Hydrogen peroxide (H2O2) plays important roles in industrial, pharmaceutical, environment protection and many other fields [1-3]. Therefore, it is of great importance to find fast and accurate methods for sensitive and selective detection of H2O2. So far, many analytical techniques have been developed for the detection of H2O2 concentration, such as fluorescence [4], spectrometry [5], chemiluminescence [6] and electrochemistry [7]. Among these methods, electrochemical sensors attracted much more attention due to its simple, sensitive, fast and cost-efficient detection of H2O2 [8-10]. In the past decades, many attentions have been paid to enzymatic sensors for the detection of H2O2 [11-13]. However, the enzyme-based sensors suffer from some serious disadvantages, such as complex immobilization procedures, limited lifetime and environmental instability [14, 15]. Moreover, enzymes were easily denatured under certain temperature and pH for electrochemical detection conditions, which affected the reproducibility and stability of the sensors and hindered the applications of the sensors. To overcome these drawbacks, a growing number of attentions have been focused on the non-enzymatic sensors. In recent years, various noble metals, including Ag, Au, Pt, and Pd have been reported as alternative electrochemical catalysts to construct non-enzymatic sensors for the detection of H2O2 [16-20]. However, due to the higher cost of noble metals, it is in urgent need of the development of a cost-effective and high-performance catalyst for H2O2 detection.
In recent years, non-noble metal materials such as metal sulfide and metal oxide have been widely used as electrocatalyst for nanoelectrochemistry [21-24]. However, the above-mentioned nanomaterials are faced with several challenges, which may prevent effective catalytic contribution of these nanostructured catalysts: (1) Aggregation, caused by the large hydrodynamic radii and the large surface area of nanoparticles, lowering their catalytic activity [25-27]. (2) Fewer electron transfer passages, caused by the poor conductivity of these nanomaterials, resulting in low catalytic activity for the application as electrochemical catalyst [28, 29]. Therefore, it is significant to design electrocatalytic catalysts with high conductivity, activity and stability.
Recently, due to its advantages of low cost, environmental friendly and electrochemical performance, CuS has attracted considerable research interest as sensors [30]. Considering the above-mentioned challenges, it is still an urgent need to construct well-dispersed CuS nanomaterials on electronically conductive substrates to facilitate the charge transfer for the application as an electrochemical sensor. Graphene has been explored as an electrode material for electrochemical applications [31, 32], due to its fascinating properties such as large surface to volume ratio, high conductivity and fast charge transfer rate. Thus, graphene can be an ideal substrate for nanoparticles to be well-dispersed on, which can accelerate electron transfer between nanoparticles and the surface of electrodes.
Inspired by this, in this paper, we synthesize well-dispersed copper sulfide (CuS) electrocatalyst on reduced graphene oxide (RGO) through a facile one-pot hydrothermal method, in which the thiourea was used as reducing agent and sulfur donor simultaneously. The CuS/RGO hybrid combines the advantages of CuS (good electrocatalytic activity, inexpensive and environmental friendly) with the advantages of graphene (large surface-tovolume ratio, high conductivity and fast charge transfer rate) and was used as H2O2 sensor. The as-prepared non-enzymatic H2O2 sensor exhibits high sensitivity, excellent stability and a low detection limit toward H2O2 detection, and the superior electrocatalytic activity arises from synergetic effects between well dispersed CuS nanoparticles and reduced graphene oxide. This work also opens up a brand new method to the synthesis of efficient electrocatalysts.
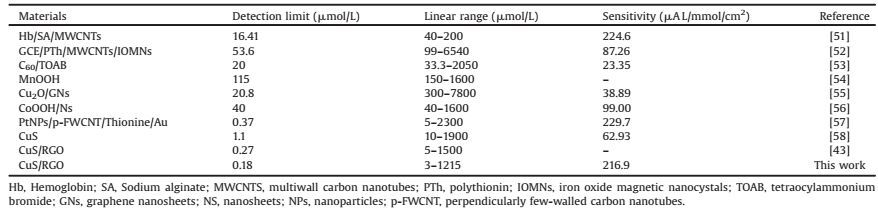
2. Results and discussionFig. 1 schematically illustrates the formation mechanism of the one-pot synthesis of CuS/RGO hybrid. Before the reaction, the graphene oxide sheets were randomly dispersed in water and in extended states, due to their electrostatic repulsion effect and strong hydrophilicity [33]. During the reaction, thiourea (TU) served as a reducing agent to reduce the grapheme oxide sheets and as a source of sulfur [34], Cu (CH3COO)2 served as a source of copper. The reaction mechanism of the above processes may be as follows: 1) Graphene oxide sheets were reduced by the reducing agent TU. 2) Cu (CH3COO)2 hydrolyze to yield Cu2+ and the generated Cu2+ reacted with the redundant S2- to form CuS nanoparticles. 3) With the reaction proceeding, the generated CuS nanoparticles interact with RGO through its carboxylic acid functional groups [35].
