b School of Chemistry and Chemical Engineering, Southeast University, Nanjing 210098, China;
c Department of Electronic Engineering, Southeast University Chengxian Colleage, Nanjing 210088, China
The photoelectrochemical (PEC) activity of nanostructured semiconductors has been intensely studied in designing solar cells and optoelectronic devices for solar hydrogen production, environmental remediation and sensors [1-5]. Carbon nanomaterials are one of the most credible materials for solar cells since they capitalize on low-cost, high-efficiency and lightweight [6, 7].However, the applications of graphene-based carbon nanomaterials are still limited due to the absence of an energy band gap. As a result, doping graphene-based materials with heteroatoms has come to be a popular solution to regulate the electronic and molecular structures towards applications in photo-energy conversion [8-12].
The two-dimensional graphite-phase polymeric carbon nitride (denoted as GPPCN), one kind of doped carbon materials, can be taken as doped graphene where some carbon atoms are replaced by nitrogen atoms. As one kind of semiconducting materials, GPPCN not only keeps the merits of traditional carbon materials such as high chemical and thermal stability, but also exhibits promising semiconducting properties for photo-energy conversion [13-22]. Despite the initial success of exploring the PEC activity of GPPCN, the effort to further improve the performance has not met with breakthroughs. A major problem in maintaining higher photo-conversion efficiency is the low electron transport rate across the particle network and subsequent high recombination rate of photo-induced electrons and holes of GPPCN [23].
To improve the photo-energy conversion activity of GPPCN, various strategies such as templating and element doping methods have been developed [24-31]. Among them, one promising strategy is to construct p-n heterojunction that promotes the separation of photo-induced electrons and holes by coupling GPPCN with other semiconductors [32-36]. To select proper components for the p-n heterojunction construction, suitable conduction and valance band levels would be a vital factor to be considered [37, 38]. As a classical semiconductor, TiO2 has been extensively investigated as a photocatalyst due to the high stability, low cost and nontoxicity [39-43]. When TiO2 and GPPCN contact, a heterojunction interface would be formed in which the conduction band (CB) and valance band (VB) positions are staggered between the two semiconductors. Upon considering the conduction and valance band levels of GPPCN (~-1.13 eV and ~+ 1.73 eV) and TiO2 (~-0.3 eV and ~+ 2.98 eV) [14], the photoinduced electrons of GPPCN could move to the CB of TiO2 and the photo-generated holes of TiO2 could move to the VB of GPPCN upon light irradiation, thereby promoting charge carrier separation and transportation in the heterojunction microstructure [44-46]. Moreover, the UVactive TiO2 combined with the visible light-active GPPCN will extend the light absorption of the final GPPCN/TiO2 composite [47].In this sense, precise controlling the microstructures of GPPCN/ TiO2 is crucial for the heterojunction construction with low recombination of electron-hole pairs, which presents a potential challenge for coupling GPPCN and TiO2 as a photocatalyst. Although many methods have been reported for the synthesis of GPPCN/TiO2 composites, e.g., electrospinning and heat-etching, ball milling and thermal transformation, it is still challenging to develop a facile and effective strategy to produce a GPPCN/TiO2 heterojunction [44, 48, 49].
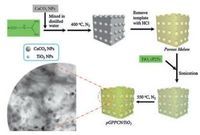
Herein, we reported the successful synthesis of GPPCN/TiO2 donor-acceptor heterojunction through a facile combination of a template technique with co-calcination process (Fig. 1). After the synthesis of porous melem by using a green template, calcium carbonate, the TiO2 nanoparticles were further assembled with porous melem and the assembly was co-calcined to form porous GPPCN/TiO2 (pGPPCN/TiO2) heterojunction. By optimizing the mass ratio of porous melem and TiO2, the light absorption and photo-energy conversion activity of pGPPCN/TiO2 nanocomposite could be largely improved. The present work would open a new avenue for the design and synthesis of various photocatalysts with high efficiency for applications in energy fields.
