b College of Chemistry and Materials Engineering Department, Wenzhou University, Wenzhou 325035, China
The nitrile moiety is a significant and abundant building block in dyes and various biologically-, pharmaceutically-, and agrichemically-active compounds as well as in many fine chemicals [1-3]. Nitriles are also important precursors in the synthesis of amides and amide derivatives, carboxylic acid derivatives, as well as numerous heterocycle compounds through multi-component reactions [4-8]. Therefore, nitirle synthesis has been a long term interest among the synthetic chemists and many methods have been reported in the literature. Traditionally, nitriles can be obtained by Sandmeyer reaction of diazonium salt derived from aryl amines [9, 10], by Rosenmund-von Braun reaction of aryl halides [11], and by ammoxidation of toluenes [12]. However, the requirement of harsh reaction conditions such as high temperatures (up to 550 ℃), the use of highly toxic cyanide salts, and the production of large amounts of wastes greatly limited the potentials of these methods in functional group tolerance and wider applications in synthesis. More recently, dehydration of amides or aldoximes at high temperatures [13, 14], transition metal (TM)-catalyzed cross-coupling reactions of CN sources with aryl halides, pseudohalides or other aryl sources [15, 16], TM-catalyzed anaerobic dehydrogenative or aerobic oxidative coupling of alcohols with ammonia [17], dehydrogenation or oxidation of amines [18-33], as well as other methods [34] have also been developed as useful alternatives for nitrile synthesis. Among these methods, dehydrogenation or oxidation of amines seems to be a direct and more practical method due to the ready availability of the amines. Therefore, oxidation methods using stoichiometric oxidants [18, 19], TM-catalyzed anaerobic dehydrogenation methods [20-22], and TM-catalyzed aerobic oxidation methods [23-33] have been described in the literature. In comparison with the stoichiometric oxidants-mediated and anaerobic dehydrogenation methods [18-22], the aerobic oxidation reactions [23-33] should be greener and more advantageous methods because oxygen or even air can be used as the oxidant without producing any wastes or byproducts other than the water. Since air is more available, more economic, safer, and more practical in use, amine oxidation with air is an attractive way for nitrile synthesis. However, since air is also less oxidative and less reactive than the pure oxygen and thus usually require harsher reaction conditions in the same oxidation reactions, the known aerobic amine oxidation reactions for nitrile synthesis mostly employed pure oxygen as the oxidant; while the use of air is still rare so far.
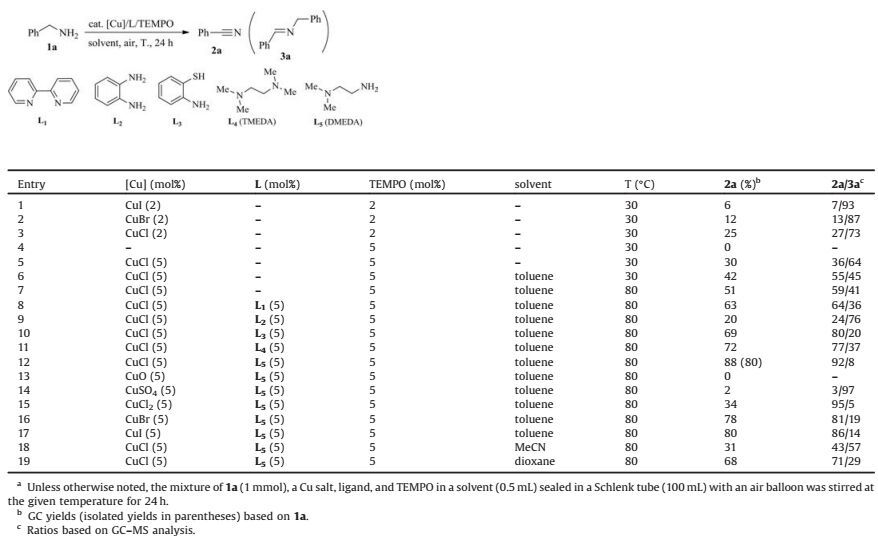
In previous studies, we have engaged in some aerobic oxidative reactions using air as the oxidant [35, 36], especially the TMcatalyzed aerobic oxidative condensation reactions of alcohols with amines [37-39] and aerobic amine oxidation reactions [39-41] for synthesis of the useful imines and benzazole heterocycles (Scheme 1A). In the Cu-catalyzed aerobic amine oxidation reactions under air, we noticed that nitriles could be observed in detectable yields in many cases (Scheme 1B) [40, 41]. Due to the importance of the nitrile compounds and the advantages of using air as the oxidant, we envisioned a new method for nitrile synthesis bya Cu-catalyzed aerobic amine oxidation reaction using air as the oxidant. As to the background of the Cu-catalyzed methods, the Kametani group had reported early in 1977 a stoichiometric CuCl-mediated aerobic amine oxidation reaction for nitriles synthesis [23]. Cu catalysts were then scarcely used in the research. Till in 2013, the Stahl group reported an efficient and highly selective but somewhat complex Cu/4, 4'-t-Bu2bpy/ABNO/ DMAP-catalyzed aerobic oxidative method for nitrile synthesis from amines [31]. However, both methods require the use of pure oxygen as the oxidant. Therefore, there is still much room for improvements of the Cu-catalyzed method by using air as the oxidant. Herein we report a readily available and relatively active CuCl/DMEDA/TEMPO catalyst system that can be utilized in the aerobic oxidation of primary amines for nitrile synthesis by using air as the oxidant under mild conditions (Scheme 1C).