|
Download:
|
| Fig. 1. Schematic illustration of the synthesis of CuS nanoparticles on RGO. | |
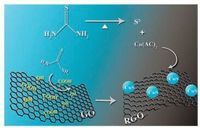
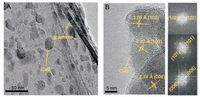
The typical SEM micrographs of the as-prepared RGO and CuS/ RGO hybrid were shown in Fig. 2. It was found that the randomly aggregated, crumpled RGO sheets loosely packed together (Fig. 2A). In the case of CuS/RGO hybrid, a large number of CuS nanoparticles anchored onto the graphene sheets were found by a magnified SEM image, as shown in inset in Fig. 2B. In addition, the typical TEM image (Fig. 3A) of the as-prepared CuS/RGO hybrid showed that the CuS nanoparticles with a homogeneous shape about 20 nm in size were anchored onto the thin graphene sheets, which was consistent with the results in Fig. 2B. The crystal structure of CuS was confirmed by the high resolution TEM (HRTEM), as shown in Fig. 3B. The HRTEM image exhibited clear crystal lattices belonging to covelline CuS. The lattice fringes with the spacing of 3.06, 3.23 and 2.74 Å corresponding to the (102), (101) and (006) planes of covelline CuS were observed, respectively. Moreover, the corresponding fast Fourier transform (FFT) images further revealed the existence of mixed planes, as shown in Fig. 3B (right).

|
Download:
|
| Fig. 2. The typical SEM images of (A) RGO and (B) CuS/RGO hybrid. Inset: highmagnification SEM image of the CuS/RGO hybrid. | |
|
Download:
|
| Fig. 3. (A) TEM image of CuS/RGO hybrid. (B) HRTEM image of the CuS nanoparticles and the corresponding fast Fourier transform (FFT) images. | |
Fig. 4A showed the X-ray diffraction (XRD) patterns of the GO and CuS/RGO nanocomposite. It was found that GO exhibited a strong peak at 11.5°, corresponding to the typical diffraction peak with a d-spacing of 0.82 nm [36]. In contrast, for the CuS/RGO hybrid, the characteristic peak of GO disappeared, which means the successful reduction of GO, indicating the restoration of the conjugation of sp2 regions. Some other peaks at 27.6, 29.2, 32.7, 47.5, 47.8, 52.5 and 59.1° were the characteristic peaks of the (101), (102), (006), (107), (110), (108), (116) planes of covelline CuS (PDF 85-0620), and the result was consistent with HRTEM in Fig. 3B.
Raman spectra of GO and CuS/RGO were shown in Fig. 4B. The Raman spectra of GO and RGO displayed D band and G band.Compared to the GO, the intensity ratio of D and G bands (ID/IG) in CuS/RGO was higher (increasing from 0.88 for GO to 1.08 for CuS/ RGO), indicating that the oxidized areas of GO sheets were partly restored by chemical reduction or GO was successfully reduced to form RGO [37]. Furthermore, compared with GO, the G band shift can also be found, supporting the formation of Cu-S-C bonds between CuS nanoparticles and graphene sheets [38, 39]. We employed XPS to further characterize the product. From the Cu 2p spectra as shown in Fig. 4C, two peaks at 932.2 and 952.3 eV were observed, corresponding to Cu 2p3/2 and Cu 2p1/2 for covelline CuS [40, 41]. The peaks at 161.8 and 162.8 eV were assigned to S 2p3/2 and S 2p1/2 [41, 42], as shown in Fig. 4D. All these observations provided direct evidence to demonstrate the presence of covelline CuS nanoparticles anchored onto the surface of RGO sheets.

|
Download:
|
| Fig. 4. (A) XRD patterns of GO and CuS/RGO hybrid. (B) Raman spectra of GO, CuS/RGO hybrid. (C) Cu 2p and (D) S 2p XPS spectra. | |
Cyclic voltammetry (CV) was firstly conducted to investigate the electrocatalytic activity of the CuS/RGO electrode toward H2O2 detection. Fig. 5 displays the CVs of GCE, RGO and CuS/RGO electrodes in a N2-saturated NaOH solution (pH 11) with and without the presence of 0.2 μmol/L H2O2 at a scan rate of 50 mV/s. As shown in Fig. 5A and B, bare GCE and RGO electrode with and without the presence of H2O2 present background current only and no obvious redox peaks were observed, indicating that the H2O2 oxidation can be hardly realized on them. Contrastingly, Fig. 5C shows that a couple of well-defined redox peaks were observed at 0.12 and 0.22 V on the CuS/RGO electrode in blank N2-saturated NaOH solution (pH 11), which should be attributed to the Cu2S/CuS redox couple [43, 44]. After 0.2 μmol/L H2O2 was added into the blank N2-saturated NaOH solution, the oxidation peak current increased dramatically for the CuS/RGO electrode, indicating that CuS/RGO electrode showed high electrocatalytic activity toward the oxidation of H2O2.