|
Download:
|
| Fig. 1. The preparation processes of the porous pGPPCN/TiO2 composite | |
2. Results and discussion
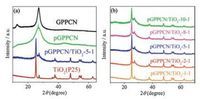
The successful preparation of pGPPCN/TiO2-m-n was confirmed by XRD analysis. As shown in Fig. 2a, both GPPCN and pGPPCN had two characteristic diffraction peaks at 12.9° and 27.3°, respectively. Generally, the peak at 27.3° could be indexed as the (002) diffraction of graphitic materials, which was a characteristic interlayer stacking peak of the GPPCN layers. Moreover, in the pGPPCN and pGPPCN/TiO2 composites, the peak at 12.9° looks depressed compared to that of GPPCN. The possible reason is that the increase of the surface area and pore structures of GPPCN would cause more defects. At the same time, it was found that TiO2 also has a small diffraction peak at 27.5°, but it is much sharper than that of GPPCN, indicative of a higher crystallinity. Nevertheless, it was found that the diffraction peak around 27.3° kept both the characteristics of GPPCN and TiO2, with a broad peak and sharp peak, suggesting that the introducing TiO2 did not change the typical structure of GPPCN. When the ratio of porous melem and TiO2 increased, the diffraction peak at 27.3° became stronger and broader, indicating a successive increase of the amount of GPPCN in the composite (Fig. 2b).Moreover, the characteristic diffraction peaks of TiO2 in the pGPPCN/TiO2 composite did not change, compared to that of pure TiO2, only with the peak at 27.5° becoming slight broader, due to the co-existence of a lower crystallinity. Therefore, the primary crystal structures of GPPCN and TiO2 were retained in the pGPPCN/ TiO2 composite.
|
Download:
|
| Fig. 2. XRD of GPPCN, pGPPCN, pGPPCN/TiO2-5-1 and TiO2 (a), and pGPPCN/TiO2 with different weight ratio of porous melem and TiO2 (b). | |
The formation of pGPPCN/TiO2 composites could be further confirmed by SEM (Fig. 3) images. As shown in Fig. 3f, the bulk GPPCN shows lamellar structures with sizes of around several micrometers. In contrast, there was evident difference for the morphology of the pGPPCN/TiO2 composites. When the w/w ratio of pGPPCN to TiO2 was low, large amount of TiO2 particles could be found both on the surface of the pGPPCN. With the increasing of the pGPPCN/TiO2 ratio, more pores of pGPPCN were exposed and TiO2 particles could be found both on the surface and inside the pores of pGPPCN. The surface morphology change of pGPPCN/TiO2-m-n was further confirmed by TEM and HRTEM. As shown in Fig. 4a, for the pGPPCN/TiO2-5-1, many TiO2 particles uniformly distributed both on the surface and in the pores of pGPPCN, while the morphology of pGPPCN in the composite is similar with that of pure pGPPCN (Fig. 4d). The X-ray energy dispersive spectrometer (EDS) analysis implies the presence of Ti in the composite. For example, in pGPPCN-TiO2-5-1, the of N, O, C and Ti was about 51.6%, 36.0%, 10.1% and 2.3%, respectively. The EDS mapping images (Fig. 4c) further show that the TiO2 uniformly dispersed in the pGPPCN, which may facilitate the formation of the micro/nanostructured pGPPCN/TiO2 heterojunctions. Moreover, some lattice fringes could be found in the pGPPCN/TiO2 with the lattice distance at ~0.35 nm (Fig. 4b), which was similar with that of anatase TiO2 (101), suggesting that the crystal texture was from TiO2 in the composite, but not from pGPPCN. More interestingly, obvious heterojunctions could be found at the interfaces of pGPPCN and TiO2 (Fig. 4b), which may be of significance for promoting charge carrier separation and transportation in the pGPPCN/TiO2 composite. Fig. 4d shows the nitrogen adsorption-desorption isotherms of the pGPPCN/TiO2 and bulk GPPCN (see summaries in Table 1). The BET surface area increased to ~4 times (38 m2/ g) of that of bulk GPPCN (10 m2/g) when the m-n is 10-1. With the increasing of the w/w ratio of pGPPCN and TiO2, the BET surface area increased gradually, and it changed to 56 m2/g when m-n was 1-1, which was higher than those of other carbon nitride/TiO2 nanocomposites prepared by thermal transformation methodology or electrospinning process [44, 48]. Therefore, the surface morphology and surface area could be regulated by the w/w ratio of pGPPCN and TiO2 in the pGPPCN/TiO2-m-n composites.

