b State Key Laboratory of Molecular Vaccinology and Molecular Diagnostics Center for Molecular Imaging and Translational Medicine, School of Public Health, Xiamen University, Xiamen 361005, China
Combination therapy, in which two or more therapeutic modalities are combined together to effectively treat cancer, has recently emerged as a promising strategy for improving the treatment efficacy and minimizing side effects. Among various combined therapies (e.g. photothermal therapy (PTT)/chemotherapy [1-6], photodynamic therapy (PDT)/chemotherapy [7] and PTT/PDT [8-11] etc.), the combination of PDT with PTT has attracted much attention owing to its non-invasive, highly localized, low systemic toxicity as well as enhanced therapeutic outcome by synergistic effects [12-15]. PDT and PTT are lightbased techniques that utilize photosensitizers (PSs) or photothermal agents to generate reactive oxygen species (ROS) (mainly singlet oxygen) or heat upon proper light irradiation to destroy cancer cells, respectively [16, 17]. However, the poor watersolubility and limited tumor selectivity of most PSs set a limitation on the wide application of PDT in clinic [18], and the higher laser power density used for PTT also will bring out some side effects in normal tissues [19]. The integration of PDT and PTT can take the advantages of each and overcome respective limitations, achieving high therapeutic efficacy [20, 21].
By loading PSs onto some near infrared (NIR) absorption photothermal nanomaterials, such as noble metal (Au, Pd) nanostructures [22-25], carbon-based nanomaterials [14, 26, 27], two-dimensional transition metal dichalcogenides (MoS2 and WS2) [28, 29] and CuS nanoparticles [30-33], the combination of PDT and PTT has been demonstrated for not only effective delivery of PSs into cancer cells, but also successfully eradicating cancer in a synergistic manner. However, such combined strategies based on multi-component nanocomposites generally suffer from prolonged and complicated treatment procedures, since two different wavelength lasers are often required for sequential exciting PDT and PTT respectively due to the absorption mismatch of PSs and photothermal agents at NIR region in most previous work [14, 28, 29, 34]. Although for simplifying this treatment procedure, simultaneous PDT and PTT treatment upon single-laser irradiation was also performed for some nanosystems by matching the absorption wavelength of the nanomaterial with the excitation wavelength of photosensitizer, the sophisticated synthetic process and raising manufacturing cost still place a limitation for their practical clinical application [10, 22, 24, 26, 31]. Thus, the development of an efficient and single agent nanomaterial for simultaneous and combinational PDT/PTT treatment is highly desirable.
Recently some NIR absorbing dyes such as indocyanine green (ICG) and naphthalocyanine (Nc) etc., which simultaneously possess great potential in converting the absorbed optical energy into heat and generating reactive oxygen species under single wavelength NIR light irradiation for combination of PTT and PDT, have attracted widespread interest [32, 35-38]. However, the clinical application of organic ICG as single treatment agent is limited by its low photostability [32, 35, 36, 39]. In contrast, owing to their considerable strong absorption band at NIR region as well as outstanding optical and thermal stability [39, 40], Nc compounds have been exploited as photothermal agents for cancer PTT [41, 42]. Later, by modifying Nc molecules with bulky organic ligands to provide steric hindrance and lower intramolecular aggregation, the application of Nc in PDT and fluorescence imaging also was demonstrated [43, 44]. More recently, by encapsulation of silicon 2, 3-naphthalocyanine bis(trihexylsilyloxide) into the interior of a generation 5 polypropylenimine (PPI G5) dendrimer that is then surrounded with the biocompatible polyethylene glycol (PEG) polymer on the surface, Taratula et al. constructed a novel theranostic nanoplatform capable of concurrent NIR fluorescence imaging and PDT/PTT combinatorial therapy of cancer [37]. These exciting results inspire us to further explore new Nc-based single agent nanoparticle systems for cancer imaging and combinatorial phototherapy.
Here, by coating silicon 2, 3-naphthalocyanine dihydroxide (SiNcOH) with DSPE-PEG and DSPE-PEG-NH2, we developed a novel theranostic nanoagent (denoted as SiNcOH-DSPE-PEG(NH2) NPs) for PDT and PTT tumor ablation under the guidance of PA imaging (Scheme 1). The prepared single agent SiNcOH-DSPE-PEG (NH2) NPs have the following advantages: 1) The synthesis process is simple, inexpensive and easily repeatable. 2) They exhibit good water solubility, significant stability and biocompatibility. 3) Upon irradiated with 808 nm NIR laser, they display high photothermal conversion efficiency (~59.8%), photostability and good ROS generation capability. 4) Besides, due to their effective NIR absorption, the SiNcOH-DSPE-PEG(NH2) NPs can act as novel contrast agent for PA imaging. The strategy of integrated PA imaging-guided combined PTT/PDT will substantially improve the accuracy, efficacy and safety of cancer treatment. We detailedly evaluated the PA imaging, and the corresponding PDT and PTT effects of SiNcOH-DSPE-PEG(NH2) NPs in both cell levels and animal models of tumor-bearing mice. Compared with monotherapy, the combination of PDT and PTT achieved an obviously enhanced tumor growth inhibition effect. We believe that the present PA imaging-guided tumor dual therapeutic properties based on single-agent nanoparticle platform will provide a promising strategy to promote the use of hydrophobic photosensitizers in tumor.

