b Key Laboratory of Road and Traffic Engineering of Ministry of Education, Tongji University, Shanghai 200092, China
Development of flexible, lightweight and environmentalfriendly energy-storage devices is urgently needed to satisfy the growing demand for sustainable and renewable power sources worldwide [1]. Of various energy-storage sources, supercapacitors with high power density, long cycling life and product safety have attracted significant attentions in mobile electronic devices, electric vehicles, etc. [2]. According to the energy storage mechanism, there are two typical types of supercapacitors: electric double-layer capacitors (EDLCs) and Faradaic supercapacitors (FSs). The common electrode materials for EDLCs are carbon-based materials, which are not electrochemically active, meaning that no electrochemical reaction occurs on the electrode materials, and exhibit excellent stability performance with relative lower capacitance [3]. And, transition mental oxides are most common electrode materials for FSs, which are electrochemically active, can directly store energy by a fast and reversible redox reactions taken place on the electrode surface and thus resulted in a far larger capacitance than EDLCs [4]. However, due to the faradaic processes slower than nonfaradaic processes, a FS normally exhibits relatively lower power density than an EDLC, and usually lacks long-term stability during cycling [5].
The capacitance of EDLCs comes from the pure physical charges stored at the electrode/electrolyte interface [6]. Porous carbon materials are commonly used as electrode materials for EDLCs due to their advantages of high surface area, good conductivity, and high electrochemical stability [7]. Pore structure in carbon electrodes is an important factor to determine the electrochemical performance of EDLCs [8]. Recently, it has been reported that ultramicropore (pore size less than 0.7 nm) can significantly improve the capacitance of carbon electrodes because that the < 1 nm micropores are related to an anomalous significant increase in specific capacitance, while, >0.5 nm pores are electroactively available in aqueous electrolytes for EDLCs [7b, 9]. On the other hand, the adjustable size and package among carbon spheres benefit the aggregation of electrolyte ion buffer reservoirs and decrease the resistance of electrolyte ion diffusion [10]. In addition, heteroatom (e.g., N, S and B) entrapped into carbon matrix facilitates the adsorption of electrolyte ions and therefore improves the electrochemical properties of the carbon-based electrodes [11]. Therefore, heteroatom functionally, ultramicroporous carbon spheres should be an optimum choice for tailoring high performance EDLC electrodes.
Transition metal oxides (e.g., RuO2 and MnO2), on the other hand, are considered as ideal candidates for FS electrode materials due to their variable oxidation states and high theoretical specific capacitances [12]. However, the toxic and high cost of RuO2 leads to difficulty in its commercialization for supercapacitors. On the contrary, MnO2 with low cost, natural abundance, environmental safety, and a high theoretical pseudocapacitance (1110 F/g), is an attractive electroactive material for FS electrodes [13]. However, MnO2 is in general kinetically unfavorable and has poor electrical conductivity, which limits its application as commercial available electroactive materials for pseudocapacitors [4b, 14]. To achieve high-performance supercapacitor devices, it is important to make good use of the synergistic effect between carbon-materials and metal oxides [15]. For example, Yang et al. prepared MnO2/ graphene nanocomposite electrodes which exhibit a high specific capacitance of 372 F/g at 0.5 A/g in 1.0 mol/L Na2SO4 aqueous electrolyte [15b]. In our previous work, we designed the combination of micro-and mesoporous carbon microsphere with MnO2 as high performance electroactive materials for energy storage system [4a].
Considering the aspects mentioned above, hybrid materials combined with nitrogen-doped, ultramicroporous carbon nanospheres, and MnO2 should be particularly interest for supercapacitor electrodes. To achieve that target, in this work, we present a facile and efficient approach to prepare MnO2/N-UCNs for supercapacitors. N-UCNs were prepared via a template-free polymerization process, in which resorcinol/formaldehyde were polymerized on the surface of phloroglucinol/terephthalaldehyde nanoparticles with hexamethylenetetramine (HMTA) acted as both catalyst and nitrogen resource. Then, MnO2 were grown on NUCNs by a redox reaction between KMnO4 and carbons. Asprepared MnO2/N-UCNs possess regular ultramicropores, high surface area, nitrogen heteroatoms, and high content of MnO2. The ultramicropores benefit fast electrolyte ion transfer and diffusion, and N heteroatom incorporated into carbon matrix can improve the electric conductivity, meanwhile the introduction of MnO2 contributes pseudocapacitance. Consequently, a typical MnO2/NUCNs as a supercapacitor electrode exhibits excellent electrochemical performance such as high specific capacitance (401 F/g at 1.0 A/g in 1.0 mol/L Na2SO4 electrolyte) and excellent charge/ discharge stability (86.3% of the initial capacitance after 10, 000 cycles at 2.0 A/g).