|
Download:
|
| Fig. 3. SEM images of pGPPCN/TiO2-m-n (a-e) with m-n = 1-1, 2-1, 5-1, 8-1, 10-1, respectively and of bulk GPPCN (f). | |

|
Download:
|
| Fig. 4. TEM images (a), HRTEM (b) and EDS mapping (c) of pGPPCN/TiO2-5-1, TEM images of pGPPCN (d), and the nitrogen adsorption-desorption isotherms (e) of GPPCN, pGPPCN and pGPPCN/TiO2-m-n. | |
|
|
Table 1 Summary of surface area and photocurrents of GPPCN, pGPPCN/TiO2-m-n and pGPPCN. |
The FT-IR spectra in Fig. 5a further revealed the chemical structures of pGPPCN/TiO2 composites and bulk GPPCN. The peaks at 809 cm-1 and 1200-1700 cm-1 could be ascribed to the breathing mode of the tri-s-triazine unites and the aromatic C-N heterocycle stretches, which were observed for all samples. Therefore, it could be concluded the pristine conjugated framework of bulk GPPCN was well maintained after the formation of pGPPCN/TiO2 composites. As shown in the UV-vis absorption spectra (Fig. 5b), the absorption of bulk GPPCN was from the UV region to the visible region up to ~460 nm. The UV-vis absorption edge of TiO2 was less than 420 nm, which was in the UV region. Compared with that of TiO2, the UV-vis absorption edges of all the pGPPCN/TiO2 composites were red-shifted, which would result in the enhanced absorption of the composites in the visible region. As shown in Fig. 5c, the band gap of pGPPCN/TiO2-5-1 was about 2.91 eV, which is between that of GPPCN (2.86 eV) and TiO2 (3.28 eV), indicating that the electronic structure of pGPPCN changed after complexing with TiO2.

|
Download:
|
| Fig. 5. FT-IR (a) and UV-vis (b) of bulk GPPCN, pGPPCN and pGPPCN/TiO2-m-n, and the UV-vis data of TiO2, pGPPCN and pGPPCN/TiO2-5-1 used for the determination of band gap (c). | |
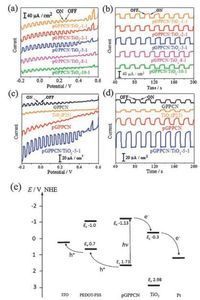
In order to investigate the photoelectrochemical activity of these materials, a standard photoelectrochemical cell was constructed by using a three-electrode configuration in an aqueous solution containing 0.1 mol/L KCl. It was observed that all the cathodic photoresponses of pGPPCN/TiO2-m-n (m-n = 1-1, 2-1, 5-1, 8-1, 10-1), GPPCN and pGPPCN gradually increased when the biased potential became more negative (Fig. 6a, c). Moreover, the photocurrents were prompt, steady, and reproducible during repeated on/off cycles under visible light irradiation (Fig. 6b, d, see summaries in Table 1). Upon changing the w/w ratio of the pGPPCN to TiO2, the photocurrent became largest when m-n was 5-1 (Fig. 6a, b), which was several ten times higher than that of carbon nitride/TiO2 composite prepared via ball milling method [49].
|
Download:
|
| Fig. 6. I-V curve of pGPPCN/TiO2-m-n (m-n=1-1, 2-1, 5-1, 8-1 and 10-1) (a) and GPPCN, TiO2, pGPPCN and pGPPCN/TiO2-5-1 (c) in 0.1mol/L KCl aqueous solution under chopped visible light, and the transient current response of pGPPCN/TiO2-m-n (m-n=1-1, 2-1, 5-1, 8-1 and 10-1) (b) and GPPCN, TiO2, pGPPCN and pGPPCN/TiO2-5-1 (d) in 0.1mol/L KCl biased at -0.2V; (e) the proposed mechanism for the photoinduced charge transfer in the pGPPCN/TiO2 composite. | |
In general, several factors such as surface area, crystallinity, pore size and morphology could influence the photoelectrochemical activity of the photoactive materials. Based on the SEM (Fig. 3) and UV-vis (Fig. 5b) results, with increasing the w/w ratio of pGPPCN and TiO2, the exposed area of pGPPCN enhanced, leading to the increased absorption in the visible region. Fig. 6e shows the possible mechanism for the enhanced photoelectric activity for pGPPCN/TiO2 composite. For the single component carbon nitride material, GPPCN and pGPPCN could be activated under 420nm visible light irradiation and generated electrons and holes. However, the photogenerated electron-hole pairs recombined easily due to the narrow energy gap, which resulted in a relative lowactivity. Moreover, since thereis noabsorptioninvisibleregion for TiO2, leading to a weak photoelectric activity under visible light irradiation. For pGPPCN/TiO2 composite with an optimized pGPPCN content, i.e. pGPPCN/TiO2-5-1, resulting in the formation of the heterostructure between pGPPCN and TiO2. Electrons on pGPPCN could transfer easily to CB of TiO2 through the intimate interface because the conduction edge potential of pGPPCN (-1.13eV vs. NHE) was lower than that of TiO2 (-0.3eV vs. NHE).