|
Download:
|
| Scheme1. Schematic illustration of the synthesis process of SiNcOH-DSPE-PEG(NH2) NPs and PA imaging-guided combinational therapy of cancer. The NPs comprise the hydrophobic SiNcOH core and the amphiphilic phospholipid-PEG shell. | |
2. Results and discussion 2.1. Synthesis and characterization of SiNcOH-DSPE-PEG(NH2) NPs
Although silicon 2, 3-naphthalocyanine dihydroxide molecule exhibits significant absorption at near-infrared (NIR) region (Fig. 1a, red line), the potential use of SiNcOH as photothermal agent or photosensitizer has been limited due to its poor water solubility and aggregation. To improve its water dispersibility as well as biocompatibility, we use amphiphilic poly(ethylene glycol)-conjugated phospholipid (DSPE-PEG and DSPE-PEG-NH2) to coat SiNcOH. The formation of hydrophilic SiNcOH-DSPE-PEG (NH2) NPs was shown in Scheme 1. SiNcOH and DSPE-PEG(NH2) were mixed and stirred in chloroform, then the hydrophobic DSPE segments tended to entangle with SiNcOH molecules by hydrophobic interaction and the hydrophilic PEG chains extended into the aqueous phase, so the hydrophobic SiNcOH (1, Inset of Fig. 1a) was transferred into the aqueous phase to form hydrophilic SiNcOH-DSPE-PEG(NH2) NPs (2, Inset of Fig. 1a). The DSPE-PEG (NH2) coating provides a great way to enhance the water solubility of SiNcOH. As shown in Fig. S1 (Supporting information), if only using DSPE-PEG or DSPE-PEG-NH2 to encapsulate SiNcOH molecules, the zeta potentials of SiNcOH-DSPE-PEG and SiNcOH-DSPEPEG-NH2 were -20.1 mV and +22.2 mV, respectively. When DSPEPEG and DSPE-PEG-NH2 were used to encapsulate SiNcOH simultaneously, the zeta potential of SiNcOH-DSPE-PEG(NH2) NPs was +8.87 mV (Fig. S1), indicating that DSPE-PEG and DSPEPEG-NH2 were successfully coated on the surface of SiNcOH. Typical transmission electron microscopy (TEM) imaging of the resultant SiNcOH-DSPE-PEG(NH2) NPs were shown in Fig. 1b, with an average diameter of approximately 120 nm. After coating, the size distribution of the SiNcOH-DSPE-PEG(NH2) NPs in aqueous solution measured by dynamic light scattering (DLS) was about 160 nm (Fig. 1c). Compared with the spectrum of pure SiNcOH in chloroform (Fig. 1a, red line), the UV-vis-NIR absorption of water dispersed SiNcOH-DSPE-PEG(NH2) NPs (Fig. 1a, blue line) became stronger and wider, which attributed to the partial aggregation of SiNcOH molecules coated by DSPE-PEG(NH2) [37]. In addition, the prepared SiNcOH-DSPE-PEG(NH2) NPs demonstrated a high stability in water, PBS, RPMI 1640 cell medium containing 10% FBS or 100% FBS. No significant spectra changes have been observed for the NPs stored in these solutions for 7 days (Fig. S2 in Supporting information). The strong and broad absorbance between 650 and 900 nm suggests that the obtained SiNcOHDSPE-PEG(NH2) NPs would be suitable for imaging, PTT and/or PDT, since irradiation in the NIR region, a transparency window for biological tissues, can penetrate tissues with higher spatial precision and sufficient intensity for inducing localized hyperthermia or generating ROS [45, 47].

|
Download:
|
| Fig. 1. (a) Absorption spectra of chloroform dispersion of SiNcOH (red line), aqueous dispersion of DSPE-PEG(NH2) (black line) and aqueous dispersion of hydrophilic SiNcOHDSPE-PEG(NH2) NPs (blue line). Inset: photographs of SiNcOH (1) and SiNcOH-DSPE-PEG(NH2) NPs (2) dispersed in water respectively. (b) Typical TEM imaging of SiNcOHDSPE-PEG(NH2) NPs. (c) Hydrodynamic size of SiNcOH-DSPE-PEG(NH2) NPs. | |
2.2. Photothermal effect and photothermal conversion efficiency of SiNcOH-DSPE-PEG(NH2) NPs
As mentioned above, the strong NIR absorption of SiNcOHDSPE-PEG(NH2) NPs makes them highly promising for cancer PTT (Fig. 1a). First, we evaluated the photothermal conversion capabilities of SiNcOH-DSPE-PEG(NH2) NPs. The photothermal effect of SiNcOH-DSPE-PEG(NH2) NPs exhibits an obvious concentration-dependent and irradiation energy-dependent manner (Fig. 2a and 2c). With the increase of the concentration of SiNcOH-DSPE-PEG(NH2) NPs from 20 to 150 ppm, the temperature change of the aqueous dispersed SiNcOH-DSPE-PEG(NH2) NPs can increase from 10.2 to 26.7 ℃ in 12 min (Fig. 2b), which is enough for tumor photothermal treatment. While under the same conditions, pure water only exhibited a negligible change (1.3 ℃). Similarly, the temperature of SiNcOH-DSPE-PEG(NH2) NPs dispersion rises as the power density increases (Fig. 2c). These results indicate that the raise of temperature is mainly contributed by SiNcOH-DSPE-PEG(NH2) NPs rather than the absorption of light by water, and the SiNcOH-DSPE-PEG(NH2) NPs can rapidly and efficiently convert the 808 nm laser energy into thermal energy.

|
Download:
|
| Fig. 2. Photothermal effect of SiNcOH-DSPE-PEG(NH2) NPs. (a) Temperature elevations of aqueous dispersions of SiNcOH-DSPE-PEG(NH2) NPs at different concentrations (20, 50, 100, and 150 ppm) as a function of irradiation time. Pure water was used as a control, and room temperature was 25 ℃. (b) Plot of temperature change (△T) over a period of 12 min versus the concentration of SiNcOH-DSPE-PEG(NH2) NPs in the aqueous dispersion. (c) Heating curves of SiNcOH-DSPE-PEG(NH2) NPs (100 ppm) under 808 nm laser irradiation with different power density. (d) The photostability of SiNcOH-DSPE-PEG(NH2) NPs solution after multiple cycles of laser-induced photothermal heating. | |
Next, the photothermal conversion efficiency (η) of SiNcOHDSPE-PEG(NH2) NPs was measured according to the method described by Roper et al. [46] and Hu et al. [45]. The η value was calculated to be 59.8% (Figs. S3a and S3b), which is relatively high or comparable with those of previously reported photothermal nanomaterials.
In addition, SiNcOH-DSPE-PEG(NH2) NPs also displayed impressive photostability. As shown in Fig. 2d, SiNcOH-DSPE-PEG (NH2) NPs remained to be a rather robust photothermal heater after five cycles of NIR laser-induced heating (808 nm laser at 1.5 W/cm2, 12 min laser irradiation for each cycle), and no significant decline of temperature increment was observed. Also, the absorption spectrum and hydrodynamic size of SiNcOH-DSPEPEG(NH2) NPs had almost no change after five cycles of NIR laser irradiation (Fig. S3c and 3d), demonstrating the excellent photothermal stability of SiNcOH-DSPE-PEG(NH2) NPs.