|
Download:
|
| Scheme1. Cu-catalyzed aerobic amine oxidation reactions with air. | |
2. Results and discussion
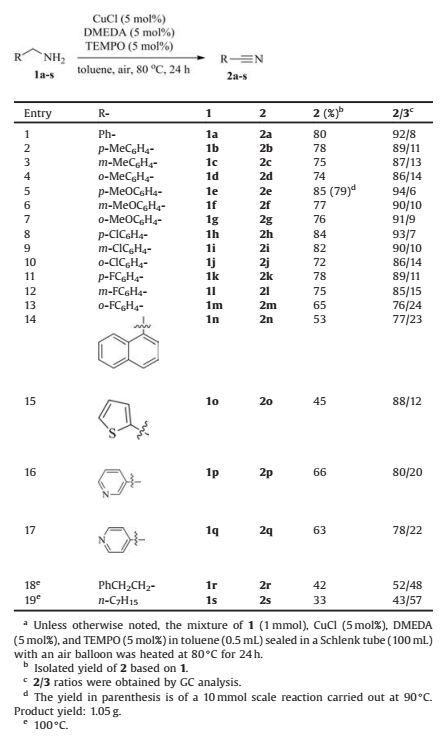
As shown in Table 1, an initial copper catalyst evaluation in aerobic oxidation of benzylamine (1a) with air under the imination conditions at room temperature (entries 1-3, with 2mol% TEMPO) showed that CuCl maybe the best Cu sourcefor nitrile synthesis for it gave the highest GC yield (25%) and selectivity (27%) of benzonitrile (2a) among the copper catalysts tested (entry 3) [40]. This also indicates that the Cu source or the anion of the copper salts is significant in the reaction. Indeed, no product could be observed under the same conditions in the absence of a Cu catalyst even with a higher loading of TEMPO (entry 4). CuCl was then used in further optimization the reaction conditions. Higher loadings of both CuCl and TEMPO (5mol%) were then firstly tested, but improvements of the 2a's yield and selectivity were not obvious (entry 5). Then, addition of toluene as the solvent (entry 6) and 1, 1'-bipyridine as the ligand (entry 8) as wellas elevation of the reaction temperature to 80 ℃ (entry 7) were all found to work to improve 2a's yield and selectivity. Realizing that the ligand and the anion of the copper salts that also work as the ligand for the copper center can both affect the catalyst's activity significantly, more ligands were further evaluated. Screening of other bidentate ligands such as o-diaminobenzene, o-aminobenzenethiol, N, N, N', N'-tetramethylethane-1, 2-diamine (TMEDA), N, N-dimethylethane-1, 2-diamine (DMEDA) showed that DMEDA is the best ligand for the reaction (entries 9-12). The optimal conditions (run 12) were then employed for further evaluation of the copper sources (entries 13-17) and solvents (entries 18-19). However, No better results could be obtained using other Cu catalysts such as CuO, CuSO4 and CuCl2, CuBr, and CuI and solvents like MeCN and dioxane. Thus, the use of 5 mol% of CuCl, DMEDA, and TEMPO as the catalyst system and running the reaction at 80 ℃ in toluene afforded 2a in the highest 80% isolated yield and 92% selectivity (entry 12).