As a result, under light irradiation, more electrons and holes appeared in the composite of pGPPCN-TiO2-5-1, and the electrons transferred more quickly from the conduction band (CB) of pGPPCN to valent band (VB) of TiO2, which in turn generated higher photocurrent (see the mechanism Scheme in Fig. 6e). When further increasing theratioof pGPPCN and TiO2, the conductivityof the composite decreased due to the reduced amount of TiO2, hence, the amount of photo-induced electron transferred from pGPPCN to TiO2 reduced. Thus, upon engineering the w/w ratio of pGPPCN and TiO2, pGPPCN/TiO2-5-1 was found to be the best for photocurrent generation among all the as-prepared pGPPCN/TiO2-m-n composites. As shown in Fig. 6d, the photocurrent of pGPPCN/ TiO2-5-1 was about 2.5 times that of pGPPCN, 5.3 times that of bulk GPPCNand 8 times thatof TiO2 when biased at -0.2V, respectively.
3. ConclusionIn summary, we have demonstrated a promising strategy for fabricating a pGPPCN/TiO2 nanocomposite. By using CaCO3 as a template, porous melem could be facilely synthesized, where the template was gently removed with diluted hydrochloride. The assemblyand subsequent co-calcination of porous melem and TiO2 resulted in the uniform dispersion of TiO2 in the porous GPPCN. The pGPPCN/TiO2 showed enhanced surface area and light absorption compared to GPPCN and pGPPCN. More importantly, thepGPPCN/TiO2 presentedhigh photo-energyconversion activity, which can be ascribed to the formation of the donor-acceptor heterojunction microstructures in the nanocomposite. This work demonstrated a new route for designing and constructing heterojunction photoelectrodes for practical photo-energy conversion applications.
4. Experimental 4.1. Synthesis of porous melem and pGPPCN/TiO2 compositesThe CaCO3 particles (340nm of diameter) were heated in a muffle furnace in air at 550 ℃ for 12h to remove organic species on the surfaces. Porous melem was synthesized by using CaCO3 as a template [31]. Briefly, DCDA (10g), CaCO3 (2.5g) were stirred in a glassy beaker with 50mL of water for 4h at room temperature. Then the solid mixture of DCDA and CaCO3 was obtained by centrifugation and washed with EtOH, and heated at 60 ℃ in vacuum for 12h. Subsequently, the as-obtained solids were calcined in N2 at 400 ℃ for 4h at a heating rate of 2.3 ℃/min. Afterwards, the solid was put in an agate mortar and ground into fine powders, followed by reacting with hydrochloric acid solution (1.0mol/L) for 12h to remove the CaCO3 templates, and washing with sufficient water and ethanol. Finally, the porous melem was obtained by heating the as-obtained solid in vacuum at 60 ℃ for 12h.
Different amount of porous melem and TiO2 (P25, Degussa AG, Germany) (the mass ratio of porous melem and P25 was 1:1, 2:1, 5:1, 8:1 and 10:1, respectively) were sonicated for 4h. Then the mixture was centrifuged, washed with ethanol, and dried at 60 ℃ in vacuum for 12h. The solids were grinded and calcined in N2 at 550 ℃ for 4h at a heating rate of 2.3 ℃/min. The as-obtained porous GPPCN/TiO2 was denoted as pGPPCN/TiO2-m-n, where the m-n was the mass ratio of porous melem and TiO2.