2. Results and discussionPhloroglucinol and terephthalaldehyde are polymerized to prepare polymer colloids, which can produce ultramicropores after carbonization [7b]. Resorcinol and HMTA were reacted on the surfaces of phloroglucinol/terephthalaldehyde polymer colloids to obtain N-UCNs, in which HMTA acted as catalyst and nitrogen source. N-UCNs were served as both supporting substrate and reductant, and MnO2 was directly grown on the surfaces of N-UCNs by a simple redox reaction between carbon and KMnO4: 4 KMnO4 + 3 C + H2O = 4MnO2 + 2KHCO3 + K2CO3. TGA curves of MnO2/N-UCNs shown in Fig. S1 (Supporting information) indicate the mass percentages of MnO2 in the samples are 36, 57 and 83 wt.%, respectively, which depends on different concentrations of KMnO4 during the fabrication process. The obtained MnO2/N-UCNs were denoted as MnO2(x%)/N-UCNs in which x% represents the mass percentage of MnO2 in the nanohybrids. Fig. 1 and Fig.S2 (Supporting information) shows SEM images of N-UCNs and MnO2/N-UCNs. N-UCNs have a uniform spherical morphology with a mean diameter of ~40 nm, as shown in Fig. 1a. A typical SEM image of MnO2/N-UCNs shown in Fig. 1b suggests that nanorodlike MnO2 is grown onto the N-UCNs.

|
Download:
|
| Fig. 1. SEM images of N-UCNs (a) and MnO2(57%)/N-UCNs (b). | |
XRD patterns of N-UCNs and MnO2/N-UCNs were shown in Fig. S3 (Supporting information). For N-UCNs, two broad peaks with 2θ at about 24° and 44° indicate the amorphous carbon. In the patterns of MnO2/N-UCNs, peaks can be ascribed to MnO2 (PDF 65-2861) besides some relatively weak diffraction peaks indexed to Mn2O3 (PDF 24-0508), indicating the successful introduction of MnO2 into carbon matrix via the redox reaction of KMnO4 and carbon [15a, 16]. With the increasing doping content of MnO2, the diffraction peaks were weak and broad, suggesting the poor crystallization of the nanocomposites and the crystallization transformation from α-MnO2 to δ-MnO2 [17]. A TEM image of N-UCNs shown in Fig. 2a indicates that N-UCNs have a uniform diameter of about 40 nm which is consistent with that of SEM observation. Fig. 2b shows a typical TEM image of MnO2(57%)/NUCNs. It is clearly to observe the marked contrast between N-UCNs and nanorod-like MnO2. The inset image of Fig. 2b shows the lattice fringes with interplanar distance of ~0.7 nm which corresponds to the (110) plane of tetragonal a-MnO2 structure [18].

|
Download:
|
| Fig. 2. TEM images of N-UCNs (a) and MnO2(57%)/N-UCNs (b). The inset of (b) is the HRTEM image of MnO2. | |
To further characterize the surface chemical states of MnO2/NUCNs, XPS spectra were conducted, and the results were shown in Fig. 3. The wide XPS spectrum demonstrates that there are C, N, O, and Mn atoms in the MnO2/N-UCNs samples (Fig. 3a). The compositions of these elements are 56.76, 1.20, 28.29 and 13.74 wt.%, respectively. Fig. 3b presents a fitted high-resolution XPS spectrum of N 1s in MnO2(57%)/N-UCNs. The nitrogen species can be fitted as pyridinic N (398.5 eV), pyrrolic N (400.1 eV), and graphitic N (401.2 eV). Pyridinic N and pyrrolic N are indexed as electrochemically active and electron donor tendency and thus improve electrochemical capacitance of the electrode [19]. And the graphitic N can efficiently accelerate electron transfer and enhance the conductivity of carbon materials [20]. Fig. 3c exhibits the highresolutionspectrumofMn2p.Thepeaks at642.2 and653.7 eVcanbe indexed as Mn 2p3/2 and Mn 2p1/2, respectively. The spin-energy separation of 11.5 eV is a typical characteristic of MnO2, and also suggests that the predominant oxidation of Mn is +4 [21].