|
|
Table 1 Condition optimization for the Cu-catalyzed aerobic amine oxidation with air for nitrile synthesis.a |
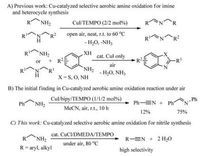
The above optimized conditions (Table 1, entry 12) were then applied to various primary amines to extend the scope of this Cucatalyzed aerobic oxidation method with air. Similar to the model reaction (entry 1), both benzylamines bearing electron-donating substituent (entries 2-7) and electron-withdrawing substituent (entries 8-13) on the phenyl ring could be smoothly oxidized by air under the standard conditions to give the desired aryl nitriles in generally good to high selectivities and good yields. The results showed that the ortho-substituted benzylamines generally gave lower yields of the products (entries 4, 7, 10, 13) than corresponding para-and meta-ones (entries 2, 3, 5, 6, 8, 9, 11, 12). As shown in the table, this method could tolerate a series of functional groups such as the reactive fluoro and chloro groups (entries 8-13). In addition, despite the steric effect of naphthyl ring, the reaction of 1-naphthylmethylamine still gave a moderate yield of target product (entry 14). The method could also be successfully extended to heteroaryl methylamines such as 2-thiophenemethylamine, 3-pyridylmethylamine as well as 4-pyridylmethylamine (entries 15-17), but only moderate yields of the corresponding heteroaryl nitriles were obtained. For alkyl amines such as 3-phenylpropylamine and n-octylamine, the reaction gave poor yields of target alkyl nitriles under the standard conditions, even if the reaction temperature was elevated to 100 ℃ (entries 18, 19).Finally, to test the scalability of the method, a 10 mmol scale reaction of 1e was performed. The reaction afforded target 2e in 79% yield (1.05 g) at an elevated temperature of 90 ℃, revealing potential of the method in large scale synthesis (Table 2).
|
|
Table 2 Scope of the Cu-catalyzed aerobic amine oxidation method.a |
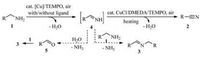
In comparison with the previous CuI/TEMPO-catalyzed imineselective aerobic amine oxidation reaction with air [40], by replacing CuI with CuCl, adding ligand DEMDA, and enhancing the reaction temperature, the present CuCl/DMEDA/TEMPO catalyst combination seems to be more active and oxidative that can lead to the selective production of the nitriles. Therefore, a possible reaction path was postulated in Scheme 2 to show the selective generation of nitriles from amines. Thus, the [Cu]/TEMPO system with or without a ligand has already been an active catalyst that can efficiently catalyze the aerobic amine oxidation to give imine intermediate 4 even at room temperature [40]. Then, in the presence of the more active CuCl/DMEDA/TEMPO catalyst, 4 was effectively oxidized again to selectively give nitriles and at an elevated temperature, rather than undergoing hydrolysis or condensation reactions to give imine byproducts 3 as in the previous cases [40]. Thus, nitriles can be selectively produced by the present method.
|
Download:
|
| Scheme2. Potential reaction paths for selective nitrile and imine synthesis. | |
3. Conclusion
In conclusion, we developed a direct method for selective synthesis of nitriles from primary amines by a simple CuCl/ DMEDA/TEMPO-catalyzed aerobic amine oxidation reaction using air as an advantageous oxidant. Since the catalyst system is readily available form commercial sources and the method tolerates aryl, heteroaryl, and aliphatic amines and several reactive functional groups and can be performed under relatively mild conditions, all these makes the method an easily applicable and practical alternative for nitrile synthesis from amines. Further applications of the Cu-based catalysts in aerobic oxidation reactions are still ongoing in this group.
4. ExperimentalTypical procedures for Cu-catalyzed aerobic oxidation of primary amines with air for nitrile synthesis: A mixture of benzylamine 1a (107.0 mg, 1.0 mmol), CuCl (5.0 mg, 0.05 mmol, 5 mol%), 2, 2, 6, 6-tetramethyl-1-piperidyloxy (TEMPO, 7.8 mg, 0.05 mmol, 5 mol%), and N, N-dimethylethane-1, 2-diamine (DMEDA, 4.4 mg, 0.05 mmol, 5 mol%) in toluene (0.5 mL) sealed in a Schlenk tube (100 mL) with an air balloon was stirred at 80 ℃ for 24 h. The reaction was then monitored by TLC and/or GC-MS. After completion of the reaction, solvent was evaporated under vacuum. The residue was purified by flash column chromatography on silica gel using petroleum ether and ethyl acetate (0-100/1) as the eluent, giving product 2a in 80% isolated yield.