As control experiments, pGPPCN was prepared by heating the porous melem in N2 at 550 ℃ for 4h at a heating rate of 2.3 ℃/min, and the conventional bulk GPPCN was synthesized bycalcining the DCDA in N2 at 550 ℃ for 4h at a heating rate of 2.3 ℃/min.
4.2. CharacterizationScanning electron microscopy (SEM) images were recorded bya Phenom ProX scanning electron microscope (The Netherlands), coupled with an X-ray energy dispersive spectrometer (EDS), and transmission electron microscopy (TEM) images were obtained from a JEOL-JEM-2100 microscope. Brunauer-Emmett-Teller (BET) surface area was calculated from N2 adsorption-desorption isotherms collected at 77K using a Nova 1200e (Quantachrome, USA). Fourier transform infrared spectra (FT-IR) was obtained by using a Nicolet 4700 FT-IR spectrometer (Thermo, USA) equipped with an attenuated total reflection (ATR) setup. X-ray diffraction (XRD) analysis was performed on a XPERT-PRO diffractometer (PANalytical, the Netherlands). Ultraviolet-visible (UV-vis) absorption spectra were taken with a Cary 100 UV-visible spectrophotometer (Agilent, USA) using BaSO4 as a reference.
4.3. Photoelectrochemical experimentsThe photoelectrodes consisting of pGPPCN/TiO2-m-n, bulk GPPCN, and TiO2 were prepared by spreading aqueous slurries of pGPPCN/TiO2-m-n, bulk GPPCN or TiO2 over 0.49 cm2 the ITO glass substrates ( < 7Ω/cm) with a glass rod by using adhesive tape as spaces. Suspensions were obtained by grinding 10 mg of bulk pGPPCN/TiO2-m-n, bulk GPPCN or TiO2 powder with 30mL of water and 30μL of Orgacon PEDOT inkjet ink (PEDOT/PSS, IJ-1005, Agfa-Gevaert N.V, Belgium). Then, the films were annealed in air at 80 ℃ for half an hour. The photoelectrochemical experiments were performed in a three-electrode system with a platinum wire as the counter electrode and an Ag/AgCl (saturated KCl) electrode as the reference electrode. A 150 W Xe lamp (Beijing NBeT Co., Ltd.) was used as the light source with a Schott optical filter GG420 to produce visible light (λ > 420 nm). The photoelectrodes were illuminated from the backside (1000 W/m2).
AcknowledgmentThis work is supported by the National Natural Science Foundation of China (Nos. 21675022, 21305065, 91333110, 31400751), Program from the Natural Science Foundation of Jiangsu Province (No. BK20160028, BK20140622) and the Fundamental Research Funds for the Central Universities.
| [1] | X.G. Yu, T.J. Marks, A. Facchetti. Metal oxides for optoelectronic applications. Nat. Mater. 15(2016)383–396. DOI:10.1038/nmat4599 |
| [2] | A. Polman, M. Knight, E.C. Garnett, B. Ehrler, W.C. Sinke. Photovoltaic materials:present efficiencies and future challenges. Science 352(2016)307–317. |
| [3] | Z. Zhou, Q. Shang, Y. Shen, et al., Chemically modulated carbon nitride nanosheets for highly selective electrochemiluminescent detection of multiple metal-ions. Anal. Chem. 88(2016)6004–6010. DOI:10.1021/acs.analchem.6b01062 |
| [4] | Q. Shang, Z. Zhou, Y. Shen, et al., Potential-modulated electrochemiluminescence of carbon nitride nanosheets for dual-signal sensing of metal ions. ACS Appl. Mater. Interfaces 7(2015)23672–23678. DOI:10.1021/acsami.5b07405 |
| [5] | J.Q. Tian, Q. Liu, A.M. Asiri, Al-Youbi A.O., X.P. Sun. Ultrathin graphitic carbon nitride nanosheet:a highly efficient fluorosensor for rapid ultrasensitive detection of Cu2+. Anal. Chem. 85(2013)5595–5599. DOI:10.1021/ac400924j |
