b University of Chinese Academy Sciences, Beijing 100049, China;
c National Center for Nanoscience and Technology, Beijing 100190, China
Styrylquinoxaline (SQ) derivatives are widely used dye molecules because of their acidichromism, photochemical sensitivities, and potential biological activities [1-5]. SQ contains a vinyl group, which can undergo reversible trans-cis isomerization or [2+2] dimerization upon photo-illumination. Photoisomerization of SQ mainly takes place in methanol solutions; while photodimerization can be achieved in Langmuir-Blodgett (LB) films because of the two dimensionally confined environments [2, 3]. The [2+2] photodimerization is a topo-chemical reaction which is sensitive to the geometrical arrangement between molecules [6, 7]. Previous studies have demonstrated that the length of alkyl chain in the amphiphilic SQ derivatives can greatly affect the occurrence of photodimerizations in LB films [3].
In addition, LB films of SQ derivatives have exhibited interesting acidichromism phenomena [2, 3]. When exposed to HCl gas and NH3 gas, the color of SQ films changes reversibly. Acidichromism of photochemical species has potential applications in pH sensors, photo-and chemical-switching systems and gas controlled reversible color-change devices [8]. Accompanied by the color changes of acidichromism, the contact with acidic gases also alters optical absorption characteristics, molecular structures, surface morphologies and supramolecular chirality of the LB films [2]. However, to our best knowledge, the influences of acidity on photochemical properties of SQ derivatives have not been explored yet.
In the present work, we focused on the effects of subphase acidity on the morphologies and the photodimerization kinetics of a SQ derivative, 3-(4-(octadecyloxy)styryl)quinoxalin-2(1H)-one (SQC18, Scheme 1) in single-layered LB films. Surface pressure-area (π-A) isotherms and AFM measurements were used to characterize the phase transition behaviors and surface topography, while the real-time second harmonic generation (SHG) was employed to monitor the photodimerization kinetics. The SHG technique, similar to sum frequency generation (SFG) spectroscopy, is a second-order nonlinear optical probe with unique surface selectivity and submonolayer sensitivity [9-13]. Previous studies have shown that the real time SHG is well suited for studying the photochemical reactions in ultrathin films or monolayers, especially for the chromophores with large hyperpolarizabilities [14-17]. In this work, the SHG measurements revealed the strong dependence of photodimerization processes of SQC18 monolayers on the subphase pH. The possible mechanisms of the acidic effects were proposed.
|
Download:
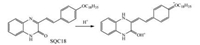
|
| Scheme1. Protonation reaction of SQC18 under acidic conditions. | |
2. Results and discussion 2.1. Monolayer formation at the air/water interface
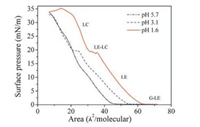
Fig. 1 shows the π-A isotherms of SQC18 on the subphases with different pH values at room temperature. SQC18 molecules formed stable monolayers at the air/aqueous interfaces. At pH 3.1 or 1.6, several regions can be identified: a coexistence of the gas (G) phase and the liquid expanded (LE) phase, a LE phase, a plateau characteristic of the coexistence of the LE and the liquid condensed (LC) phase, and the pure LC phase [21, 22]. For pH 5.7, there was no obvious transition from the LE phase to the LE-LC coexistence phase.
|
Download:
|
| Fig. 1. Surface pressure-area (π-A) isotherms of SQC18 on aqueous subphases with different pH values at room temperature. | |
By extrapolating the linear part of the isotherms to zero surface pressure, we obtained the limiting areas per molecule, which were 0.44 nm2, 0.53 nm2 and 0.62 nm2 for pH 5.7, 3.1 and 1.6, respectively. According to the Corey-Pauling-Koltun (CPK) model [2, 3, 23], the areas occupied by the vertically and flatly oriented aromatic ring of SQC18 are 0.25 and 0.78 nm2 per molecule, respectively. Therefore the aromatic rings of SQC18 were tilted at the air/aqueous interfaces and oriented more towards the surfaces at lower pH.