|
Download:
|
| Fig. 5. CVs of the GCE (A) and RGO (B) and CuS/RGO (C) in N2-saturated NaOH (pH 11) with and without the presence of 0.2 μmol/L H2O2. The potential scan rate is 50 mV/s.(D) CVs of the CuS/RGO in N2-saturated NaOH (pH 11) at different scan rates (from a to g: 20, 30, 40, 50, 60, 70, 80 mV/s). Inset: peak current vs. scan rate1/2. | |
To further investigate the electrochemical kinetics of the CuS/ RGO electrode, the CVs of the electrode were examined at different scan rates from 20 mV/s to 80 mV/s, as shown in Fig. 5D. It can be seen that the oxidation peak currents increased obviously with the increasing of scan rate and were linearly proportional to the square root of scan rate as shown in Fig. 5D (inset), indicating that the redox reaction on the surface of the CuS/RGO was diffusion controlled [45]. Moreover, both oxidation peak potential and reduction peak potential almost didn't change with the increase of scan rate, indicating that the excellent electrochemical reaction ability and fast electron transfer kinetics of the CuS/RGO electrode [32].
The enhanced electrocatalytic activity of the CuS/RGO hybrid may be attributed to the following reasons. First, the welldispersed and large quantities of CuS nanoparticles successfully deposited on the thin graphene sheets, which provide more accessible sites for H2O2 oxidation. Second, the conductive graphene substrate accelerate the charge transfer from CuS surface to electrode [46, 47]. Third, the small size CuS nanoparticles may also contribute to the enhanced electrocatalytic activity of the CuS/ RGO hybrid, which provide a high catalytic surface [48, 49]. The excellent electrocatalytic activity of CuS/RGO hybrid makes it a promising material for H2O2 detection.
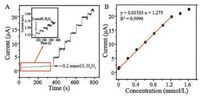
For the purpose of acquiring the information of repeatability and sensitivity, the optimum potential was selected at 0.2 V for amperometric detection of H2O2, which is in accord with relationshipbetweenthe potential and the electrocatalytic oxidationcurrent of H2O2. The optimization of experiment conditions for H2O2 detection was shown in Fig. S1 in Supporting information. Fig. 6A displays the typical amperometric i-t curve of H2O2 oxidation at CuS/ RGO electrode after the successive addition of H2O2 into NaOH solution (pH 11) at 0.2 V. It can be seen that the sensor responded rapidly to the change of H2O2 concentration and the oxidation current rose steeply to reach a stable value within 3 s. Fig. 6B shows the corresponding calibration curve and the linear regression equation is as follows: I (μA) = 0.01533C (μmol/L) + 1.275 (R2=0.9996). The linear range of this sensor to H2O2 concentration was from 3 to 1215 μmol/L, with a high sensitivity of 216.9 μAL/ mmol/cm2 (the slope of the calibration plot divided by geometric surface area of the electrode).
|
Download:
|
| Fig. 6. (A) Amperometric responses of the CuS/RGO upon successive addition of H2O2 in N2-saturated NaOH (pH 11) under an applied potential of 0.2 V (vs. Ag/AgCl).(B) The corresponding calibration curve of the CuS/RGO for the H2O2 detections. | |
The limit of detection (LOD) of the H2O2 was calculated on the basis of the signal-to-noise of 3, which means three times the standard deviation for the average measurements of blank samples (LOD=3 ×RSD/slope) [50]. Therefore, the LOD was calculated to be 0.18μmol/L. A comparison of detection limit, linear range and sensitivity of the prepared sensor with other sensors reported previously were shown in Table 1. As seen in Table 1, the present modifiede lectrode showed an excellent performance in comparison with several typical enzymatic and non-enzymatic H2O2 sensors reported previously. Especially for the lowest detection limit (0.18 μmol/L). To the best of our known, our CuS/RGO hybrid shows lower detection limit than certain enzymes and noble metal nanomaterial ever reported for the H2O2 detection. We also investigated the high reproducibility and stability of CuS/RGO hybrid, which are also very important factors for the practical applications of the electrochemical sensors. As can be seen from Fig. S2 in Supporting information, five successive experiments were carried out for the same electrode, and the relative standard deviation (RSD) was less than 4.21% (when the detected concentration of H2O2 was 0.2μmol/L), indicating a good reproducibility of the H2O2 sensor. The stability measurements showed that the amperometric response of the sensor has almost no change after 2 weeks of storageat 4 ℃ (Fig.S3 in Supporting information).Thus, itwassafely concluded that the as-prepared sensor exhibits a satisfactory stability. Therefore, the CuS/RGO composite might be an attractive electrochemical material for H2O2 detection with high sensitivity, low detection limit, good reproducibility and long-term stability.