| [6] | Y.F. Shen, T. Nakanishi. Fullerene assemblies toward photo-energy conversions. Phys. Chem. Chem. Phys. 16(2014)7199–7204. DOI:10.1039/C4CP00221K |
| [7] | Y.F. Shen, A. Saeki, S. Seki, et al., Exfoliation of graphene and assembly formation with alkylated-C-60:a nanocarbon hybrid towards photo-energy conversion electrode devices. Adv. Opt. Mater. 3(2015)925–930. DOI:10.1002/adom.201400619 |
| [8] | Y.J. Zhang, T. Mori, J.H. Ye, M. Antonietti. Phosphorus-doped carbon nitride solid:enhanced electrical conductivity and photocurrent generation. J. Am. Chem. Soc. 132(2010)6294–6295. DOI:10.1021/ja101749y |
| [9] | Y. Wang, J.S. Zhang, X.C. Wang, M. Antonietti, H.R. Li. Boron-and fluorinecontaining mesoporous carbon nitride polymers:metal-free catalysts for cyclohexane oxidation. Angew. Chem. Int. Ed. 49(2010)3356–3359. DOI:10.1002/anie.201000120 |
| [10] | P. Niu, L.C. Yin, Y.Q. Yang, G. Liu, H.M. Cheng. Increasing the visible light absorption of graphitic carbon nitride (melon) photocatalysts by homogeneous self-modification with nitrogen vacancies. Adv. Mater. 26(2014)8046–8052. DOI:10.1002/adma.v26.47 |
| [11] | Z.G. Mou, Y.J. Wu, J.H. Sun, et al., TiO2 nanoparticles-functionalized N-doped graphene with superior interfacial contact and enhanced charge separation for photocatalytic hydrogen generation. ACS Appl. Mater. Interfaces 6(2014)13798–13806. DOI:10.1021/am503244w |
| [12] | A. Vinu, P. Srinivasu, D.P. Sawant, et al., Three-dimensional cage type mesoporous CN-based hybrid material with very high surface area and pore volume. Chem. Mater. 19(2007)4367–4372. DOI:10.1021/cm070657k |
| [13] | X.C. Wang, K. Maeda, A. Thomas, et al., A metal-free polymeric photocatalyst for hydrogen production from water under visible light. Nat. Mater. 8(2009)76–80. DOI:10.1038/nmat2317 |
| [14] | Y.J. Zhang, M. Antonietti. Photocurrent generation by polymeric carbon nitride solids:an initial step towards a novel photovoltaic system. Chem. Asian J. 5(2010)1307–1311. |
| [15] | Y. Zheng, J. Liu, J. Liang, M. Jaroniec, S.Z. Qiao. Graphitic carbon nitride materials:controllable synthesis and applications in fuel cells and photocatalysis. Energy Environ. Sci. 5(2012)6717–6731. DOI:10.1039/c2ee03479d |
| [16] | S.C. Yan, Z.S. Li, Z.G. Zou. Photodegradation performance of γ-C3N4 fabricated by directly heating melamine. Langmuir 25(2009)10397–10401. DOI:10.1021/la900923z |
| [17] | Q.L. Cui, J.S. Xu, X.Y. Wang, et al., Phenyl-modified carbon nitride quantum dots with distinct photoluminescence behavior. Angew. Chem. Int. Ed. 55(2016)3672–3676. DOI:10.1002/anie.201511217 |
| [18] | M. Shalom, S. Inal, C. Fettkenhauer, D. Neher, M. Antonietti. Improving carbon nitride photocatalysis by supramolecular preorganization of monomers. J. Am. Chem. Soc. 135(2013)7118–7121. DOI:10.1021/ja402521s |
| [19] | S.W. Cao, J.X. Low, J.G. Yu, M. Jaroniec. Polymeric photocatalysts based on graphitic carbon nitride. Adv. Mater. 27(2015)2150–2176. DOI:10.1002/adma.201500033 |
| [20] | W.J. Ong, L.L. Tan, Y.H. Ng, S.T. Yong. Chai S.P.. Graphitic carbon nitride (γ-C3N4)-based photocatalysts for artificial photosynthesis and environmental remediation:are we a step closer to achieving sustainability? Chem. Rev. 116(2016)7159–7329. |