Benzonitrile (2a): Colourless oil. 1H NMR (500 MHz, CDCl3) : δ 7.65-7.59 (m, 3H), 7.49-7.46 (m, 2H); 13C NMR (125 MHz, CDCl3) : δ 132.83, 132.11, 129.16, 118.84, 112.37; MS (EI): m/z (%) 104 (8), 103 (100), 77 (6), 76 (38), 75 (9), 74 (4), 73 (2), 63 (3), 52 (5), 51 (10), 50 (17).
AcknowledgmentWe thank NNSFC (Nos. 51502174, 21672163), ZJNSF (No.LR14B020002), and Postdoctoral Science Foundation of China (Nos. 2015 M582401, 2016M592520) for financial support. This work was also partially supported by Science and Technology Project of Shenzhen (Nos. JCYJ20150324141711616, JCYJ20150626090504916) and Science and Technology Planning Project of Guangdong Province (No. 2016B050501005).
| [1] | G. Pollak, F. Romeder, H. P. Hagedorn, Ullmann's Encyclopedia of Industrial Chemistry, Wiley-VCH, Weinheim, Germany, 2012. |
| [2] | A. J. Fatiadi, Preparation and Synthetic Applications of Cyano Compounds, in: S. Patai, Z. Rappaport (Eds. ), Triple-Bonded Functional Groups, Wiley, New York, 1983, pp. 1057-1303. |
| [3] | J.S. Miller, J.L. Manson. Designer magnets containing cyanides and nitriles. Acc. Chem. Res. 34(2001)563–570. DOI:10.1021/ar0000354 |
| [4] | K. Yamaguchi, M. Matsushita, N. Mizuno. Efficient hydration of nitriles to amides in water, catalyzed by ruthenium hydroxide supported on alumina. Angew. Chem. Int. Ed. 43(2004)1576–1580. DOI:10.1002/(ISSN)1521-3773 |
| [5] | J.N. Moorthy, N. Singhal. Facile and highly selective conversion of nitriles to amides via indirect acid-catalyzed hydration using TFA or AcOH-H2SO4. J. Org. Chem. 70(2005)1926–1929. DOI:10.1021/jo048240a |
| [6] | G.K. Jnaneshwara, V.H. Deshpande, M. Lalithambika, T. Ravindranathan, A.V. Bedekar. Natural Kaolinitic clay catalyzed conversion of nitriles to 2-oxazolines. Tetrahedron Lett. 39(1998)459–462. DOI:10.1016/S0040-4039(97)10575-5 |
| [7] | H. Chen, W. Dai, Y. Chen, et al., Efficient and selective nitrile hydration reaction in water catalyzed by unexpected dimethylsulfinyl anion generated in situ from CsOH and DMSO. Green Chem. 16(2014)2136–2141. DOI:10.1039/C3GC42310G |
| [8] | Y. Li, H. Chen, J. Liu, X. Wan, Q. Xu. Clean synthesis of primary to tertiary carboxamides by CsOH-catalyzed aminolysis of nitriles in water. Green Chem. 18(2016)4865–4870. DOI:10.1039/C6GC01565D |
| [9] | R.G.R. Bacon, H.A.O. Hill. Metal ions and complexes in organic reactions. Part I. Substitution reactions between aryl halides and cuprous salts in organic solvents. J. Chem. Soc. (1964)1097–1107. DOI:10.1039/jr9640001097 |
| [10] | P. Benz, R. Muntwyler, R. Wohlgemuth. Chemoenzymatic synthesis of chiral carboxylic acids via nitriles. J. Chem. Technol. Biotechnol. 82(2007)1087–1098. DOI:10.1002/(ISSN)1097-4660 |
| [11] | G.P. Ellis, T.M.R. Alexander. Cyanation of aromatic halides. Chem. Rev. 87(1987)779–794. DOI:10.1021/cr00080a006 |
| [12] | R.K. Grasselli. Advances and future trends in selective oxidation and ammoxidation catalysis. Catal. Today 49(1999)141–153. DOI:10.1016/S0920-5861(98)00418-0 |
| [13] | H. Yamaguchi, Y. Fujiwara, M. Ogasawara. A tungsten-tin mixed hydroxide as an efficient heterogeneous catalyst for dehydration of aldoximes to nitriles. Angew. Chem. Int. Ed. 46(2007)3922–3925. DOI:10.1002/(ISSN)1521-3773 |