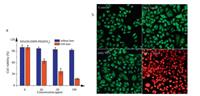
2.3. Photodynamic properties of the SiNcOH-DSPE-PEG(NH2) NPsBesides the photothermal effect, SiNcOH-DSPE-PEG(NH2) NPs have excellent capability to generate singlet oxygen (1O2) under 808 nm laser irradiation with low power density simultaneously. The 1O2 generation capability of SiNcOH-DSPE-PEG(NH2) NPs was assessed by using 1, 3-diphenylisobenzofuran (DPBF) as a probe molecule [24, 48]. DPBF reacts irreversibly with 1O2 to cause a decrease in the intensity of DPBF absorption at around 410 nm. As demonstrated in Fig. 3a, in the presence of SiNcOH-DSPE-PEG (NH2) NPs, the DPBF absorption at 410 nm significantly decreased with the time under the irradiation of 808 nm laser at a power density of 0.01 W/cm2, suggesting these nanoparticles are highly efficient in the generation of 1O2. In addition, the intracellular ROS production by SiNcOH-DSPE-PEG(NH2) NPs was measured using 20, 70-dichlorofluorescein diacetate (DCFH-DA). Upon irradiation with 808 nm laser, the ROS generation of SiNcOH-DSPE-PEG(NH2) NPs can oxidize DCFH, a non-fluorescent part of DCFH-DA, to 2, 7-dichlorofluorescein (DCF) and emit bright green fluorescence in HeLa cells (Fig. 3b). While no obvious fluorescence was observed from untreated cells, laser-treated cells or NPs-treated cells. These results indicate clearly that SiNcOH-DSPE-PEG(NH2) NPs can generate ROS under the irradiation of 808 nm light, and have great potential for application in PDT therapy.

|
Download:
|
| Fig. 3. Determination of reactive oxygen species (ROS) levels. (a) Absorption spectra of DPBF in the presence of SiNcOH-DSPE-PEG(NH2) NPs after irradiation for different times with an 808 nm laser source at 0.01 W/cm2. (b) Confocal fluorescence images of HeLa cells to detect oxidative stress using DCFH-DA, a fluorogenic marker for ROS. The four groups are: untreated, only treated with SiNcOH-DSPE-PEG(NH2) NPs solution, only irradiation with NIR laser, and treated with SiNcOH-DSPE-PEG(NH2) NPs solution followed by irradiation with NIR laser, respectively. The cells showing green fluorescence color represent oxidatively stressed cells affected with ROS. | |
Based on the above researches, the single-agent SiNcOH-DSPEPEG(NH2) nanoplatform would be promising as an ideal candidate for simultaneous PTT and PDT therapy upon single 808 nm laser irradiation.
2.4. Evaluation of the cellular uptake of SiNcOH-DSPE-PEG(NH2) NPsAs mentioned above, SiNcOH-DSPE-PEG(NH2) NPs exhibit high photothermal conversion efficiency and excellent 1O2 generation capability, whether can these SiNcOH-DSPE-PEG(NH2) NPs be taken up effectively by cancer cells to achieve a good PTT/PDT combined therapeutic effect? In order to test this, SiNcOH-DSPEPEG(NH2) NPs were first modified with fluorescent molecules rhodamine B isothiocyanate (RBITC) by amidation reaction of isothiocyanate and amino group, and the successful conjugation of RBITC was confirmed by the appearance of the characteristic absorption peak of RBITC (λ = 554 nm) at the UV-Vis-NIR absorption spectra of SiNcOH-DSPE-PEG-RBITC NPs (Fig. S4a in Supporting information). As shown in Fig. 4a and Fig. S4b (Supporting information), HeLa cells treated with SiNcOH-DSPE-PEG-RBITC NPs for different time exhibit obvious fluorescence signal compared to the untreated HeLa cells. Meanwhile, confocal microscopy imaging was carried out to detect the internalization of SiNcOH-DSPE-PEG-RBITC NPs. Bright red fluorescence could be clearly observed in HeLa cells after incubation with SiNcOH-DSPEPEG-RBITC NPs (Fig. 4b). These results clearly indicated that the SiNcOH-DSPE-PEG(NH2) NPs could be effectively taken up by cancer cells, which was beneficial for the following phototherapy since the cells can be easily destroyed by both 1O2 and heat produced from the nanoparticles.

|
Download:
|
| Fig. 4. Cellular uptake of SiNcOH-DSPE-PEG(NH2) NPs. (a) Flow cytometry analysis of untreated HeLa cells and HeLa cells treated with SiNcOH-DSPE-PEG-RBITC NPs for 12 h. (b) CLSM images of HeLa cells treated with SiNcOH-DSPE-PEG-RBITC NPs for 12 h. | |
2.5. In vitro cytotoxicity assay and PTT/PDT-induced cell death
To investigate the PTT/PDT combined treatment efficiency based on SiNcOH-DSPE-PEG(NH2) NPs in vitro, HeLa cells were incubated with different concentrations of SiNcOH-DSPE-PEG (NH2) NPs for 24 h and then irradiated by the 808 nm laser with a power density of 1.0 W/cm2 for 6 min. MTT assay was used to assess the cell viabilities. Without laser, SiNcOH-DSPE-PEG(NH2) NPs demonstrated good biocompatibility (Fig. 5a), which was better than the free SiNcOH (Fig. S5), while a significant decrease of the cell viability was observed with the increase of SiNcOH-DSPE-PEG (NH2) NPs concentrations under laser irradiation. More than 80% of cells were destroyed when the concentration of SiNcOH-DSPE-PEG (NH2) NPs was 100 ppm, confirming that SiNcOH-DSPE-PEG(NH2) NPs possessed good combination therapy effect in vitro. In addition, after irradiation the cells were stained with both Calcein AM and Propidium iodide (PI), and imaged by a confocal fluorescence microscope. As shown in Fig. 5b, significant cell death indicated by red fluorescence can only be observed in the group of cells incubated with SiNcOH-DSPE-PEG(NH2) NPs and followed by NIR irradiation (1.0 W/cm2, 6 min). While green fluorescence was observed in the groups of untreated cells, cells treated with either SiNcOH-DSPE-PEG(NH2) NPs or laser alone, indicating negligible cell death. These results suggested that SiNcOH-DSPE-PEG(NH2) NPs could effectively kill cancer cells through the simultaneous photodynamic and photothermal effect induced by NIR irradiation.
