b College of Science, Agricultural University of Hebei, Baoding 071001, China;
c Medical School, Hebei University, Baoding 071002, China;
d Department of Chemistry and Center for Diagnostics and Therapeutics, Georgia State University, Atlanta, GA 30303, USA
Fluorescent sensors are important tools because they are useful probes for mapping the spatial and temporal distribution of biological analytes [1, 2]. Boronic acid-based molecular receptors for saccharides have attracted considerable interests due to their ability to bind saccharides in aqueous media [3-6]. In this case, the most common interactions are with cis-1, 2-or 1, 3-saccharide diols to form five-or six-membered rings with the boronic acid moiety. Formation of such cyclic esters leads to an increase in the Lewis acidity of a central boron atom, and this property has been exploited to create saccharide sensing molecules [7]. The strength of boronic acid binding to saccharidesis determined by the orientation and relative positions of hydroxyl groups; thus boronic acids can differentiate structurally similar saccharide molecules. For almost a decade, efforts have been made to achieve and improve selectivity for agiven saccharide target by using properly positioned boronic acids and by offering multivalent interactions that enhance both affinity and selectivity [8].
Cell surface carbohydrates, such as sialyl Lewis X (sLex), sialyl Lewis A (sLea) and Lewis Y (Ley) (Fig. 1) [9], as part of glycosylated proteins, have been associated with different types of cells. These surface carbohydrates present a characteristic signature of the development and progression of many forms of cancer, such as hepatocellular carcinoma (HCC), one of the most common carcinomas worldwide [10, 11]. Tumor-selective diagnostic agents rely on physiological differences between tumor cells and normal, healthy cells [12]. The over expression of certain cell-surface receptors and other biomarkers by tumor cells provides a convenient mean for selective imaging of malignancies with fluorescent probes [12]. Biosensors that could both recognize occult metastasis and provide targeted delivery of treatment could potentially improve the chance for success in therapy. Therefore, the exploration for early diagnostic biomarkers of cancer has become a research hotspot [13].

|
Download:
|
| Fig. 1. Structures of Lewis oligosaccharides and bisboronic acid compound 1. | |
Boron-based probes in particular have been of interest as early diagnostics for disease epitopes [14] due to their selective carbohydrate recognition. For example, the development of sensors to recognize sLex is invaluable for the diagnosis and early detection of cancers [15-17]. In 2002, Wang and co-workers [18] designed and synthesized a series of fluorescent anthracene-basedbisboronic acid probes by changing the linker between the bisboronic acid units, producing receptors for the detection of sialyl Lewis X (sLex). Among them, compound 1 (Fig. 1) with the strongest affinity for sLex displayed selective labeling of sLex expressing HepG2 cells, whilst non-sLex-expressing cells were not labeled in the control experiments. The monomer of 1 is based on a system that Shinkai developed [19, 20], which was proposed as a photoinduced electron transfer (PET) [15] moiety with the detailed mechanism still being a subject of research (Scheme 1) [21-23].

|
Download:
|
| Scheme1. Illustration of the anthracene-based photoinduced electron transfer system. | |
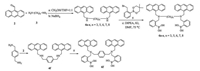
Inspired by these positive results, herein we would like to report a series of novel fluorescence sensors 6(a-f) (Scheme 2), in which two anthracene-based boronic acid units were linked by alkyl chains. Linkers with different length, rigidity, and spatial orientation play key roles in search of an optimal arrangement of the two boronic acid units, which would improve the selectivity for mono/oligosaccharide. The modular nature of 6 makes it easy to vary the linker structure.
|
Download:
|
| Scheme2. Synthesis of the bisboronic acid compounds 6a-f. | |
2. Results and discussion
To validate the complexation of the synthesized bisboronic acids and diol, fluorescence binding experiments of the bisboronic sensors with various mono/disaccharides were conducted in a mixture (v/v, 3:2) of MeOH and PBS (0.1 mol/L phosphate buffer solution, pH 7.4). The concentration of the sensors was fixed at 1 μmol/L, while the concentration of the mono/disaccharide was gradually increased. Based on the principles of PET (photoelectron transfer) [19-24], binding of the boronic acid sensors with the target sugars should increase their respective fluorescence intensities. As expected, the fluorescence intensities of 6(a-f) increased significantly with the addition of saccharides (Shown in supporting information). The apparent binding constants (K) of the bisboronic acids were calculated [15] by fitting the emission intensity at 415 nm versus concentration and are given in Table 1 and Fig. 2. As shown in the 3D view (Fig. 2), most of the tested saccharides expressed low binding affinity with the bisboronic acids, expect 6d and 6e with glucose and fructose, respectively. The marked selectivity of 6d and 6e made them have useful sensors in glucose or fructose recognitions, which suggested that separation of the two anthracene-based boronic acid units by a long distance (n = 7, 8) [25, 26] would be beneficial for recognizing glucose or fructose for such bisboronic acids. It should be noted that the apparent binding constants of 6d, 6e for glucose put their sensitive detection range near the physiological concentrations of glucose.
|
|
Table 1 Binding constants K for the saccharides complexes with the bisboronic acids 6(a-f). |