| [14] | K. Ishihara, Y. Furuya, H. Yamamoto. Rhenium (Ⅶ) oxo complexes as extremely active catalysts in the dehydration of primary amides and aldoximes to nitriles. Angew. Chem. Int. Ed. 41(2002)2983–2986. DOI:10.1002/1521-3773(20020816)41:16<2983::AID-ANIE2983>3.0.CO;2-X |
| [15] | Y. Zhu, L. Li, Z. Shen. Cu-catalyzed cyanation of arylboronic acids with acetonitrile:a dual role of TEMPO. Chem. A Eur. J. 21(2016)13246–13252. |
| [16] | B. Mondal, K. Acharyya, P. Howlader, P.S. Mukherjee. Molecular cage impregnated palladium nanoparticles:efficient additive-free heterogeneous catalysts for cyanation of aryl halides. J. Am. Chem. Soc. 138(2016)1709–1716. DOI:10.1021/jacs.5b13307 |
| [17] | B.L. Ryland, S.S. Stahl. Practical aerobic oxidations of alcohols and amines with homogeneous copper/TEMPO and related catalyst systems. Angew. Chem. Int. Ed. 53(2014)8824–8838. DOI:10.1002/anie.201403110 |
| [18] | J. Zhang, Z. Wang, Y. Wang, et al., A metal-free catalytic system for the oxidation of benzylic methylenes and primary amines under solvent-free conditions. Green Chem. 11(2009)1973–1978. DOI:10.1039/b919346b |
| [19] | G. Bagherzade, A. Zali, A. Shokrolahi. Preparation of aromatic nitriles via direct oxidative conversion of benzyl alcohols, aldehydes and amines with pentylpyridinium tribromide in aqueous NH4OAc. Chin. Chem. Lett. 26(2015)603–606. DOI:10.1016/j.cclet.2015.01.009 |
| [20] | W.H. Bernskoetter, M. Brookhart. Kinetics and mechanism of iridiumcatalyzed dehydrogenation of primary amines to nitriles. Organometallics 27(2008)2036–2045. DOI:10.1021/om701148t |
| [21] | K.N.T. Tseng, A.M. Rizzi, N.K. Szymczak. Oxidant-free conversion of primary amines to nitriles. J. Am. Chem. Soc. 135(2013)16352–16355. DOI:10.1021/ja409223a |
| [22] | D. Damodara, R. Arundhathi, P.R. Likhar. Copper nanoparticles from copper aluminum hydrotalcite:an efficient catalyst for acceptor-and oxidant-free dehydrogenation of amines and alcohols. Adv. Synth. Catal. 356(2014)189–198. DOI:10.1002/adsc.201300453 |
| [23] | T. Kametani, K. Takahashi, T. Ohsawa, M. Ihara. Oxidation of amines to nitriles or aldehydes using copper (Ⅰ) chloride. Synthesis (1977)245. |
| [24] | E.C. Corker, U.V. Mentzel, J. Mielby, A. Riisager, R. Fehrmann. An alternative pathway for production of acetonitrile:ruthenium catalysed aerobic dehydrogenation of ethylamine. Green Chem. 15(2013)928–933. DOI:10.1039/c3gc36513a |
| [25] | O.D. Pavel, P. Goodrich, L. Cristian, et al., Direct oxidation of amines to nitriles in the presence of ruthenium-terpyridyl complex immobilized on ILs/SILP. Catal. Sci. Technol. 5(2015)2696–2704. DOI:10.1039/C5CY00011D |
| [26] | D.S. Ovoshchnikov, B.G. Donoeva, V.B. Golovko. Visible-light-driven aerobic oxidation of amines to nitriles over hydrous ruthenium oxide supported on TiO2. ACS Catal. 5(2015)34–38. DOI:10.1021/cs501186n |
| [27] | C. Hammond, M.T. Schuemperli, I. Hermans. Insights into the oxidative dehydrogenation of amines with nanoparticulate iridium oxide. Chem. Eur. J. 19(2013)13193–13198. DOI:10.1002/chem.v19.39 |