|
Download:
|
| Fig. 5. The photothermal/photodynamic cytotoxicity of SiNcOH-DSPE-PEG(NH2) NPs. (a) The cytotoxicity of different concentrations of SiNcOH-DSPE-PEG(NH2) NPs on HeLa cells without or with exposure to laser (808 nm, 1.0 W/cm2) for 6 min. (b) Confocal images of Calcein AM (green, live cells) and Propidium iodide (red, dead cells) co-stained HeLa cells after laser irradiation (1.0 W/cm2). | |
2.6. Photoacoustic imaging of SiNcOH-DSPE-PEG(NH2) NPs in vitro and in vivo
In addition to the treatment function, our SiNcOH-DSPE-PEG (NH2) NPs can also be used for imaging tracking. Photoacoustic (PA) imaging is a hybrid biomedical imaging modality through measuring the ultrasonic waves induced by endogenous or exogenous PA contrast agents to convert NIR light into heat for acoustic imaging [47, 49, 50]. To investigate the PA imaging properties of SiNcOH-DSPE-PEG(NH2) NPs, in vitro PA imaging was performed in vials using a PA instrument at the wavelength of 730 nm. As shown in Fig. 6a and b, SiNcOH-DSPE-PEG(NH2) NPs exhibited significant PA contrast effect, which motivated us to use PA imaging to visualize the in vivo circulation and accumulation behaviors of SiNcOH-DSPE-PEG(NH2) NPs with high spatial resolution and anatomical localization.

|
Download:
|
| Fig. 6. . (a) PA imaging of SiNcOH-DSPE-PEG(NH2) NPs solution with different concentrations and (b) quantification photoacoustic signals. (c) PA imaging of SiNcOH-DSPE-PEG (NH2) NPs in tumor sites at different time. | |
Experimentally, SiNcOH-DSPE-PEG(NH2) NPs was intravenously injected at a dose of 15 mg/kg (300 mL, 1 mg/mL) and PA images of tumors were recorded at 1 h, 2 h, 4 h, 8 h and 24 h post injection. From Fig. 6c (before injection), only major blood vessels could be observed without SiNcOH-DSPE-PEG(NH2) NPs. After injection, at first the PA signals in tumor sites were strong due to the higher blood concentration of SiNcOH-DSPE-PEG(NH2) NPs in blood circulation. Afterword, as the blood circulation went on, some SiNcOH-DSPE-PEG(NH2) NPs might be absorbed by the RES organs such as live and spleen etc., so the PA signals somewhat decreased with the lower blood concentrations of SiNcOH-DSPE-PEG(NH2) NPs. But at the same time, some SiNcOH-DSPE-PEG(NH2) NPs also gradually accumulated at the tumor site. Obvious PA signals could be observed in tumor tissue instead of blood vessels at 8 h and 24 h (Fig. 6c) compared with the control group. Therefore, the PA image results of SiNcOH-DSPE-PEG(NH2) NPs can be used to guide the in vivo tumor therapy.
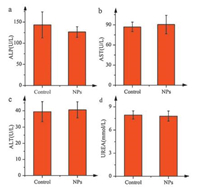
2.7. Biosafety assessment of SiNcOH-DSPE-PEG(NH2) NPs in vivoThe biosafety assessment is necessary for the application of SiNcOH-DSPE-PEG(NH2) NPs in mice. Established serum biochemistry assays were performed to evaluate the potential toxicity to organs, especially liver and kidney. As shown in Fig. 7, the three important hepatic function indicators, alanine aminotransferase (ALT), aspartate aminotransferase (AST) and alkaline phosphatase (ALP), as well as one indicator related to kidney functions, blood urea nitrogen (BUEA), were all in the normal range. These results indicated that SiNcOH-DSPE-PEG(NH2) NPs possess no obvious toxicity to mice in vivo, implying the promise of using them for in vivo cancer therapy.
|
Download:
|
| Fig. 7. Serum biochemistry results obtained from mice injected with SiNcOH-DSPEPEG(NH2) NPs at 24 h (n = 5, test) and no injected mice (n = 5, control). (a) The blood levels of alkaline phosphatase (ALP), (b) aspartate aminotransferase (AST) and (c) alanine aminotransferase (ALT) as liver function markers. (d) Blood urea nitrogen (BUEA) levels in the blood marking kidney functions. | |
2.8. In vivo photothermal/photodynamic therapy
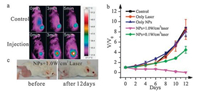
In vivo phototherapy tests using SiNcOH-DSPE-PEG(NH2) NPs were performed on tumor-bearing mice. First, S180 tumor-bearing mice were intravenously injected SiNcOH-DSPE-PEG(NH2) NPs (200 mL, 2 mg/mL) and exposed to 808 nm wavelength laser irradiation for 5 min at 24 h post-injection. The temperature changes of the tumors were recorded by a visual IR thermometer. As observed from Fig. 8a, for the 20 mg/kg SiNcOH-DSPE-PEG(NH2) NPs injected group, the temperature in the tumor region increases with irradiation time, and reaches near 52.7 ℃ after laser irradiation for 5 min (Fig. 8a). In comparison, under the same irradiation conditions, the tumor surface temperature rises only ~4 ℃ for saline injected mice.
|
Download:
|
| Fig. 8. (a) Infrared thermal imaging after the intravenous injection of SiNcOH-DSPE-PEG(NH2) NPs into tumor-bearing mice and the control group. (b) Time-dependent tumor growth curves of the mice with different designs: control, only laser, only SiNcOH-DSPE-PEG(NH2) NPs, SiNcOH-DSPE-PEG(NH2) NPs with laser (1.0 W/cm2) and SiNcOHDSPE-PEG(NH2) NPs with laser (0.1 W/cm2). (c) Photographs of the tumor-bearing mice with SiNcOH-DSPE-PEG(NH2) NPs injection before and after treated with SiNcOHDSPE-PEG(NH2) NPs and laser (1.0 W/cm2). | |
The therapeutic efficacy of SiNcOH-DSPE-PEG(NH2) NPs was further investigated in vivo. Five groups of mice were treated differently, including one group without any treatment, one group with only NIR irradiation, one group with only injection of SiNcOHDSPE-PEG(NH2) NPs, one group with both injection of SiNcOHDSPE-PEG(NH2) NPs and low NIR irradiation (0.1 W/cm2), and one group with both injection of SiNcOH-DSPE-PEG(NH2) NPs and high NIR irradiation (1 W/cm2). The PTT and PDT efficacy was assessed by measuring tumor size changes and observing the tumor necrosis within 12 days after treatment. As shown in Fig. 8b and 8c, for the mice treated with both SiNcOH-DSPE-PEG(NH2) NPs and 808 nm laser (1.0 W/cm2) irradiation for 10 min, the tumors sizes rapidly reduced and completely disappeared after 12 days of treatment. While the group that received the injection of SiNcOH-DSPE-PEG(NH2) NPs and low power density (0.1 W/cm2) irradiation exhibited slower growth of tumor, indicating the only PDT effect of SiNcOH-DSPE-PEG(NH2) NPs could not completely eliminate tumor. In contrast, tumors in the other three control groups (Fig. 8b and Fig. S6) showed rapid growth rates and were still intact without any sign of tumor necrosis, implying only SiNcOH-DSPE-PEG(NH2) NPs injection or NIR laser irradiation did not affect the tumor development. Therefore, the PTT/PDT combined treatment using SiNcOH-DSPE-PEG(NH2) NPs was more effective in destroying tumors than PDT alone, and SiNcOH-DSPEPEG(NH2) NPs are a type of promising PTT/PDT agent for in vivo cancer therapy.