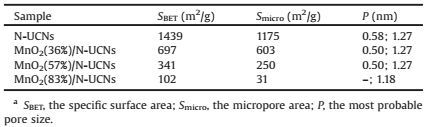
Fig. 4 exhibits nitrogen adsorption and desorption isotherms and pore size distribution curves of N-UCNs and MnO2/N-UCNs. The isotherms of N-UCNs and MnO2/N-UCNs shown in Fig. 4a can be classified to type I curves. The increasing steep in the adsorbed volume at very low relative pressure (P/P0 < 0.05) indicates the presence of abundant micropores in the samples [7b]. Besides, at a relative pressure (P/P0) of 0.90, a nitrogen condensation step with a hysteresis loop was appeared, suggesting the existence of mesopores due to the interparticle cavities resulted from the packing of MnO2/N-UCNs nanohybrids. N-UCNs and MnO2/NUCNs exhibit regular ultramicropores at ~0.5 nm and secondary micropores (or supermicropores) at ~1.27 nm, as shown in Fig. 4b.The ~0.5 nm ultramicropores are resulted from phloroglucinol/ terephthalaldehyde derived carbons, and the ~1.27 nm secondary micropores can be ascribed to the decomposition of resorcinol/ formaldehyde polymer during carbonization [7b, 9b]. MnO2/NUCNs shows smaller specific surface area of 697 (MnO2(36%)/NUCNs), 341 (MnO2(57%)/N-UCNs) and 102 (MnO2(83%)/N-UCNs) m2/g than that of N-UCNs (1439 m2/g), as shown in Table 1. And the pore volumes also show the same trend in N-UCNs and MnO2/NUCNs. With the increasing doping content of MnO2, the pore structure parameters of MnO2/N-UCNs nanohybrids are systematically decreased, suggesting the blockage of some pores in N-UCNs.

|
Download:
|
| Fig. 3. XPS spectrum of MnO2(57%)/N-UCNs (a), fitted high-resolution XPS spectra of N 1s (b) and Mn 2p (c) in MnO2(57%)/N-UCNs. | |

|
Download:
|
| Fig. 4. Nitrogen adsorption/desorption isotherms (a) and pore size distribution (b) of N-UCNs and MnO2/N-UCNs. | |
|
|
Table 1 Pore structure parameters of N-UCNs and MnO2/N-UCNs.a |
Fig. 5 shows the Nyquist plots of N-UCNs and MnO2/N-UCNs. Each plot consists of a straight line, a nearly 45° diagonal line, and a quasi-semicircle, suggests that both N-UCN and MnO2/N-UCNs can be well used as the electrode materials for supercapacitors. A nearly vertical line in the low-frequency region indicates that MnO2/N-UCNs electrode has a Warburg resistance of charge saturation, and conductive network of N-UCNs benefits rapid electron transferation. The semicircle in high-frequency region represnts the charge transfer resistance (Rct) and bulk electrolyte resistance (Rs). And the first intersection point on the Z' axis denotes the equivalent series resistance (ESR) of the electrode including Rs, the internal resistance and the interfacial contact resistance of active material/current collector. N-UCNs shows a lower ESR value of 4.59Ω. With the increasing doping content of MnO2, MnO2/M-UCNs exihibit an incremental ESR values of 5.04 (MnO2(36%)/N-UCNs), 8.53 (MnO2(57%)/N-UCNs) and 10.64V (MnO2(83%)/N-UCNs) because of the poor conductivity of MnO2.