|
|
Table 1 Comparison of several typical non-enzymatic and enzymatic H2O2 sensors. |
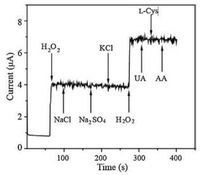
Since the possible electrochemical active interferents such as UA, L-cysteine and AA possiblyinterfere the determination of H2O2, selectivity is also one of the important factors for the practical applications of the H2O2 sensor. Therefore, the effects of these possible interfering species were thus evaluated by chronoamperometric measurements. As shown in Fig. 7, evident current step was observed when injecting 0.2mmol/L H2O2. However, no obvious current response was observed when interfering species were added into the reaction system. Effect of other possible interferes (cations and anions) on the determination of H2O2 was also evaluated, which indicated that these ions also have no interference in the determination of H2O2. These above results confirm that interference for such species is negligible, showing that the CuS/RGO sensor was highly selective to H2O2.
|
Download:
|
| Fig. 7. Amperometric responses (at 0.2V vs. Ag/AgCl) on CuS/RGO by adding H2O2 (0.2mmol/L), NaCl (0.1mmol/L), Na2SO4 (0.1mmol/L), KCl (0.1mmol/L), UA (0.1mmol/L), L-Cys (0.1mmol/L) and AA (0.1mmol/L) in NaOH (pH 11) solution. | |
3. Conclusion
In summary, we havesuccessfully prepared the CuS/RGO hybrid through a facile one-pot hydrothermal method. Owning to the good electrocatalytic activity of dispersed CuS nanoparticles and the exceptional properties of graphene, the resulting CuS/RGO electrode shows lower detection (0.18 μmol/L), high sensitivity (216.9 μAL/mmol/cm2) as well almost little interference from UA, L-Cys, AA and other possible interferes. Consequently, the electrochemical measurements showed that the CuS/RGO hybrid can be a promising material for non-enzymatic H2O2 detection in terms of simple preparation, excellent catalytic performance, good reproducibility and long-term stability and fast response.
4. Experimental 4.1. Chemicals and reagentsThiourea (TU, ≥98.0%) and ethylene glycol (EG, ≥99.0%) were acquired from Sinopharm Chemical Reagent Co., Ltd. Copper acetate monohydrate (Cu (CH3COO)2·H2O, ≥99.0%) was acquired from Lanbo Industrial Co., Ltd. Graphite powders (C, ≥98.0%), hydrogen peroxide (H2O2, 30%), uric acid (UA), L-cysteine and ascorbic acid (AA) were purchased from Aladin Ltd. Sodium chloride (NaCl, ≥99.5%), sodium sulfate (Na2SO4, ≥99%), potassium chloride (KCl, ≥98%) were obtained from Shanghai Chemical Reagent Company. All other reagents were at analytical grade and used without further purification. In addition, ultrapure water (18.2MΩ cm) was used for all the experiments.
4.2. CharacterizationThe morphologies of samples were observed by a SU70 scanning electron microscopy (SEM) (Hitachi, Japan). Transmission electron microscopy (TEM) images were gained using a 2100F field emission transmission electron microscope (JEOL, Japan) coupled with energy-dispersive X-ray spectrometer at an accelerating voltage of 200 kV. Raman spectra were obtained on a DXR Smart Raman (ThermoFisher Scientific, USA) spectrometer with a 532 nm wavelength incident laser light. X-ray diffraction (XRD) data was collected on a XRD-6000 diffractometer (Shimadzu, Japan) using Cu Ka source. X-ray photoelectron spectra (XPS) was carried out on Escalab 250Xi (Thermo Fisher Scientific, USA).
Electrochemical measurements were carried out using a computer-controlled VSP electrochemical analyzer (Bio-Logic, France) with a conventional three-electrode system. The modified glassy carbon electrode was used as the working electrode, a platinum (Pt) wire and an Ag/AgCl (saturated KCl) electrode as counter and reference electrode, respectively. All experiments were conducted in a cell filled with 80 mL NaOH solution (pH 11) and the electrolyte was purged with N2 gas for at least 15 min prior to the electrochemical measurements.