3. ConclusionIn summary, we have constructed a novel SiNcOH-DSPE-PEG (NH2) NPs-based theranostic nanoplatform and demonstrated their excellent performance in PA imaging and combinatorial PTT/ PDT therapy of cancer. The aqueous dispersion of the SiNcOHDSPE-PEG(NH2) NPs exhibits good photothermal conversion efficiency and singlet oxygen generation capability upon a single 808 nm laser irradiation. In addition, the strong absorption at NIR region also endows these nanoparticles PA imaging functionality. After intravenous injection, the accumulation of SiNcOH-DSPEPEG(NH2) NPs in tumors can be monitored by PA imaging, and the tumors can be effectively ablated by PTT/PDT combination therapy under the irradiation of 1 W/cm2 808 nm laser. Our study provides a promising strategy for the design single-reagent based theranostic nanoplatform and application in molecular imaging-guided tumor phototherapy.
4. Experimental 4.1. MaterialsAll chemicals were obtained from commercial suppliers and used without further purification. Silicon 2, 3-naphthalocyanine dihydroxide and methylthiazolyldiphenyl-tetrazolium bromide (MTT) were purchased from Sigma-Aldrich. 1, 2-Distearoyl-snglycero-3-phosphoethanolamine-N-[methoxy(polyethyleneglycol)-2000] (ammonium salt) (DSPE-PEG) and 1, 2-distearoylsn-glycero-3-phosphoethanolamine-N-[amino(polyethylene-glycol)-2000] (ammonium salt) (DSPE-PEG-NH2) were obtained from Xiamen Sinopeg Biotech Co., Ltd.
4.2. CharacterizationSizes and morphologies of the nanoparticles were determined by a high-resolution transmission electron microscope (HRTEM, JEM-1400). UV-vis absorption spectra were measured on a Shimadzu UV2550 ultraviolet-visible spectrophotometer (Shimadzu) using quartz cuvettes with an optical path of 1 cm. Zeta potential and dynamic light scattering (DLS) were carried out on a Nano-ZS (Malvern Instruments). An optical fiber-coupled 808 nm diode laser (maximal power = 5 W, BWT Beijing Co., Ltd., China) was used to perform photothermal therapy. MTT experiments were carried out on an Infinite 200 PRO ELIASA.
4.3. Synthesis of SiNcOH-DSPE-PEG(NH2) NPsA solution of the hydrophobic SiNcOH (2 mg) in chloroform (2 mL) and a solution of DSPE-PEG (2 mg) and DSPE-PEG-NH2 (2 mg) in chloroform (4 mL) were mixed together with magnetic stirring for up to 10 h. Then the solution was spinned dry using the rotary evaporator in vacuum. Finally, the hydrophilic NPs were dispersed in water or phosphate buffer solution (PBS, pH 7.4) for further use.
4.4. Photothermal effect measurementFor investigating the photothermal effect induced by the NIR irradiation, 1 mL aqueous solutions containing different concentrations of SiNcOH-DSPE-PEG(NH2) NPs (20, 50, 100 and 150 ppm) were irradiated by an 808 nm laser (1.5 W/cm2) for 12 min, or the same concentration (100 ppm) was irradiated by an 808 nm laser with different power density (0.5, 1.0, 1.5 and 2.0 W/cm2) for 12 min. The temperatures of the solutions were monitored using a submerged thermocouple microprobe and recorded one time per 30 s. The photostability of SiNcOH-DSPE-PEG(NH2) NPs was measured by multiple cycles of laser-induced photothermal heating. The samples were irradiated with 808 nm laser for 10 min (laser on), followed by naturally cooling to room temperature without NIR laser irradiation for 30 min (laser off). This cycle repeated for five times. The photothermal conversion efficiency of SiNcOH-DSPE-PEG(NH2) NPs was determined according to previous method [45, 46]. Detailed calculation was given as following:
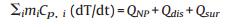
|
(1) |
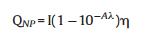
|
(2) |
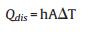
|
(3) |
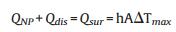
|
(4) |
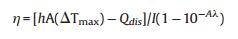
|
(5) |

|
(6) |

|
(7) |
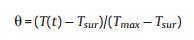
|
(8) |
m and Cp are the mass and heat capacity of water, respectively, T is the solution temperature, QNP is the energy inputted by SiNcOHDSPE-PEG(NH2) NPs, Qdis is the baseline energy inputted by the sample cell, and Qsur is heat conduction away from the system surface by air. I is incident laser power, η is the conversion efficiency from incident laser energy to thermal energy, and Aλ is the absorbance of the SiNcOH-DSPE-PEG(NH2) NPs at wavelength of 808 nm. h is heat transfer coefficient, A is the surface area of the container, and △T is ambient temperature of the surroundings.
4.5. Cytotoxicity assayThe in vitro cytotoxicity was measured using the methyl thiazolyl tetrazolium (MTT) assay in human cervical carcinoma cell line HeLa. HeLa cell line was obtained from the cell storeroom of Chinese Academy of Science and grown in RPMI-1640 culture medium containing 10% calf serum and 1% penicillin/streptomycin at 37 ℃ in the presence of 5% CO2 for 24 h. Then the cells were incubated with fresh medium containing the SiNcOH-DSPE-PEG (NH2) NPs at different concentrations (i.e., 0, 20, 50 and 100 ppm) for 24 h at 37 ℃. Subsequently, 15 mL of MTT (5 mg/mL) was added to each well of the 96-well assay plate and incubated for more 4 h at 37 ℃. And the cell medium was replaced by 150 mL of dimethyl sulfoxide (DMSO). Finally, the 96-well assay plates were gently shaken for 20 min at room temperature before measuring the absorbance at 490 nm.