|
Download:
|
| Fig. 5. Nyquist plots of N-UCN and MnO2/N-UCNs electrodes in 1.0 mol/L Na2SO4 aqueous solution. | |
Fig. S4a (Supporting information) shows CV curves of N-UCNs and MnO2/N-UCNs electrodes at a scan rate of 5 mV/s. The N-UCNs electrode show good rectangular shapes, which is characteristic of the EDLCs performance. The shape of the CV curves of MnO2/NUCNs electrodes is pseudo-rectangular as expected for MnO2 presenting capacitive behavior with an unclear redox peaks at about 0.2-0.4 V [15a]. The CV curves of MnO2(57%)/N-UCNs at different scan rates were shown in Fig. S4b (Supporting information). When the scan rate increasing to 100 mV/s, the CV curve of MnO2(57%)/N-UCNs electrode shows a obvious shuttle type, resulted from a large polarization.
GCD curves of N-UCNs and MnO2/N-UCNs at current density of 1.0 A/g in the voltage window from 0 to 1 V are shown in Fig. 6a.These GCD profiles exhibit relatively triangular symmetrical distribution versus Ag/AgCl electrode, indicating good capacitive properties for supercapacitor. N-UCNs electrode shows a specific capacitance of 195 F/g. The good electrochemical performance of N-UCNs electrode is due to the unique pore structure that a developed network of ultramicropores and secondary micropores is particularly suitable for charging the double layer [9b]. Besides, N functional group can efficiently accelerate electron transfer and improve the conductively of samples. The specific capacitances of MnO2/N-UCNs, calculated from the discharge curves, are 328, 401 and 201 F/g for MnO2(36%)/N-UCNs, MnO2(57%)/N-UCNs, and MnO2(83%)/N-UCNs, respectively. The electrochemical capacitances of MnO2/N-UCNs electrodes are larger than that of N-UCNs due to the introduction of MnO2. When increasing the Mn source, MnO2/N-UCNs should show a higher capacitance resulted from the pseudocapacitance of MnO2. However, in this case MnO2/N-UCNs have decreased specific surface areas due to the blockage of ultramicropores and micropores in N-UCNs caused by MnO2 (Table 1), which significantly reduces the double layer capacitance.In addition, the conductivity of the nanohybrids are also decreased, as shown in Fig. 5. MnO2(57%)/N-UCNs electrode achieves an optimum balance between pseudocapacitance and double layer capacitance due to the synergistic effect of N-UCNs and MnO2, leading to the best electrochemical performance.

|
Download:
|
| Fig. 6. GCD curves of N-UCN and MnO2/N-UCNs electrodes at 1.0 A/g (a), and MnO2(57%)/N-UCNs electrode at different current densities (b) in 1.0 mol/L Na2SO4 aqueous solution. | |
Fig. 6b exhibits the GCD curves of MnO2(57%)/N-UCNs electrode at various densities. MnO2(57%)/N-UCNs electrode shows a specific capacitance of 283 F/g at 2.0 A/g and 124 F/g at 10 A/g. The excellent electrochemical performances of MnO2/N-UCNs nanostructures can be ascribed to the introduction of MnO2, the N-doping and unique ultramicropore structure. The MnO2 grown on the surface of N-UCNs can make full use of active material, shorten the transportation path of the electrolyte ion and thus enhance the intercalation of ions at the interface of electroactive material/ electrolyte [16b] MnO2 provides high pseudocapacitance. The existent of N element improves the surface properties and enhances electric conductivity [19, 20]; while the unique ultramicropore structure benefits the fast transportation and diffusion of electrolyte ions [7b]. The MnO2(57%)/N-UCNs electrode shows a higher specific capacitance than many other MnO2/carbon electrodes reported in the literatures (Table S1 in Supporting information). The electrode durability is an important for practical application. Fig. 7 shows the cycle stability of MnO2/N-UCNs electrodes performed by GCD measurement up to 10, 000 cycles at 2.0 A/g. The specific capacitance of MnO2(57%)/N-UCNs electrode retains 244 F/g (86.3% retention) after 10, 000 charge/discharge cycles (Fig. 7 and Fig. S5 in Supporting information), suggesting an excellent cycle stability; While MnO2(36%)/N-UCNs and MnO2(83%)/N-UCNs have a capacitance retention of 87.9 and 78.0%, respectively.