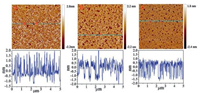
2.2. Effect of the acidity on monolayer morphologiesFig. 2 shows the AFM images of the single-layer LB films of SQC18 transferred at different pH values (pH 5.7, 3.1 and 1.6). The transfer pressure of monolayers was kept at 22 mN/m for all of the three pH values. Stick-like structures were clearly observed in the LB film deposited from pure water subphase (pH 5.7). For LB films transferred from the acidic subphases, the stick-like structures became smaller and sparser, especially for pH 1.6 where the sticklike structures were almost replaced by granular domains. The disappearance of stick-like structures indicated that closely packed face-to-face aggregation of SQC18 was inhibited on acidic subphases [2].
|
Download:
|
| Fig. 2. AFM images of SQC18 LB monolayers on mica substrates deposited from subphases with pH of (a) 5.7, (b) 3.1, and (c) 1.6. The scan areas were 5 μm × 5 μm for all cases. | |
The lower panels in Fig. 2 show the heights of aggregated structures along the blue dotted lines in the AFM images of the upper panels. From the depth analysis, the heights of the domains were mostly distributed in the range of 1.75-3.0 nm for pH 5.7, 1.5-2.5 nm for pH 3.1 and 1.5-2.0 nm for pH 1.6. These changes of height distributions implied that SQC18 molecules tilted further away from the surface normal on acidic subphases, which was consistent with the results of the π-A isotherm measurements.
The above morphological rearrangements of the SQC18 LB monolayer can be explained by the changes of molecular structures when SQC18 is protonated. The chemical changes of SQ derivative LB films when exposed to HCl gas has been identified by FT-IR and XRD in previous studies [2, 3]. The similar reaction between the SQC18 monolayer and subphase acid are shown in Scheme 1. The conjugation between the quinoxaline and benzene ring is destroyed during the reaction, possibly causing the destruction of face-to-face aggregation of SQC18 as observed in the AFM images. Additionally, the quinoxaline nitrogen is protonated to form the more hydrophilic N-H group, resulting in the reorientation of quinoxaline ring towards the surface.
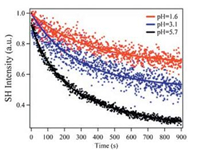
2.3. Effect of the acidity on photodimerization kineticsTo evaluate the acidic effect on the photodimerization kinetics of SQC18, the monolayers at different subphase pH values were transferred to the fused silica substrates, followed by the time dependent SHG measurements. As displayed in Fig. 3, the SHG signals from SQC18 LB monolayers gradually decayed before reaching equilibrium. The initially large SHG signals at time zero were due to the near-resonant enhancement of SHG signals for SQC18 monomers [18]. This was confirmed by the UV-vis measurements of SQC18 monomer showing a broad absorption maximum centered at 382 nm [2, 3], of which the absorption range fully covered the second harmonic wavelength of 395 nm. After photodimerization, the formation of dimer damaged the conjugated structure, and the absorption cross section around 382 nm significantly decreased for the dimers [2, 3]. This led to smaller molecular hyperpolazabilities and SHG intensities for the dimers at the second harmonic wavelength. Therefore, the time-dependent decay of SHG signals in Fig. 3 was a result of the SQ photodimerization. In the current study, the photodimerization of SQC18 was triggered by the fundamental laser beam at 790 nm via a twophoton process, which was confirmed by an additional laser power dependent experiment. This phenomenon was similar to the twophoton induced spiropyran-merocyanine isomerization at the air/ water interface reported in a recent SHG study [18].
|
Download:
|
| Fig. 3. Typical time dependence of normalized SHG intensities of SQC18 LB monolayers on fused silica substrates deposited from subphases with different pH values. | |
The two-photon induced dimeraiztion of SQC18 in the LB monolayer showed the first-order dependence on the surface molecular densities and the second-order dependence on the incident laser power. Therefore the time-dependent SHG curves in Fig. 3 can be fit by Eq. (1).

|
(1) |
where I(t) is the normalized SHG intensity at time t, k is the photodimeriation rate constant, while the ratio of a/(1 + a) defines the percentages of monomers that do not convert to the dimers at the photodimerization equilibrium. In other words, the percentage yields of the photodimerization can be quantified by the ratio of 1/ (1 + a). The detailed derivation of Eq. (1) is beyond the scope of this short letter and will be described in a follow-up article. The SQC18 monolayer at both the air/water interface and solid substrates was not spatially uniform. Microstructured domains were randomly distributed on the surfaces. Therefore we measured at least 20 different positions for each LB film. The statistical results for the photodimerization rate constant k as well as the a/(1 + a) ratios were summarized in Figs. 4 and 5.