4.3. Preparation of CuS/RGO hybridGraphene oxide (GO) was prepared from graphite powders based on Hummers method [32]. The CuS/RGO hybrid was prepared by a facile one-pot hydrothermal method. To prepare CuS/RGO hybrid directly from GO, in a typical synthesis, 17.5 mg GO powder was dispersed into 35 mL ethylene glycol (EG) solution followed by sonication for 1 h. Next, Cu (CH3COO)2·H2O (99.83 mg) and TU (0.114 g) were slowly added into the above 35 mL mixed solution at room temperature. After vigorous stirring for about 30 min, these components were transferred into a 50 mL Teflonlined stainless steel autoclave and reacted under 180 ℃ for 12 h.Then the solution was cooled to ambient temperature naturally and the obtained solution was then washed with ethanol several times to remove non-reacted reactants, residual EG and CuS nanoparticles not bound to the graphene sheet. Finally the CuS/ RGO hybrid was centrifuged at 5000 rpm, and dried under vacuum at 50 ℃ overnight.
4.4. Construction of CuS/RGO modified glass carbon electrodePrior to use, the glass carbon electrode (GCE) were polished with 1.0mm alumina powders on the polishing cloth and sonicated in ethanol and ultrapure water for 30 min, respectively. The cleaned electrode dried with a stream of nitrogen. The modified electrodes were prepared by a simple casting method. Typically, an amount of the CuS/RGO hybrid suspension (2 mg/mL in ethanol) was dropped onto the GCE and then dried in air for 15 min to form CuS/RGO modified GCE. For comparative studies, we have prepared bare GCE and the RGO-modified GCE in the same way.
AcknowledgmentsThe financial support was received from the National Natural Science Foundation of China (Nos. 21522606, 21676246, 21476201, 21436007, U1462201, and 21376216). This research was also supported by Zhejiang Provincial Natural Science Foundation of China (No. LR17B060003) and Major Science and Technology Project of Water Pollution Control and Management (No.2017ZX07101).
Appendix A. Supplementary dataSupplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.cclet.2017.04.032.
| [1] | J. Ma, W. Song, C. Chen, et al., Fenton degradation of organic compounds promoted by dyes under visible irradiation. Environ. Sci. Technol. 39(2005)5810–5815. DOI:10.1021/es050001x |
| [2] | O.S. Keen, S. Baik, K.G. Linden, D.S. Aga, N.G. Love. Enhanced biodegradation of carbamazepine after UV/H2O2 advanced oxidation. Environ. Sci. Technol. 46(2012)6222–6227. DOI:10.1021/es300897u |
| [3] | A. Piwkowska, D. Rogacka, M. Jankowski, K. Kocbuch, S. Angielski. Hydrogen peroxide induces dimerization of protein kinase G type Ia subunits and increases albumin permeability in cultured rat podocytes. J. Cell. Physiol. 227(2012)1004–1016. DOI:10.1002/jcp.22810 |
| [4] | L. Yuan, W. Lin, Y. Xie, B. Chen, S. Zhu. Single fluorescent probe responds to H2O2 NO, and H2O2/NO with three different sets of fluorescence signals. J. Am. Chem. Soc. 134(2011)1305–1315. |
| [5] | A. Lobnik, M. Cajlakovi-ć. Sol-gel based optical sensor for continuous determination of dissolved hydrogen peroxide. Sens. Actuators B:Chem. 74(2001)194–199. DOI:10.1016/S0925-4005(00)00733-4 |
| [6] | D.W. King, W.J. Cooper, S.A. Rusak, et al., Flow injection analysis of H2O2 in natural waters using acridinium ester chemiluminescence:method development and optimization using a kinetic model. Anal. Chem. 79(2007)4169–4176. DOI:10.1021/ac062228w |
| [7] | Y. Peng, D. Jiang, L. Su, et al., Mixed monolayers of ferrocenylalkanethiol and encapsulated horseradish peroxidase for sensitive and durable electrochemical detection of hydrogen peroxide. Anal. Chem. 81(2009)9985–9992. DOI:10.1021/ac901833s |