| [21] | Z. Dong, T. Wang, J. Zhao, et al., Catalytic performance of iron oxide loaded on electron-rich surfaces of carbon nitride. J. Energy Chem. 25(2016)1021–1026. DOI:10.1016/j.jechem.2016.10.005 |
| [22] | J.H. Wang, Y.F. Shen, Y. Li, S.Q. Liu, Y.J. Zhang. Crystallinity modulation of layered carbon nitride for enhanced photocatalytic activities. Chem. Eur. J. 22(2016)12449–12454. DOI:10.1002/chem.v22.35 |
| [23] | Q.J. Xiang, J.G. Yu, M. Jaroniec. Preparation and enhanced visible-light photocatalytic H-2-production activity of graphene/C3N4 composites. J. Phys. Chem. C 115(2011)7355–7363. DOI:10.1021/jp200953k |
| [24] | Y.J. Zhang, T. Mori, J.H. Ye, M. Antonietti. Phosphorus-doped carbon nitride solid:enhanced electrical conductivity and photocurrent generation. J. Am. Chem. Soc. 132(2010)6294–6295. DOI:10.1021/ja101749y |
| [25] | N. Sagara, S. Kamimura, T. Tsubota, T. Ohno. Photoelectrochemical CO2 reduction by a p-type boron-doped γ-C3N4 electrode under visible light. Appl. Catal. B-Environ. 192(2016)193–198. DOI:10.1016/j.apcatb.2016.03.055 |
| [26] | Z. Yang, Y.J. Zhang, Z. Schnepp. Soft and hard templating of graphitic carbon nitride. J. Mater. Chem. A 3(2015)14081–14092. DOI:10.1039/C5TA02156A |
| [27] | Y.J. Zhang, A. Thomas, M. Antonietti, X.C. Wang. Activation of carbon nitride solids by protonation:morphology changes, enhanced ionic conductivity, and photoconduction experiments. J. Am. Chem. Soc. 131(2009)50–51. DOI:10.1021/ja808329f |
| [28] | H.J. Yan. Soft-templating synthesis of mesoporous graphitic carbon nitride with enhanced photocatalytic H-2 evolution under visible light. Chem. Commun. 48(2012)3430–3432. DOI:10.1039/c2cc00001f |
| [29] | G. Liu, P. Niu, C.H. Sun, et al., Unique electronic structure induced high photoreactivity of sulfur-doped graphitic C3N4. J. Am. Chem. Soc. 132(2010)11642–11648. DOI:10.1021/ja103798k |
| [30] | D.J. Martin, K.P. Qiu, S.A. Shevlin, et al., Highly efficient photocatalytic H-2 evolution from water using visible light and structure-controlled graphitic carbon nitride. Angew. Chem. Int. Ed. 53(2014)9240–9245. DOI:10.1002/anie.201403375 |
| [31] | J.H. Wang, C. Zhang, Y.F. Shen, et al., Environment-friendly preparation of porous graphite-phase polymeric carbon nitride using calcium carbonate as templates, and enhanced photoelectrochemical activity. J. Mater. Chem. A 3(2015)5126–5131. DOI:10.1039/C4TA06778A |
| [32] | J.G. Hou, C. Yang, H.J. Cheng, et al., High-performance p-Cu2O/n-TaON heterojunction nanorod photoanodes passivated with an ultrathin carbon sheath for photoelectrochemical water splitting. Energy Enviro. Sci. 7(2014)3758–3768. DOI:10.1039/C4EE02403F |
| [33] | J. Yan, Z.G. Chen, H.Y. Ji, et al., Construction of a 2D graphene-like MoS2/C3N4 heterojunction with enhanced visible-light photocatalytic activity and photoelectrochemical activity. Chem. Eur. J. 22(2016)4764–4773. DOI:10.1002/chem.201503660 |
| [34] | X. Chen, Q. Liu, Q.L. Wu, et al., Incorporating graphitic carbon nitride (γ-C3N4) quantum dots into bulk-heterojunction polymer solar cells leads to efficiency enhancement. Adv. Funct. Mater. 26(2016)1719–1728. DOI:10.1002/adfm.v26.11 |
| [35] | C.S. Pan, J. Xu, Y.J. Wang, D. Li, Y.F. Zhu. Dramatic activity of C3N4/BiPO4 photocatalyst with core/shell structure formed by self-assembly. Adv. Funct. Mater. 22(2012)1518–1524. DOI:10.1002/adfm.v22.7 |