|
Download:
|
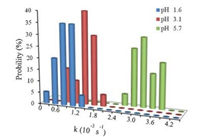
| Fig. 4. Statistics of rate constants (k) for different pH. | |
|
Download:
|
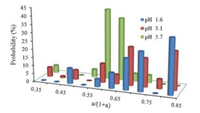
| Fig. 5. Statistics of the a/(1 + a) ratios at different pH values. This ratio defines the percentages of monomers that did not participate in the photodimerization reactions. | |
As seen from Fig. 4, the reaction rate constants for pure water subpahse (pH 5.7) were in the range of 2.7-3.9 ×10-3 s-1. But for the acidic subphases with pH 3.1 and 1.6, the rate constants dropped dramatically, mainly falling in the range of 1.2-1.8 × 10-3 s-1 for pH 3.1 and in the range of 0.6-1.2 ×10-3 s-1 for pH 1.6. These results indicated the slower photodimerization of SQC18 molecules in acidic environments.
The percentages of monomers that didn't participate in the photodimerizations were shown in Fig. 5. For pH 5.7, about 50%-65% monomers remained unreacted upon the laser radiation, while for pH 3.1 and 1.6, this percentage raised to 65%-85%. Therefore, less number of chromophores participated in the photodimerizations at lower pH.
2.4. Mechanism of acidic effects on photodimerizationThe increasing a/(1 + a) ratios for lower pH values can be easily understood. As shown by Scheme 1, a large number of SQC18 molecules are expected to be protonated on acidic subphases. The change of conjugation structures makes photodimerization difficult for the protonated molecules at the current laser wavelength. As a result, only the remaining small number of unprotonated SQC18 molecules can effectively participate in the photodimerizations. The fact that the photodimerization was still observed on acidic subphases implies that the protonation is not 100% even at pH as low as 1.6.
On the other hand, the decreasing dimerization rate constants with the smaller number of unprotonated SQC18 at lower pH are unexpected at some extent. The change of reactant concentrations is not supposed to affect the rate constants in gas phase or bulk solution reactions. However, the photodimerization of SQC18 occurred within the LB monolayer. Unlike the bulk species that can freely diffuse, SQC18 molecules were loosely anchored on solid substrate and only had the limited fluidity. As a result, the unprotonated SQC18 molecules at lower pH were surrounded by the protonated molecule and had few chances to encounter with each other. This made the dimerization significantly more difficult because the formation of dimer requires the participation of two closely spaced monomers.
The partial protonation of SQC18 affected the dimerization rate constant also through the change of molecular aggregation structures. As we discussed earlier in this letter, the conjugated π-electron distributions were disturbed by the protonation, causing the destruction of the π-π stacking and face-to-face aggregation structures [2, 24]. Moreover, the change of hydrophilicity of the quinoxaline ring upon protonation made the headgroup lie flat on the surfaces. Both of these changes resulted in the increasing average intermolecular distances between SQC18 molecules at lower pH, which was also confirmed by the π-A isotherm measurements in Fig. 1. According to the "topochemical postulate" [25], photodimerizations can occur only if the reacting double bonds are parallel to each other and their distance is less than 4.2 Å. The increasing intermolecular distances and the destruction of the face-to-face configurations are both expected to lower the photodimerization rate constants. Therefore from the current study, we confirm that the photochemical reactions within the LB monolayers are sensitive to the intermolecular interactions and geometrical arrangements within the aggregations.
3. ConclusionIn summary, we studied the effects of acidity on the morphologies and photodimerization kinetics of the SQC18 LB monolayers. We found that SQC18 tilted more towards the surface and the photodimerization rates were slower in LB monolayers deposited from subphases with lower pH. We proposed that the acid changed the hydrophilicity and conjugated π-electron distributions of the SQC18 headgroups, which subsequently broke the face-to-face aggregation structures and hindered the photodimerization processes. These findings demonstrate that the photodimerization process in ultrathin films could be controlled by adjusting the pH of the subphase. The observed dependence of photodimerization rate constant on surface densities of unprotonated SQC18 molecules and its aggregation structures indicate that the reaction mechanisms at surfaces are significantly different from those in the bulk.
4. ExperimentalSQC18 was synthesized according to the procedure described previously [2]. Chloroform and hydrochloride acid were analytical reagents and were used without further purification. Millipore water (18 MV cm) was used in all cases. To avoid the trans-to-cis isomerization of SQC18, all experiments were carried out in the dark.