4.6. Cellular uptake of SiNcOH-DSPE-PEG(NH2) NPsRBITC exhibits intense red fluorescence under PE inspire channel. To testify if SiNcOH-DSPE-PEG(NH2) NPs can be taken up by HeLa cells, the NPs were modified with RBITC by the interaction of amino groups and SCN. After incubation HeLa cells with SiNcOH-DSPE-PEG-RBITC NPs in medium for 12 h, the cells were washed three times with PBS and transferred to serum-free medium to examine and observe the fluorescence signal by the flow cytometry and confocal laser scanning microscopy.
4.7. Determination of intracellular reactive oxygen species (ROS) levelsIntracellular ROS levels were measured using 2', 7'-dichlorofluorescin diacetate (DCFH-DA). DCFH-DA is a fluorogenic marker for ROS, which permeates live cells and is deacetylated by intracellular esterases. In the presence of ROS, the reduced fluorescein compound is oxidized and emits bright green fluorescence. Pre-seeded HeLa cells were incubated with SiNcOH-DSPE-PEG(NH2) NPs suspension (100 μg/mL) for 12 h at 37 ℃ with 5% CO2. After washing the cells with PBS, 10 mmol/L DCFH-DA in serum free medium was added and incubated for 30 min. Next, the cells were washed twice with PBS to remove all excess DCFH-DA that had not penetrated into the cells. Then, the cells were irradiated by the 808 nm (0.1 W/cm2) laser for 10 min. Fluorescence intensity was measured on a confocal fluorescence microscope.
4.8. Photothermal/photodynamic cytotoxicityHeLa cells were incubated in 96-well plates at 37 ℃ with 5% CO2 for 24 h and exposed to various concentrations of SiNcOH-DSPEPEG(NH2) NPs for another 24 h. Then they were washed three times by the PBS to remove NPs that had not penetrated into the cells. Next, the cells were irradiated by 808 nm laser (0.1 W/cm2 or 1 W/cm2) for 6 min and the cell viabilities were determined by the standard MTT assay mentioned above. To visualize the photothermal cytoxicity of SiNcOH-DSPE-PEG(NH2) NPs, after irradiation by the 808 nm laser at 1 W/cm2 for 6 min, live and dead cells were stained by Calcein AM and Propidium iodide (PI). Confocal imaging of cells was performed using a Leica SP5 laser scanning confocal microscope.
4.9. Mouse tumor modelFemale Balb/c mice (weight ≈20 g) were obtained from Shanghai SLAC laboratory Animal Co., Ltd. All animals were performed in accordance with the Animal Management Rules of the Ministry of Health of the People's Republic of China and all the experiments were carried out under protocols approved by Xiamen University Laboratory Animal Center. The S180 tumor models were generated by subcutaneous injection (2 ×106 S180 cells in 50 μL phosphate buffer solution (PBS)) onto the right rear flanks of each mouse.
5. In vivo PA imagingPA imaging was performed using Nexus 128 scanner manufactured by Endra Life Sciences (Ann Arbor, MI, USA). 730 nm were chosen as the working laser wavelength with 30 pulses averaging. During the scanner, the water heating system maintains the water temperature at 38 ℃ to keep the mice comfort. PA scans were performed before the injection of the contrast agent. Then, SiNcOH-DSPE-PEG(NH2) NPs were injected into the mouse tail vein and the PA imaging was performed at different time.
5.1. Hematology studiesFemale Balb/c mice were randomly divided into test and control groups (n = 5). In the test group, the mice received an intravenous injection of SiNcOH-DSPE-PEG(NH2) NPs (200 μL, 1 mg/mL), while the mice with no injection were used as the control group. Blood were harvested from treatment and control groups after 24 h. Three important hepatic indicators (alanine aminotransferase (ALT), aspartate aminotransferase (AST) and alkaline phosphatase (ALP)) and one indicator for kidney functions (blood urea nitrogen (UREA)) in the blood were measured by the Automatic microplate reader TECAN Infinite 200 PRO.
5.2. In vivo NIR imagingTo image the temperature of tumors when they were exposed to laser, an infrared thermography (HM-300, Guangzhou SAT Infrared Technology Co., Ltd.) was used to capture the temperature change on the sites of the tumors.
5.3. Photothermal and photodynamic therapy in vivo using NIR laserThe photothermal and photodynamic therapy in vivo were carried out by using an optical fiber-coupled 808 nm high power laser diode (BWT Beijing Co., Ltd.). Experimentally, the tumor on each mouse was exposed to the 808 nm NIR laser with a power density of 0.1 W/cm2 or 1.0 W/cm2 for 5 min. Then, the tumor sizes were measured by a caliper every other day and calculated as the volume = (tumor length) × (tumor width)2/2. Relative tumor volumes were defined as the tumor change during the experiment, which were calculated as V/V0 (V0 was the initiated tumor volume when the treatment started, while V was the tumor volume during the treatment).
AcknowledgmentsThe work was supported by the National Natural Science Foundation of China (No. 21101131), National Basic Research Foundation (973) of China (No. 2014CB932004) and Natural Science Foundation of Fujian Province (No. 2016J01073). We thank Kang Ning in Lei Ren Professor's group and Ming-Xia Zhang in Chao-Yong Yang's group for help with the measurement of flow cytometry analysis.