| [8] | Y. Han, J. Zheng, S. Dong. A novel nonenzymatic hydrogen peroxide sensor based on Ag-MnO2-MWCNTs nanocomposites. Electrochim. Acta 90(2013)35–43. DOI:10.1016/j.electacta.2012.11.117 |
| [9] | A. Afraz, A.A. Rafati, A. Hajian. Analytical sensing of hydrogen peroxide on Ag nanoparticles-multiwalled carbon nanotube-modified glassy carbon electrode. J. Solid State Electr. 17(2013)2017–2025. DOI:10.1007/s10008-013-2057-8 |
| [10] | L. Zhang, D.B. Tian, J.J. Zhu. Third generation biosensor based on myoglobin-TiO2/MWCNTs modified glassy carbon electrode. Chin. Chem. Lett. 19(2008)965–968. DOI:10.1016/j.cclet.2008.04.027 |
| [11] | A.A. Karyakin, E.E. Karyakina, L. Gorton. Amperometric biosensor for glutamate using Prussian blue-based artificial peroxidase as a transducer for hydrogen peroxide. Anal. Chem. 72(2000)1720–1723. DOI:10.1021/ac990801o |
| [12] | Y. Xiao, H.X. Ju, H.Y. Chen. Hydrogen peroxide sensor based on horseradish peroxidase-labeled Au colloids immobilized on gold electrode surface by cysteamine monolayer. Anal. Chim. Acta 391(1999)73–82. DOI:10.1016/S0003-2670(99)00196-8 |
| [13] | A.K. Williams, J.T. Hupp. Sol-gel-encapsulated alcohol dehydrogenase as a versatile, environmentally stabilized sensor for alcohols and aldehydes. J. Am. Chem. Soc. 120(1998)4366–4371. DOI:10.1021/ja973772c |
| [14] | Z. Liu, B. Zhao, Y. Shi, et al., Novel nonenzymatic hydrogen peroxide sensor based on iron oxide-silver hybrid submicrospheres. Talanta 81(2010)1650–1654. DOI:10.1016/j.talanta.2010.03.019 |
| [15] | E. Shoji, M.S. Freund. Potentiometric sensors based on the inductive effect on the p K a of poly (aniline):a nonenzymatic glucose sensor. J. Am. Chem. Soc. 123(2001)3383–3384. DOI:10.1021/ja005906j |
| [16] | Y. Wang, X. Yang, J. Bai, X. Jiang, G. Fan. High sensitivity hydrogen peroxide and hydrazine sensor based on silver nanocubes with rich {100} facets as an enhanced electrochemical sensing platform. Biosens. Bioelectron. 43(2013)180–185. DOI:10.1016/j.bios.2012.10.099 |
| [17] | X. Sun, S. Guo, Y. Liu, S. Sun. Dumbbell-like PtPd-Fe3O4 nanoparticles for enhanced electrochemical detection of H2O2. Nano Lett. 12(2012)4859–4863. DOI:10.1021/nl302358e |
| [18] | F. Jiang, R. Yue, Y. Du, J. Xu, P. Yang. A one-pot 'green' synthesis of Pd-decorated PEDOT nanospheres for nonenzymatic hydrogen peroxide sensing. Biosens. Bioelectron. 44(2013)127–131. DOI:10.1016/j.bios.2013.01.003 |
| [19] | A. Kafi, A. Ahmadalinezhad, J. Wang, D.F. Thomas, A. Chen. Direct growth of nanoporous Au and its application in electrochemical biosensing. Biosens. Bioelectron. 25(2010)2458–2463. DOI:10.1016/j.bios.2010.04.006 |
| [20] | M.X. Kan, X.J. Wang, H.M. Zhang. Detection of H2O2 at a composite film modified electrode with highly dispersed Ag nanoparticles in Nafion. Chin. Chem. Lett. 22(2011)458–460. DOI:10.1016/j.cclet.2010.12.009 |
| [21] | S. Guo, D. Wen, Y. Zhai, S. Dong, E. Wang. Platinum nanoparticle ensemble-ongraphene hybrid nanosheet:one-pot, rapid synthesis, and used as new electrode material for electrochemical sensing. ACS Nano 4(2010)3959–3968. DOI:10.1021/nn100852h |
| [22] | X. Liu, M.T. Swihart. Heavily-doped colloidal semiconductor and metal oxide nanocrystals:an emerging new class of plasmonic nanomaterials. Chem. Soc. Rev. 43(2014)3908–3920. DOI:10.1039/C3CS60417A |
| [23] | S. Bai, Y. Xiong. Some recent developments in surface and interface design for photocatalytic and electrocatalytic hybrid structures. Chem. Commun. 51(2015)10261–10271. DOI:10.1039/C5CC02704G |
| [24] | H.L. Xu, W.D. Zhang. Graphene oxide-MnO2 nanocomposite-modified glassy carbon electrode as an efficient sensor for H2O2. Chin. Chem. Lett. 28(2017)143–148. DOI:10.1016/j.cclet.2016.10.008 |