| [28] | V. Mahadevan, DuBois J.L., B. Hedman, K.O. Hodgson, T.D.P. Stack. Exogenous substrate reactivity with a [Cu(Ⅲ)2O2]2+ core:structural implications. J. Am. Chem. Soc. 121(1999)5583–5584. DOI:10.1021/ja983635v |
| [29] | L.M. Mirica, D.J. Rudd, M.A. Vance, et al., μ-η2:η2-Peroxodicopper (Ⅱ) complex with a secondary diamine ligand:a functional model of tyrosinase. J. Am. Chem. Soc. 128(2006)2654–2665. DOI:10.1021/ja056740v |
| [30] | J. Wang, S. Lu, X. Cao, H. Gu. Common metal of copper (0) as an efficient catalyst for preparation of nitriles and imines by controlling additives. Chem. Commun. 50(2014)5637–5640. DOI:10.1039/c4cc01389a |
| [31] | J. Kim, S.S. Stahl. Cu/Nitroxyl-catalyzed aerobic oxidation of primary amines into nitriles at room temperature. ACS Catal. 3(2013)1652–1656. DOI:10.1021/cs400360e |
| [32] | M. Varyani, P.K. Khatri, S.L. Jain. Amino acid derived ionic liquid supported iron schiff base catalyzed greener approach for the aerobic oxidation of amines to nitriles. Tetrahedron Lett. 57(2016)723–727. DOI:10.1016/j.tetlet.2015.12.082 |
| [33] | R.V. Jagadeesh, H. Junge, M. Beller. Nanorust-catalyzed benign oxidation of amines for selective synthesis of nitriles. ChemSusChem 8(2015)92–96. DOI:10.1002/cssc.201402613 |
| [34] | J.Q. Ye, Z.L. Zhang, Z.G. Zha, Z.Y. Wang. A green and efficient access to aryl nitriles via an electrochemical anodic oxidation. Chin. Chem. Lett. 25(2014)1112–1114. DOI:10.1016/j.cclet.2014.04.024 |
| [35] | X. Ma, C. Su, Q. Xu. N-Alkylation by hydrogen autotransfer reactions in hydrogen transfer reactions:reductions and beyond (Eds. Guillena G., D. J. Ramón). Top. Curr. Chem. 374(2016)1–74. |
| [36] | Q. Xu, Q. Li. Recent advances of transition metal-catalyzed aerobic dehydrative reactions of alcohols and amines and related researches. Chin. J. Org. Chem. 33(2013)18–35. DOI:10.6023/cjoc201208016 |
| [37] | L. Jiang, L. Jin, H. Tian, et al., Direct and mild palladium-catalyzed aerobic oxidative synthesis of imines from alcohols and amines under ambient conditions. Chem. Commun. 47(2011)10833–10835. DOI:10.1039/c1cc14242a |
| [38] | H. Tian, X. Yu, Q. Li, J. Wang, Q. Xu. General, green, and scalable synthesis of imines from alcohols and amines by a mild and efficient copper-catalyzed aerobic oxidative reaction in open air at room temperature. Adv. Synth. Catal. 354(2012)2671–2677. DOI:10.1002/adsc.v354.14/15 |
| [39] | E. Zhang, H. Tian, S. Xu, X. Yu, Q. Xu. Iron-catalyzed direct synthesis of imines from amines or alcohols and amines via aerobic oxidative reactions under air. Org. Lett. 15(2013)2704–2707. DOI:10.1021/ol4010118 |
| [40] | B. Huang, H. Tian, S. Lin, et al., Cu (Ⅰ)/TEMPO-catalyzed aerobic oxidative synthesis of imines directly from primary and secondary amines under ambient and neat conditions. Tetrahedron Lett. 54(2013)2861–2864. DOI:10.1016/j.tetlet.2013.03.098 |
| [41] | J. Liu, C. Wang, X. Ma, et al., Simple synthesis of benzazoles by substratepromoted CuI-catalyzed aerobic oxidative cyclocondensation of o-thio/amino/hydroxyanilines and amines under air. Catal. Lett. 146(2016)2139–2148. DOI:10.1007/s10562-016-1818-2 |
 2017, Vol. 28
2017, Vol. 28