Appendix A. Supplementary dataSupplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.cclet.2017.01.007.
| [1] | H. Liu, D. Chen, L. Li, et al., Multifunctional gold nanoshells on silica nanorattles:a platform for the combination of photothermal therapy and chemotherapy with low systemic toxicity. Angew. Chem. Int. Ed. 50(2011)891–895. DOI:10.1002/anie.201002820 |
| [2] | W. Fang, J. Yang, J. Gong, et al., Photo-and pH-Triggered Release of Anticancer Drugs from Mesoporous Silica-Coated Pd@Ag Nanoparticles. Adv. Funct. Mater. 22(2012)842–848. DOI:10.1002/adfm.201101960 |
| [3] | C. Wang, H. Xu, C. Liang, et al., Iron oxide@polypyrrole nanoparticles as a multifunctional drug carrier for remotely controlled cancer therapy with synergistic antitumor effect. ACS Nano 7(2013)6782–6795. DOI:10.1021/nn4017179 |
| [4] | M. Zheng, C. Yue, Y. Ma, et al., Single-step assembly of DOX/ICG loaded lipid-polymer nanoparticles for highly effective chemo-photothermal combination therapy. ACS Nano 7(2013)2056–2067. DOI:10.1021/nn400334y |
| [5] | S. Tang, M. Chen, N. Zheng. Multifunctional ultrasmall Pd nanosheets for enhanced near-infrared photothermal therapy and chemotherapy of cancer. Nano Res. 8(2014)165–174. |
| [6] | S. Shi, X. Chen, J. Wei, et al., Platinum(ⅳ) prodrug conjugated Pd@Au nanoplates for chemotherapy and photothermal therapy. Nanoscale 8(2016)5706–5713. DOI:10.1039/C5NR09120A |
| [7] | W. Miao, G. Shim, S. Lee, et al., Safety and tumor tissue accumulation of pegylated graphene oxide nanosheets for co-delivery of anticancer drug and photosensitizer. Biomaterials 34(2013)3402–3410. DOI:10.1016/j.biomaterials.2013.01.010 |
| [8] | M. Zhang, T. Murakami, K. Ajima, et al., Fabrication of ZnPc/protein nanohorns for double photodynamic and hyperthermic cancer phototherapy. Proc. Natl. Acad. Sci. U. S. A. 105(2008)14773–14778. DOI:10.1073/pnas.0801349105 |
| [9] | B. Jang, J.-Y. Park, C.-H. Tung, et al., Gold nanorod-photosensitizer complex for near-infrared fluorescence imaging and photodynamic/photothermal therapy in vivo. ACS Nano 5(2011)1086–1094. DOI:10.1021/nn102722z |
| [10] | S. Wang, P. Huang, L. Nie, et al., Single continuous wave laser induced photodynamic/plasmonic photothermal therapy using photosensitizer-functionalized gold nanostars. Adv. Mater. 25(2013)3055–3061. DOI:10.1002/adma.v25.22 |
| [11] | Z.X. Zhao, Y.Z. Huang, S.G. Shi, et al., Cancer therapy improvement with mesoporous silica nanoparticles combining photodynamic and photothermal therapy. Nanotechnology 25(2014)285701. DOI:10.1088/0957-4484/25/28/285701 |
| [12] | L. Cheng, C. Wang, L. Feng, et al., Functional nanomaterials for phototherapies of cancer. Chem. Rev. 114(2014)10869–10939. DOI:10.1021/cr400532z |
| [13] | M. Sun, L. Xu, W. Ma, et al., Hierarchical plasmonic nanorods and upconversion core-satellite nanoassemblies for multimodal imaging-guided combination Phototherapy. Adv. Mater. 28(2016)898–904. DOI:10.1002/adma.v28.5 |
| [14] | B. Tian, C. Wang, S. Zhang, et al., Photothermally enhanced photodynamic therapy delivered by nano-graphene oxide. ACS Nano 5(2011)7000–7009. DOI:10.1021/nn201560b |
| [15] | T.Y. Lin, W. Guo, Q. Long, et al., HSP90 Inhibitor encapsulated phototheranostic nanoparticles for synergistic combination cancer therapy. Theranostics 6(2016)1324–1335. DOI:10.7150/thno.14882 |
| [16] | R. Bonnett. Photosensitizers of the porphyrin and phthalocyanine series for photodynamic therapy. Chem. Soc. Rev. 24(1995)19–33. DOI:10.1039/cs9952400019 |
| [17] | X. Huang, I.H. El-Sayed, W. Qian, et al., Cancer cell imaging and photothermal therapy in the near-infrared region by using gold nanorods. J. Am. Chem. Soc. 128(2006)2115–2120. DOI:10.1021/ja057254a |
| [18] | Y.N. Konan, R. Gurny, E. Allémann. State of the art in the delivery of photosensitizers for photodynamic therapy. J. Photochem. Photobio. B 66(2002)89–106. DOI:10.1016/S1011-1344(01)00267-6 |
| [19] | J. Mou, T. Lin, F. Huang, et al., Black titania-based theranostic nanoplatform for single NIR laser induced dual-modal imaging-guided PTT/PDT. Biomaterials 84(2016)13–24. DOI:10.1016/j.biomaterials.2016.01.009 |
| [20] | L. Gao, J. Fei, J. Zhao, et al., Hypocrellin-loaded gold nanocages with high twophoton efficiency for photothermal/photodynamic cancer therapy in vitro. ACS Nano 6(2012)8030–8040. DOI:10.1021/nn302634m |
| [21] | J.C. Kah, R.C. Wan, K.Y. Wong, et al., Combinatorial treatment of photothermal therapy using gold nanoshells with conventional photodynamic therapy to improve treatment efficacy:an in vitro study. Laser. Surg. Med. 40(2008)584–589. DOI:10.1002/lsm.v40:8 |
| [22] | W.S. Kuo, Y.T. Chang, K.C. Cho, et al., Gold nanomaterials conjugated with indocyanine green for dual-modality photodynamic and photothermal therapy. Biomaterials 33(2012)3270–3278. DOI:10.1016/j.biomaterials.2012.01.035 |
| [23] | S. Shi, X. Zhu, Z. Zhao, et al., Photothermally enhanced photodynamic therapy based on mesoporous Pd@Ag@mSiO2 nanocarriers. J. Mater. Chem. B 1(2013)1133. DOI:10.1039/c2tb00376g |
| [24] | Z. Zhao, S. Shi, Y. Huang, et al., Simultaneous photodynamic and photothermal therapy using photosensitizer-functionalized Pd nanosheets by single continuous wave laser. ACS Appl. Mater. Interface 6(2014)8878–8885. DOI:10.1021/am501608c |
| [25] | Y. Huang, X. Chen, S. Shi, et al., Effect of glutathione on in vivo biodistribution and clearance of surface-modified small Pd nanosheets. Sci. China Chem. 58(2015)1753–1758. |