| [36] | W. Yu, D. Xu, T. Peng. Enhanced photocatalytic activity of γ-C3N4 for selective CO2 reduction to CH3OH via facile coupling of ZnO:a direct Z-scheme mechanism. J. Mater. Chem. A 3(2015)19936–19947. DOI:10.1039/C5TA05503B |
| [37] | Y. Na, B. Hu, Q.L. Yang, et al., CdS quantum dot sensitized p-type NiO as photocathode with integrated cobaloxime in photoelectrochemical cell for water splitting. Chin. Chem. Lett. 26(2015)141–144. DOI:10.1016/j.cclet.2014.09.011 |
| [38] | Y. Shi, H.Y. Li, L. Wang, W. Shen, H.Z. Chen. Novel α-Fe2O3/CdS cornlike nanorods with enhanced photocatalytic performance. ACS Appl. Mater. Interfaces 4(2012)4800–4806. DOI:10.1021/am3011516 |
| [39] | J. Tian, Z.H. Zhao, A. Kumar, R.I. Boughton, H. Liu. Recent progress in design, synthesis, and applications of one-dimensional TiO2 nanostructured surface heterostructures:a review. Chem. Soc. Rev. 43(2014)6920–6937. DOI:10.1039/C4CS00180J |
| [40] | H. Xu, S.X. Ouyang, L.Q. Liu, et al., Recent advances in TiO2-based photocatalysis. J. Mater. Chem. A 2(2014)12642–12661. DOI:10.1039/C4TA00941J |
| [41] | J.M. Liu, Q.C. Zhang, J.C. Yang, et al., Facile synthesis of carbon-doped mesoporous anatase TiO2 for the enhanced visible-light driven photocatalysis. Chem. Commun. 50(2014)13971–13974. DOI:10.1039/C4CC05544F |
| [42] | W.G. Ma, L.N. Wang, N. Zhang, et al., Biomolecule-free, selective detection of odiphenol and its derivatives with WS2/TiO2-based photoelectrochemical platform. Anal. Chem. 87(2015)4844–4850. DOI:10.1021/acs.analchem.5b00315 |
| [43] | J. Li, Y. Xu, W. Yue, et al., Synthesis and study of Bi2Ti2O7/TiO2 composites as efficient visible-light-active photocatalysts in the reduction of aqueous Cr(Ⅵ). Chin. Chem. Lett. 27(2016)867–870. DOI:10.1016/j.cclet.2016.01.031 |
| [44] | K. Sridharan, E. Jang, T.J. Park. Novel visible light active graphitic C3N4-TiO2 composite photocatalyst:synergistic synthesis growth and photocatalytic treatment of hazardous pollutants. Appl. Catal. B-Environ. 142(2013)718–728. |
| [45] | J. Xu, G. Wang, J. Fan, et al., g-C3N4 modified TiO2 nanosheets with enhanced photoelectric conversion efficiency in dye-sensitized solar cells. J. Power Sources 274(2015)77–84. DOI:10.1016/j.jpowsour.2014.10.033 |
| [46] | J.G. Yu, S.H. Wang, J.X. Low, W. Xiao. Enhanced photocatalytic performance of direct Z-scheme g-C3N4-TiO2 photocatalysts for the decomposition of formaldehyde in air. Phys. Chem. Chem. Phys. 15(2013)16883–16890. DOI:10.1039/c3cp53131g |
| [47] | S. Pany, K.M. Parida. A facile in situ approach to fabricate N, S-TiO2/g-C3N4 nanocomposite with excellent activity for visible light induced water splitting for hydrogen evolution. Phys. Chem. Chem. Phys. 17(2015)8070–8077. DOI:10.1039/C4CP05582A |
| [48] | C. Han, Y.D. Wang, Y.P. Lei, et al., In situ synthesis of graphitic-C3N4 nanosheet hybridized N-doped TiO2 nanofibers for efficient photocatalytic H-2 production and degradation. Nano Res. 8(2015)1199–1209. DOI:10.1007/s12274-014-0600-2 |
| [49] | J.W. Zhou, M. Zhang, Y.F. Zhu. Photocatalytic enhancement of hybrid C3N4/TiO2 prepared via ball milling method. Phys. Chem. Chem. Phys. 17(2015)3647–3652. DOI:10.1039/C4CP05173D |
 2017, Vol. 28
2017, Vol. 28 