| [25] | R.P. Bagwe, L.R. Hilliard, W. Tan. Surface modification of silica nanoparticles to reduce aggregation and nonspecific binding. Langmuir 22(2006)4357–4362. DOI:10.1021/la052797j |
| [26] | J.C. Jin, Z.Q. Xu, P. Dong, et al., One-step synthesis of silver nanoparticles using carbon dots as reducing and stabilizing agents and their antibacterial mechanisms. Carbon 94(2015)129–141. DOI:10.1016/j.carbon.2015.05.084 |
| [27] | D. Jiang, X. Du, Q. Liu, et al., One-step thermal-treatment route to fabricate well-dispersed ZnO nanocrystals on nitrogen-doped graphene for enhanced electrochemiluminescence and ultrasensitive detection of pentachlorophenol. ACS Appl. Mater. Interfaces 7(2015)3093–3100. DOI:10.1021/am507163z |
| [28] | X. Zhang, G. Wang, A. Gu, Y. Wei, B. Fang. CuS nanotubes for ultrasensitive nonenzymatic glucose sensors. Chem. Commun. (2008)5945–5947. |
| [29] | L. Jiang, M. Yao, B. Liu, et al., Controlled synthesis of CeO2/graphene nanocomposites with highly enhanced optical and catalytic properties. J. Phys. Chem. C 116(2012)11741–11745. DOI:10.1021/jp3015113 |
| [30] | J. Liu, D. Xue. Rapid and scalable route to CuS biosensors:a microwave-assisted Cu-complex transformation into CuS nanotubes for ultrasensitive nonenzymatic glucose sensor. J. Mater. Chem. 21(2011)223–228. DOI:10.1039/C0JM01714K |
| [31] | Y. Wang, Y. Shao, D.W. Matson, J. Li, Y. Lin. Nitrogen-doped graphene and its application in electrochemical biosensing. ACS Nano 4(2010)1790–1798. DOI:10.1021/nn100315s |
| [32] | J. Ju, W. Chen. In situ growth of surfactant-free gold nanoparticles on nitrogendoped graphene quantum dots for electrochemical detection of hydrogen peroxide in biological environments. Anal. Chem. 87(2015)1903–1910. DOI:10.1021/ac5041555 |
| [33] | Y. Xu, K. Sheng, C. Li, G. Shi. Self-assembled graphene hydrogel via a one-step hydrothermal process. ACS Nano 4(2010)4324–4330. DOI:10.1021/nn101187z |
| [34] | C.K. Chua, M. Pumera. Chemical reduction of graphene oxide:a synthetic chemistry viewpoint. Chem. Soc. Rev. 43(2014)291–312. DOI:10.1039/C3CS60303B |
| [35] | G. Williams, B. Seger, P.V. Kamat. TiO2-graphene nanocomposites. UV-assisted photocatalytic reduction of graphene oxide. ACS Nano 2(2008)1487–1491. DOI:10.1021/nn800251f |
| [36] | D.A. Dikin, S. Stankovich, E.J. Zimney, et al., Preparation and characterization of graphene oxide paper. Nature 448(2007)457–460. DOI:10.1038/nature06016 |
| [37] | H. Wang, J.T. Robinson, X. Li, H. Dai. Solvothermal reduction of chemically exfoliated graphene sheets. J. Am. Chem. Soc. 131(2009)9910–9911. DOI:10.1021/ja904251p |
| [38] | N.A. Zubir, C. Yacou, J. Motuzas, X. Zhang. J.C.D. da Costa, Structural and functional investigation of graphene oxide-Fe3O4 nanocomposites for the heterogeneous Fenton-like reaction. Sci. Rep. 4(2014)4594. |
| [39] | X. Xu, H. Li, Q. Zhang, et al., Self-sensing, ultralight, and conductive 3D graphene/iron oxide aerogel elastomer deformable in a magnetic field. ACS Nano 9(2015)3969–3977. DOI:10.1021/nn507426u |
| [40] | B. Li, Y. Xie, Y. Xue. Controllable synthesis of CuS nanostructures from selfassembled precursors with biomolecule assistance. J. Phys. Chem. C 111(2007)12181–12187. |
| [41] | Y. Zhang, J. Tian, H. Li, et al., Biomolecule-assisted environmentally friendly, one-pot synthesis of CuS/reduced graphene oxide nanocomposites with enhanced photocatalytic performance. Langmuir 28(2012)12893–12900. DOI:10.1021/la303049w |
| [42] | C. Wu, S.H. Yu, S. Chen, G. Liu, B. Liu. Large scale synthesis of uniform CuS nanotubes in ethylene glycol by a sacrificial templating method under mild conditions. J. Mater. Chem. 16(2006)3326–3331. DOI:10.1039/b606226a |