Measurements of surface pressure-area (π-A) isotherms were performed on a KSV NIMA minitrough (Helsinki, Finland). The Langmuir monolayers of SQC18 were formed by spreading 30mL chloroform solution (0.9 mmol/L) on the aqueous subphases. The pH of the subphase was adjusted by adding hydrochloric acid and measured with a pH meter (FE20K, Mettler Toledo). After evaporation of chloroform for 20 min, the surface pressure-area (π-A) isotherms were measured with two barriers compressing the films at a speed of 10 mm/min. The LB monolayer deposition was also carried out using the same minitrough, where the monolayer at the air/aqueous interface was transferred to either a fused silica substrate or cleaved mica by a vertical lifting method at speed of ~11 mm/min. In this study, all the LB monolayers were prepared at the surface pressure of 22 mN/m.
To characterize the morphologies of the SQ18 monolayers, the atomic force microscopy (AFM) measurements of the LB monolayers on mica substrates were performed by a Digital Instrument Nanoscope IIIa Multimode system (Santa Barbara, CA). The AFM images were recorded using the tapping mode with silicon cantilever probes.
Real-time SHG measurements were used to record the photodimerization kinetics of the SQC18 monolayers on fused silica substrates. A detailed description of the SHG setup can be found in previous papers [18-20]. Briefly, a laser beam centered at 780 nm (82 MHz, pulse width ~80 fs) was focused on the sample surface with an incident angle of 70° against the sur-face normal. The SHG signals at 390 nm were collected in a reflective geometry with a photo-multiplier (R585, Hamamatsu) and a photon counter (SR400, Stanford). A short-pass filter and a monochromator were used to remove the fundamental light from the SHG signals. The incident light was p-polarized while the SHG signals with all possible polarizations were collected.
AcknowledgmentsThis work was supported by the National Natural Science Foundation of China (Nos. 21473217, 21227802 and 21303216) and the Chinese Ministry of Science and Technology (No. 2013CB834504).
| [1] | M.S. Kim, K.T. Lee, B.M. Jeong, et al., Photochemical trans-cis isomerization of 6-styrylquinoxaline. Photochem. Photobiol. 54(1991)517–524. DOI:10.1111/php.1991.54.issue-4 |
| [2] | M.F. Yin, H.F. Gong, B.W. Zhang, et al., Photochemical reaction, acidichromism, and supramolecular nanoarchitectures in the langmuir-blodgett films of an amphiphilic styrylquinoxaline derivative. Langmuir 20(2004)8042–8048. DOI:10.1021/la0490745 |
| [3] | L. Liu, L. Zhang, T. Wang, et al., Interfacial assembly of amphiphilic styrylquinoxalines:alkyl chain length tunable topochemical reactions and supramolecular chirality. Phys. Chem. Chem. Phys. 15(2013)6243–6249. DOI:10.1039/c3cp50384d |
| [4] | R.D. Dillard, D.E. Pavey, D.N. Benslay. Synthesis and antiinflammatory activity of some 2, 2-dimethyl-1, 2-dihydroquinolines. J. Med. Chem. 16(1973)251–253. DOI:10.1021/jm00261a019 |
| [5] | E.N. Gulakova, A.G. Sitin, L.G. Kuz'mina, et al., Synthesis and structure of styrylsubstituted azines. Russ. J. Org. Chem. 47(2011)245–252. DOI:10.1134/S107042801102014X |
| [6] | L.P. Xu, C.J. Yan, L.J. Wan, et al., Light-induced structural transformation in selfassembled monolayer of 4-(amyloxy)cinnamic acid investigated with scanning tunneling microscopy. J. Phys. Chem. B 109(2005)14773–14778. DOI:10.1021/jp052959k |
| [7] | H. Fan, X. Zhu, L. Gao, et al., The photodimerization of a cinnamoyl moiety derivative in dilute solution based on the intramolecular chain interaction of gemini surfactant. J. Phys. Chem. B 112(2008)10165–10170. DOI:10.1021/jp8017666 |