|
Download:
|
| Fig. 7. Cycle stability of MnO2/N-UCNs electrodes at 2.0 A/g in 1.0 mol/L Na2SO4 aqueous solution. | |
3. Conclusion
In conclusion, we demonstrate a highly efficient fabrication of MnO2/N-UCNs through a simple and template-free polymerization of resorcinol/formaldehyde on phloroglucinol/terephthalaldehyde colloids with the presence of hexamethylenetetramine, followed by carbonization and then a redox reaction between KMnO4 and carbons. The resultant MnO2/N-UCNs nanostructure combines the advantages of regular geometry (~40 nm in diameter) and ultramicropores, high surface area, nitrogen functional heteroatoms, and high content of MnO2. With a suitable doping content of MnO2 (57 wt.%), the obtained MnO2(57%)/N-UCNs have a surface area of 341 m2/g, regular ultramicropores of 0.50 nm, and nitrogen heteroatom (1.20 at.%), and achieve an optimum balance between pseudocapacitance and double layer capacitance due to the synergistic effect of N-UCNs and MnO2. As a result, MnO2(57%)/ N-UCNs electrode shows a high specific capacitance of 401 F/g at 1.0 A/g in 1.0 mol/L Na2SO4 electrolyte solution. Besides, the electrode exhibits good rate capability and excellent cycling stability (86.3% retention at 2.0 A/g after 10, 000 cycles). Highperformance MnO2/N-UCNs electrode material synthesized by a simple and efficient approach provides new opportunities for advanced energy storage.
4. ExperimentalAll reagents used in this experiment are of analytical grade. NUCNs were fabricated using an extended Stöber method [22] with some modifications. Typically, 0.126 g phloroglncinol and 0.10 g terephthalaldehyde were mixed in 56 mL distilled water and further stirred at 70 ℃ for 1 h, followed by 0.33 g resorcinol adding into the mixture and stirring for 30 min. Then, 0.0939 g HMTA and 0.3 mL formaldehyde solution (37 wt. %) were added and stirred for 24 h at 35 ℃. After a hydrothermal process in an autoclave (100 ℃ for 24 h), polymer nanospheres were obtained by filtering, washing and drying. The polymer nanospheres were carbonized at 850 ℃ (5 ℃/min) for 4 h in N2 atmosphere flow to obtain N-UCNs. 100 mg of as-prepared N-UCNs were dispersed into 35 mL of KMnO4 aqueous solution (0.01-0.05 mol/L) under ultrasonication for 60 min. Then, the suspension was transferred to an autoclave and maintained at 180 ℃ for 1 h. Finally, MnO2/N-UCNs were obtained by centrifugation and cleaned by three centrifugation/ washing/re-dispersion cycles in water, followed by drying. The resultant MnO2/N-UCNs were denoted as MnO2(x%)/N-UCNs in which x% represents the mass percentage of MnO2 in the hybrids.
Thermogravimetric analysis (TGA) was achieved by using a STA409 PC instrument with a heating rate of 5 ℃/min in air. Scanning electron microscopy (SEM) images were obtained using a Hitachi S-4800 equipment. AJEM-2100 equipment operated at 200 kV was used to obtain the transmission electron microscopy (TEM) images of samples. X-ray photoelectron spectroscopy (XPS) spectra (Kratos Axis Ultra DLD) were employed using monochromatic Al Kα X-rays. Power X-ray diffraction (XRD) datum were collected on a Focus D8 Advance diffractometer (Bruker, Germany) with Cu Kα radiation (40 kV, λ = 0.154 nm) source. N2 adsorption-desorption isotherms were obtained using the Micromeritics ASAP 2460 gas adsorption analyzer at -196 ℃. The Brumauer-Emmett-Teller (BET) method was used to calculate the specific surface area of samples. And the micropore volume was achieved from a Dubinin-Radushkevich plot. Nonlocal density functional theory (NLDFT) was adopted to obtain pore size distribution of samples.The electrochemical performance (electrochemical impedance spectroscopy (EIS), cyclic voltammetry (CV), galvanostatic charge/ discharge (GCD), and life cyclability) of MnO2/N-UCNs was measured by a conventional three-electrode system in a 1.0 mol/L Na2SO4 aqueous solution on a CHI660D workstation. A Pt wire and Ag/AgCl electrodes were served as the counter electrode and reference electrode, respectively. The working electrode was fabricated as follows: MnO2/N-UCNs, graphite and polytetrafluoroethylene graphite (PTFE, 60 wt.%) were mixed in a mass ratio of 8:1:1 and dispersed in ethanol to form a slurry. Then, the slurry was pressed into a nickel foam at 20 MPa to obtain an electrode, followed by dried at an oven (>100 ℃) for more than 24 h. The typical mass loading of the electrode materials was about 2.1 mg/cm2. The potential window of CV and GCD measurement were 0-1 V.The specific capacitance (Cm, F/g) of the electrode was calculated from GCD curves using the equation of C = I△t/(△Vm), where C represents the calculated capacitance (F); m represents the mass of electroactive material (g); t is the discharge time (s); and △V represents the potential window of the charge/discharge process (Ⅴ).