| [43] | J. Bai, X. Jiang. A facile one-pot synthesis of copper sulfide-decorated reduced graphene oxide composites for enhanced detecting of H2O2 in biological environments. Anal. Chem. 85(2013)8095–8101. DOI:10.1021/ac400659u |
| [44] | Y.J. Yang, W. Li, X. Wu. Copper sulfide|reduced graphene oxide nanocomposite for detection of hydrazine and hydrogen peroxide at low potential in neutral medium. Electrochim. Acta 123(2014)260–267. DOI:10.1016/j.electacta.2014.01.046 |
| [45] | S. Tian, A. Baba, J. Liu, et al., Electroactivity of polyaniline multilayer films in neutral solution and their electrocatalyzed oxidation of β-nicotinamide adenine dinucleotide. Adv. Funct. Mater. 13(2003)473–479. DOI:10.1002/adfm.200304320 |
| [46] | K.K. Manga, Y. Zhou, Y. Yan, K.P. Loh. Multilayer hybrid films consisting of alternating graphene and titania nanosheets with ultrafast electron transfer and photoconversion properties. Adv. Funct. Mater. 19(2009)3638–3643. DOI:10.1002/adfm.v19:22 |
| [47] | A. Cao, Z. Liu, S. Chu, et al., A facile one-step method to produce graphene-CdS quantum dot nanocomposites as promising optoelectronic materials. Adv. Mater. 22(2010)103–106. DOI:10.1002/adma.v22:1 |
| [48] | D. Dey, T. Bhattacharya, B. Majumdar, et al., Carbon dot reduced palladium nanoparticles as active catalysts for carbon-carbon bond formation. Dalton Trans. 42(2013)13821–13825. DOI:10.1039/c3dt51234g |
| [49] | W. Wei, W. Chen. Naked Pd nanoparticles supported on carbon nanodots as efficient anode catalysts for methanol oxidation in alkaline fuel cells. J. Power Sources 204(2012)85–88. DOI:10.1016/j.jpowsour.2012.01.032 |
| [50] | C. Cao, J.P. Kim, B.W. Kim, et al., A strategy for sensitivity and specificity enhancements in prostate specific antigen-a 1-antichymotrypsin detection based on surface plasmon resonance. Biosens. Bioelectron. 21(2006)2106–2113. DOI:10.1016/j.bios.2005.10.014 |
| [51] | H. Zhao, W. Zheng, Z. Meng, et al., Bioelectrochemistry of hemoglobin immobilized on a sodium alginate-multiwall carbon nanotubes composite film. Biosens. Bioelectron. 24(2009)2352–2357. DOI:10.1016/j.bios.2008.12.004 |
| [52] | Y. Miao, H. Wang, Y. Shao, et al., Layer-by-layer assembled hybrid film of carbon nanotubes/iron oxide nanocrystals for reagentless electrochemical detection of H2O2. Sens. Actuators B:Chem. 138(2009)182–188. DOI:10.1016/j.snb.2008.12.045 |
| [53] | W. Liu, X. Gao. C60 trianion-mediated electrocatalysis and amperometric sensing of hydrogen peroxide. Electrochem. Commun. 10(2008)1377–1380. DOI:10.1016/j.elecom.2008.06.031 |
| [54] | X. Cao, N. Wang, L. Wang, et al., A novel non-enzymatic hydrogen peroxide biosensor based on ultralong manganite MnOOH nanowires. Sens. Actuators B:Chem. 147(2010)730–734. DOI:10.1016/j.snb.2010.03.087 |
| [55] | M. Liu, R. Liu, W. Chen. Graphene wrapped Cu2O nanocubes:non-enzymatic electrochemical sensors for the detection of glucose and hydrogen peroxide with enhanced stability. Biosens. Bioelectron. 45(2013)206–212. DOI:10.1016/j.bios.2013.02.010 |
| [56] | K.K. Lee, P.Y. Loh, C.H. Sow, W.S. Chin. CoOOH nanosheet electrodes:simple fabrication for sensitive electrochemical sensing of hydrogen peroxide and hydrazine. Biosens. Bioelectron. 39(2013)255–260. DOI:10.1016/j.bios.2012.07.061 |
| [57] | M. Ma, Z. Miao, D. Zhang, et al., Highly-ordered perpendicularly immobilized FWCNTs on the thionine monolayer-modified electrode for hydrogen peroxide and glucose sensors. Biosens. Bioelectron. 64(2015)477–484. DOI:10.1016/j.bios.2014.09.057 |
| [58] | A.K. Dutta, S. Das, P.K. Samanta, et al., Non-enzymatic amperometric sensing of hydrogen peroxide at a CuS modified electrode for the determination of urine H2O2. Electrochimi. Acta 144(2014)282–287. DOI:10.1016/j.electacta.2014.08.051 |
 2017, Vol. 28
2017, Vol. 28