| [26] | G. Gollavelli, Y.C. Ling. Magnetic and fluorescent graphene for dual modal imaging and single light induced photothermal and photodynamic therapy of cancer cells. Biomaterials 35(2014)4499–4507. DOI:10.1016/j.biomaterials.2014.02.011 |
| [27] | P. Zhang, H. Huang, J. Huang, et al., Noncovalent ruthenium(Ⅱ) complexessingle-walled carbon nanotube composites for bimodal photothermal and photodynamic therapy with near-infrared irradiation. ACS Appl. Mater. Interfaces 7(2015)23278–23290. DOI:10.1021/acsami.5b07510 |
| [28] | T. Liu, C. Wang, W. Cui, et al., Combined photothermal and photodynamic therapy delivered by PEGylated MoS 2 nanosheets. Nanoscale 6(2014)11219–11225. DOI:10.1039/C4NR03753G |
| [29] | Y. Yong, L. Zhou, Z. Gu, et al., WS2 nanosheet as a new photosensitizer carrier for combined photodynamic and photothermal therapy of cancer cells. Nanoscale 6(2014)10394. DOI:10.1039/C4NR02453B |
| [30] | X. Tan, X. Pang, M. Lei, et al., An efficient dual-loaded multifunctional nanocarrier for combined photothermal and photodynamic therapy based on copper sulfide and chlorin e6. Int. J. Pharm. 503(2016)220–228. DOI:10.1016/j.ijpharm.2016.03.019 |
| [31] | S. Wang, A. Riedinger, H. Li, et al., Plasmonic copper sulfide nanocrystals exhibiting near-infrared photothermal and photodynamic therapeutic effects. ACS Nano 9(2015)1788–1800. DOI:10.1021/nn506687t |
| [32] | L. Han, Y. Zhang, X.-W. Chen, et al., Protein-modified hollow copper sulfide nanoparticles carrying indocyanine green for photothermal and photodynamic therapy. J. Mater. Chem. B 4(2016)105–112. |
| [33] | Y. Huang, Y. Lai, S. Shi, et al., Copper sulfide nanoparticles with phospholipid-PEG coating for in vivo near-infrared photothermal cancer therapy. Chem. Asian. J. 10(2015)370–376. DOI:10.1002/asia.v10.2 |
| [34] | Y.K. Kim, H.K. Na, S. Kim, et al., One-pot synthesis of multifunctional Au@graphene oxide nanocolloid core@shell nanoparticles for Raman bioimaging, photothermal, and photodynamic therapy. Small 11(2015)2527–2535. DOI:10.1002/smll.v11.21 |
| [35] | E. Crescenzi, L. Varriale, M. Iovino, et al., Photodynamic therapy with indocyanine green complements and enhances low-dose cisplatin cytotoxicity in MCF-7 breast cancer cells. Mol. Cancer. Ther. 3(2004)537–544. |
| [36] | L. Wu, S. Fang, S. Shi, et al., Hybrid polypeptide micelles loading indocyanine green for tumor imaging and photothermal effect study. Biomacromolecules 14(2013)3027–3033. DOI:10.1021/bm400839b |
| [37] | O. Taratula, C. Schumann, T. Duong, et al., Dendrimer-encapsulated naphthalocyanine as a single agent-based theranostic nanoplatform for near-infrared fluorescence imaging and combinatorial anticancer phototherapy. Nanoscale 7(2015)3888–3902. DOI:10.1039/C4NR06050D |
| [38] | C.K. Lim, J. Shin, Y.D. Lee, et al., Phthalocyanine-aggregated polymeric nanoparticles as tumor-homing near-infrared absorbers for photothermal therapy of cancer. Theranostics 2(2012)871–879. DOI:10.7150/thno.4133 |
| [39] | S. Karan, B. Mallik. Templating effects and optical characterization of copper (Ⅱ) phthalocyanine nanocrystallites thin film:nanoparticles, nanoflowers, nanocabbages, and nanoribbons. J. Phys. Chem. C. 111(2007)7352–7365. DOI:10.1021/jp070302o |
| [40] | M. Triesscheijn, P. Baas, J.H. Schellens, et al., Photodynamic therapy in oncology. Oncologist 11(2006)1034–1044. DOI:10.1634/theoncologist.11-9-1034 |
| [41] | S. Mathew, T. Murakami, H. Nakatsuji, et al., Exclusive photothermal heat generation by a gadolinium bis (naphthalocyanine) complex and inclusion into modified high-density lipoprotein nanocarriers for therapeutic applications. ACS Nano 7(2013)8908–8916. DOI:10.1021/nn403384k |
| [42] | A.K. Singh, M.A. Hahn, L.G. Gutwein, et al., Multi-dye theranostic nanoparticle platform for bioimaging and cancer therapy. Int. J. Nanomed. 7(2012)2739–2750. |
| [43] | Y. Jin, F. Ye, M. Zeigler, et al., Near-infrared fluorescent dye-doped semiconducting polymer dots. ACS Nano 5(2011)1468–1475. DOI:10.1021/nn103304m |
| [44] | L. Song. Naphthalocyanine-reconstituted LDL nanoparticles for in vivo cancer imaging and treatment. Int. J. Nanomed. 2(2007)767–774. |
| [45] | Q. Tian, F. Jiang, R. Zou, et al., Hydrophilic Cu9S5 nanocrystals:A photothermal agent with a 25.7% heat conversion efficiency for photothermal ablation of cancer cells in vivo. ACS Nano 5(2011)9761–9771. DOI:10.1021/nn203293t |
| [46] | D.K. Roper, W. Ahn, M. Hoepfner. Microscale heat transfer transduced by surface plasmon resonant gold nanoparticles. J. Phys. Chem. C 111(2007)3636–3641. DOI:10.1021/jp064341w |
| [47] | G. Ku, M. Zhou, S. Song, et al., Copper sulfide nanoparticles as a new class of photoacoustic contrast agent for deep tissue imaging at 1064nm. ACS Nano 6(2012)7489–7496. DOI:10.1021/nn302782y |
| [48] | M. Li, X. Yang, J. Ren, et al., Using grapheneoxide highnear-infrared absorbance for photothermal treatment of Alzheimer's disease. Adv. Mater. 24(2012)1722–1728. DOI:10.1002/adma.201104864 |
| [49] | C. Kim, C. Favazza, L.V. Wang. In vivo photoacoustic tomography of chemicals:high-resolution functional and molecular optical imaging at new depths. Chem. Rev. 110(2010)2756–2782. DOI:10.1021/cr900266s |
| [50] | M. Chen, S. Tang, Z. Guo, et al., Core-shell Pd@Au nanoplates as theranostic agents for in-vivo photoacoustic imaging CT imaging, and photothermal therapy. Adv. Mater. 26(2014)8210–8216. DOI:10.1002/adma.201404013 |
 2017, Vol. 28
2017, Vol. 28 


