yazdani Elah Abadi A., M.T. Maghsoodlou, M. Mohammadi. PTSAcatalyzed four-component domino reactions for the one-pot synthesis of functionalized 11H-benzo[a]benzo[6, 7] chromeno[2, 3-c]phenazine-11, 16(17H)-diones in PEG. Res. Chem. Intermed. 42(2016)5915–5959. DOI:10.1007/s11164-015-2413-7 |
| [30] | Z. Vojdani, R. Mohebat. Facile and efficient one-pot synthesis of highly functionalised 1, 2, 3, 5-tetrahydroimidazo[1, 2-a]pyrimidines. J. Chem. Res. 39(2015)203–205. |
| [31] | R. Mohebat, S. Naseri, A. Hassanabadi. One-pot synthesis of highly functionalised 1-tosylpyrazolidines. J. Chem. Res. 38(2014)175–177. DOI:10.3184/174751914X13921460686140 |
| [32] | R. Mohebat, Anary-Abbasinejad M., S. Hajmohammadi, A. Hassanabadi. Threecomponent reaction of triphenylphosphine, acetylenic esters, and 6-aminouracil or 6-amino-N, N'-dimethyluracil. Synth. Commun. 43(2013)2833–2840. DOI:10.1080/00397911.2011.627105 |
| [33] | B.C. Ranu, S. Banerjee. Ionic liquid as catalyst and reaction medium. The dramatic influence of a task-specific ionic liquid, [bmIm]OH, in Michael addition of active methylene compounds to conjugated ketones, carboxylic esters, and nitriles. Org. Lett. 7(2005)3049–3052. DOI:10.1021/ol051004h |
 2017, Vol. 28
2017, Vol. 28