AcknowledgmentThis work was financially supported by the National Natural Science Foundation of China (Nos. 21273162, 21473122, 21501135), the Science and Technology of Shanghai Municipality, China (No.14DZ2261100), the Fundamental Research Funds for the Central Universities, and the Large Equipment Test Foundation of Tongji University.
Appendix A. Supplementary dataSupplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.cclet.2017.04.007.
| [1] |
(a) M. Liu, X. Ma, L. Gan, et al. , A facile synthesis of a novel mesoporous Ge@C sphere anode with stable and high capacity for lithium ion batteries, J. Mater. Chem. 2(2014) 17107-17114 A; (b) H. Li, L. Jiang, Q. Cheng, et al. , MnO2 nanoflakes/hierarchical porous carbon nanocomposites for high-performance supercapacitor electrodes, Electrochim. Acta 164(2015) 252-259; (c) M. Wang, Y. Xu, Design and construction of three-dimensional graphene/conducting polymer for supercapacitors, Chin. Chem. Lett. 27(2016) 1437-1444; (d) F. Zhang, L. Qi, Recent progress in self-supported metal oxide nanoarray electrodes for advanced lithium-ion batteries, Adv. Sci. 3(2016) 1600049; (e) Y. Xiao, C. Long, M. Zheng, et al. , High-capacity porous carbons prepared by KOH activation of activated carbon for supercapacitors, Chin. Chem. Lett. 25(2014) 865-868; (f) G. Y. Zeng, H. Wang, J. Guo, et al. , Fabrication of Nb2O5/C nanocomposites as a high performance anode for lithium ion battery, Chin. Chem. Lett. 28(2017) 755-758; (g) H. Wang, S. Dou, S. Wang, et al. , Synthesis of electrocatalytically functional carbon honeycombs through cooking with molecule precursors, Int. J. Hydrogen Energy 42(2017) 6472-6481. |
| [2] |
(a) L. Zhang, X. Zhao, Carbon-based materials as supercapacitor electrodes, Chem. Soc. Rev. 38(2009) 2520-2531; (b) X. Wang, G. Shi, Flexible graphene devices related to energy conversion and storage, Energy Environ. Sci. 8(2015) 790-823; (c) Y. Dai, H. Jiang, Y. Hu, et al. , Controlled synthesis of ultrathin hollow mesoporous carbon nanospheres for supercapacitor applications, Ind. Eng. Chem. Res. 53(2014) 3125-3130; (d) M. Liu, L. Chen, D. Zhu, et al. , Zinc tartrate oriented hydrothermal synthesis of microporous carbons for high performance supercapacitor electrodes, Chin. Chem. Lett. 27(2016) 399-404; (e) Z. Xu, J. Wang, Z. Hu, et al. , Structure evolutions and high electrochemical performances of carbon aerogels prepared from the pyrolysis of phenolic resin gels containing ZnCl2, Electrochim. Acta 231(2017) 601-608. |
| [3] |
(a) H. Chang, H. Wu, Graphene-based nanocomposites: preparation, functionalization, and energy and environmental applications, Energy Environ. Sci. 6(2013) 3483-3507; (b) K. Pinkert, L. Giebeler, M. Herklotz, et al. , Functionalised porous nanocomposites: a multidisciplinary approach to investigate designed structures for supercapacitor applications, J. Mater. Chem. A 1(2013) 4904-4910. |
| [4] |
(a) M. Liu, L. Gan, W. Xiong, et al. , Development of MnO2/porous carbon microspheres with a partially graphitic structure for high performance supercapacitor electrodes, J. Mater. Chem. A 2(2014) 2555-2562; (b) H. Yu, J. Wu, L. Fan, et al. , An efficient redox-mediated organic electrolyte for high-energy supercapacitor, J. Power Sources 248(2014) 1123-1126; (c) M. Liu, M. Shi, D. Zhu, et al. , Core-shell reduced graphene oxide/MnOx@carbon hollow nanospheres for high performance supercapacitor electrodes, Chem. Eng. J. 313(2017) 518-526. |