| [8] | H.F. Gong, C.M. Wang, M.H. Liu, et al., Acidichromism in the langmuir-blodgett films of novel photochromic spiropyran and spirooxazine derivatives. J. Mater. Chem. 11(2001)3049–3052. DOI:10.1039/b100711o |
| [9] | C.S. Tian, Y.R. Shen. Recent progress on sum-frequency spectroscopy. Surf. Sci. Rep. 69(2014)105–131. DOI:10.1016/j.surfrep.2014.05.001 |
| [10] | W. Gan, B. Xu, H.L. Dai. Activation of thiols at a silver nanoparticle surface. Angew. Chem. Int. Ed. 50(2011)6622–6625. DOI:10.1002/anie.v50.29 |
| [11] | X. Li, G.H. Deng, R.J. Feng, et al., Salt effect on molecular orientation at air/liquid methanol interface. Chin. Chem. Lett. 27(2016)535–539. DOI:10.1016/j.cclet.2016.01.004 |
| [12] | S.R. Walter, K.L. Young, J.G. Holland, et al., Counting the number of magnesium ions bound to the surface-immobilized thymine oligonucleotides that comprise spherical nucleic acids. J. Am. Chem. Soc. 135(2013)17339–17348. DOI:10.1021/ja406551k |
| [13] | S. Ye, H. Li, W. Yang, et al., Accurate determination of interfacial protein secondary structure by combining interfacial-sensitive amide Ⅰ and amide Ⅲ spectral signals. J. Am. Chem. Soc. 136(2014)1206–1209. DOI:10.1021/ja411081t |
| [14] | W. Wu, H. Fang, F. Yang, et al., Understanding the different steps of surfactant adsorption at the oil-water interface with second harmonic generation. J. Phys. Chem. C 120(2016)6515–6523. |
| [15] | W.K. Zhang, H.F. Wang, D.S. Zheng. Quantitative measurement and interpretation of optical second harmonic generation from molecular interfaces. Phys. Chem. Chem. Phys. 8(2006)4041–4052. DOI:10.1039/b608005g |
| [16] | M. Schulze, M. Utecht, T. Moldt, et al., Nonlinear optical response of photochromic azobenzene-functionalized self-assembled monolayers. Phys. Chem. Chem. Phys. 17(2015)18079–18086. DOI:10.1039/C5CP03093E |
| [17] | M. Schulze, M. Utecht, A. Hebert, et al., Reversible photoswitching of the interfacial nonlinear optical response. J. Phys. Chem. Lett. 6(2015)505–509. DOI:10.1021/jz502477m |
| [18] | L. Lin, Z. Zhang, Z. Lu, et al., Two-photon-induced isomerization of spiropyran/merocyanine at the air/water interface probed by second harmonic generation. J. Phys. Chem. A 120(2016)7859–7864. |
| [19] | K. Lv, L. Lin, X. Wang, et al., Significant chiral signal amplification of langmuir monolayers probed by second harmonic generation. J. Phys. Chem. Lett. 6(2015)1719–1723. DOI:10.1021/acs.jpclett.5b00472 |
| [20] | L. Lin, T. Wang, Z. Lu, et al., In situ measurement of the supramolecular chirality in the langmuir monolayers of achiral porphyrins at the air/aqueous interface by second harmonic generation linear dichroism. J. Phys. Chem. C 118(2014)6726–6733. DOI:10.1021/jp4106579 |
| [21] | M. Sovago, G.W.H. Wurpel, M. Smits, et al., Calcium-induced phospholipid ordering depends on surface pressure. J. Am. Chem. Soc. 129(2007)11079–11084. DOI:10.1021/ja071189i |
| [22] | G. MaH.C. Allen. Condensing effect of palmitic acid on dppc in mixed langmuir monolayers. Langmuir 23(2007)589–597. DOI:10.1021/la061870i |
| [23] | Y. Xu, Y.Q. Liu, J. Wu, et al., Preparation and electrical conductivityof LangmuirBlodgett films of poly(3-alkylthiophene)s. J. Appl. Polym. Sci. 69(1998)1–6. DOI:10.1002/(ISSN)1097-4628 |
| [24] | A.A. Parent, D.H. Ess, J.A. Katzenellenbogen. π-π interaction energies as determinants of the photodimerization of mono-, di-, and triazastilbenes. J. Org. Chem. 79(2014)5448–5462. DOI:10.1021/jo500457n |
| [25] | S. d'Agostino, F. Spinelli, E. Boanini, et al., Single crystal to single crystal[2+2] photoreactions in chloride and sulphate salts of 4-amino-cinnamic acid via solid-solution formation:a structural and kinetic study. Chem. Commun. 52(2016)1899–1902. DOI:10.1039/C5CC09412G |
 2017, Vol. 28
2017, Vol. 28 