| [5] | G. Wang, L. Zhang, J. Zhang. A review of electrode materials for electrochemical supercapacitors. ChemInform 41(2012)797–828. |
| [6] |
(a) L. Zhang, X. Zhao, Carbon-based materials as supercapacitor electrodes, Chem. Soc. Rev. 38(2009) 2520-2531; (b) W. Qu, Y. Xu, A. Lu, et al. , Converting biowaste corncob residue into high value added porous carbon for supercapacitor electrodes, Bioresour. Technol. 189(2015) 285-291. |
| [7] |
(a) D. Qu, S. Hang, Studies of activated carbons used in double-layer capacitors, J. Power Sources 74(1998) 99-107; (b) M. Liu, J. Qian, Y. Zhao, et al. , Core-shell ultramicroporous@microporous carbon nanospheres as advanced supercapacitor electrodes, J. Mater. Chem. A 3(2015) 11517-11526; (c) D. Zhu, Y. Wang, W. Lu, et al. , A novel synthesis of hierarchical porous carbons from interpenetrating polymer networks for high performance supercapacitor electrodes, Carbon 111(2016) 667-674. |
| [8] | E. Frackowiak, F. Béguin, Carbon materials for the electrochemical storage of energy in capacitors Carbon 39(2001) 937-950. |
| [9] |
(a) J. Chmiola, G. Yushin, Y. Gogotsi, et al. , Anomalous increase in carbon capacitance at pore sizes less than 1 nanometer, Science 313(2006) 1760-1763; (b) C. Vix-Guterl, E. Frackowiak, K. Jurewicz, et al. , Electrochemical energy storage in ordered porous carbon materials, Carbon 43(2005) 1293-1302. |
| [10] | U. Jeong, Y.L. Wang, M. Ibisate, et al., Some new developments in the synthesis, functionalization, and utilization of monodisperse colloidal spheres. ChemInform 15(2006)1907–1921. |
| [11] |
(a) H. K. Jeong, M. Jin, E. J. Ra, et al. , Enhanced electric double layer capacitance of graphite oxide intercalated by poly(sodium 4-styrensulfonate) with high cycle stability, ACS Nano 4(2010) 1162-1166; (b) D. Wang, F. Li, Z. Chen, et al. , Synthesis and electrochemical property of boron-doped mesoporous carbon in supercapacitor, Chem. Mater. 20(2008) 7195-7200; (c) Q. Shi, R. Zhang, Y. Lv, et al. , Nitrogen-doped ordered mesoporous carbons based on cyanamide as the dopant for supercapacitor, Carbon 84(2015) 335-346; (d) L. Miao, H. Duan, M. Liu, et al. , Poly(ionic liquid)-derived, N, S-codoped ultramicroporous carbon nanoparticles for supercapacitors, Chem. Eng. J. 317(2017) 651-659; (e) L. Wang, C. Yang, S. Dou, et al. , Nitrogen-doped hierarchically porouscarbon networks: synthesis and applications in lithium-ion battery, sodium-ion battery and zinc-air battery, Electrochim. Acta 219(2016) 592-603. |
| [12] |
(a) C. D. Lokhande, D. P. Dubal, O. S. Joo, Metal oxide thin film based supercapacitors, Curr. Appl. Phys. 11(2011) 255-270; (b) R. Bi, X. Wu, F. Cao, et al. , Highly dispersed RuO2 nanoparticles on carbon nanotubes: facile synthesis and enhanced supercapacitance performance, J. Phys. Chem. C 114(2010) 2448-2451. |
| [13] |
(a) J. Ge, H. Yao, W. Hu, et al. , Facile dip coating processed graphene/MnO2, nanostructured sponges as high performance supercapacitor electrodes Nano Energy 2(2013) 505-513; (b) P. Wang, Y. Zhao, L. Wen, et al. , Ultrasound-microwave-assisted synthesis of MnO2 supercapacitor electrode materials, Ind. Eng. Chem. Res. 53(2014) 20116-20123. |
| [14] |
(a) S. Li, C. Wang, Design and synthesis of hierarchically porous MnO2/carbon hybrids for high performance electrochemical capacitors, J. Colloid Interface Sci. 438(2015) 61-67; (b) Z. Lei, J. Zhang, X. Zhao, Ultrathin MnO2 nanofibers grown on graphitic carbon spheres as high-performance asymmetric supercapacitor electrodes, J. Mater. Chem. 22(2011) 153-160. |
| [15] |
(a) L. Li, R. Li, S. Gai, et al. , MnO2 nanosheets grown on nitrogen-doped hollow carbon shells as a high-performance electrode for asymmetric supercapacitors, Chem. Eur. J. 21(2015) 7119-7126; (b) M. Yang, B. G. Choi, Rapid one-step synthesis of conductive and porous MnO2/graphene nanocomposite for high performance supercapacitors, J. Electroanal. Chem. 776(2016) 134-138; (c) Y. Liu, X. Cai, B. Luo, et al. , MnO2 decorated on carbon sphere intercalated graphene film for high-performance supercapacitor electrodes, Carbon 107(2016) 426-432. |
| [16] |
(a) H. Xu, Z. Qu, C. Zong, et al. , MnOx/graphene for the catalytic oxidation and adsorption of elemental mercury, Environ. Sci. Technol. 49(2015) 6823-6830; (b) Y. Zhao, Y. Meng, J. Peng, Carbon@MnO2 core-shell nanospheres for flexible high-performance supercapacitor electrode materials, J. Power Sources 259(2014) 219-226. |
| [17] | X. Feng, Z. Yan, N. Chen, et al., The synthesis of shape-controlled MnO2/graphene composites via a facile one-step hydrothermal method and their application in supercapacitors. J. Mater. Chem. A 1(2013)12818–12825. DOI:10.1039/c3ta12780j |
| [18] |
(a) S. Chen, J. Zhu, X. Wu, et al. , Graphene oxide-MnO2 nanocomposites for supercapacitors, ACS Nano 4(2010) 2822-2830; (b) D. Portehault, S. Cassaignon, E. Baudrin, et al. , Morphology control of cryptomelane type MnO2 nanowires by soft chemistry. growth mechanisms in aqueous medium, Chem. Mater. 19(2007) 5410-5417. |
| [19] | Y. Li, G. Wang, T. Wei, et al., Nitrogen and sulfur co-doped porous carbon nanosheets derived from willow catkin for supercapacitors. Nano Energy 19(2015)165–175. |
| [20] | D. Zhu, K. Cheng, Y. Wang, et al., Nitrogen-doped porous carbons with nanofiber-like structure derived from poly (aniline-co-p-phenylenediamine) for supercapacitors. Electrochim. Acta 224(2017)17–24. DOI:10.1016/j.electacta.2016.12.023 |
| [21] |
(a) Y. He, W. Chen, J. Zhou, et al. , Constructed uninterrupted charge-transfer pathways in three-dimensional micro/nanointerconnected carbon-based electrodes for high energy-density ultralight flexible supercapacitors, ACS Appl. Mater. Interfaces 6(2013) 210-218; (b) M. Liu, W. W. Tjiu, J. Pan, et al. , One-step synthesis of graphene nanoribbon-MnO2 hybrids and their all-solid-state asymmetric supercapacitors, Nanoscale 6(2014) 4233-4242. |
| [22] |
(a) J. H. Kim, D. Bhattacharjya, J. S. Yu, Synthesis of hollow TiO2@N-doped carbonwith enhanced electrochemical capacitance byan in situ hydrothermal process using hexamethylenetetramine, J. Mater. Chem. A 2(2014) 11472-11479; (b) W. Lu, M. Liu, L. Miao, et al. , Nitrogen-containing ultramicroporous carbon nanospheres for high performance supercapacitor electrodes, Electrochim. Acta 205(2016) 132-141; (c) Z. Xu, Q. Guo, A simple method to prepare monodisperse and size-tunable carbon nanospheres from phenolic resin, Carbon 52(2013) 464-467. |
 2017, Vol. 28
2017, Vol. 28