|
Download:
|
| Fig. 2. The 3D view of the K values for the saccharides complexing with 6(a--e). | |
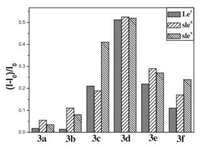
Further, to validate the complexation of the sensors 6(a-f) and cell surface carbohydrates, fluorescence binding experiments of the sensors with various Lewis oligosaccharides were conducted in a mixture (3:2, v/v) of MeOH and PBS (0.1 mol/L, pH 7.4). The concentration of sensors was fixed at 10 μmol/L, while oligosaccharides were set at 60 μmol/L chosen on the literatures precedent [18]. A favorable interaction of the probes with the sLex antigen was observed. Among them, 6c exhibited the strongest fluorescence enhancement (41%) upon binding with sLex at 10 μmol/L (Fig. 3), but relatively low increases with other Lewis oligosaccharides, which suggested that 6c recognize sLex with certain selectivity over other sugars. One could possibly state that 6c displays a two-binding site model between the interactions of hydroxyl groups of the saccharide (sLex) and the bisboronic acid units (receptor). Sensors 6d and 6e expressed high but nonselective fluorescence intensity enhancement with the three tested sugars. 6a, 6b and 6f showed low enhancement upon binding with these sugars. All the bisboronic acids 6(a-f) have a similar molecular structure, and the only structural difference among them is the length of the linker, which made these compounds different in flexibility and might be the reason for their selectivity. These results suggest that minor structural changes can significantly affect the selectivity among structurally similar sugars, which is consistent with previous findings [15-18].
|
Download:
|
| Fig. 3. Fluorescence intensity changes of the bisboronic acids 6a-f (10 μmol/L) upon binding with different oligosaccharides (60 μmol/L), 6(a-e): λex = 367 nm, λem = 415 nm; 6f: λex = 380 nm, λem = 417 nm. | |
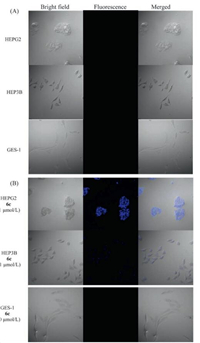
To explore the capability for the bisboronic acids to label carcinoma cells, we studied the ability for 6c to fluorescently stain HepG2 and HEP3B liver carcinoma cells as oppose to a normal GES-1fibroblast cell line. Staining by the fluorescent probe was observed using a fluorescent microscope with a blue optical filter. The results are shown in Fig. 4. Bisboronic acid 6c was able to stain the HEPG2 cell line at 1 μmol/L and label the HEP3B cell line only slightly at the same concentration. Such results indicate that 6c showed selective interaction with HepG2 at a low concentration (1 μmol/L); but the selectivity of 6c toward HEPG2 and HEP3B cells at higher concentrations diminished (Shown in Supporting information). As a comparison, 6d can stain all three cell lines significantly at similar concentrations (Shown in Supporting information). Such results are consistent with the solution binding studies with the Lewis oligosaccharides (Fig. 3).
|
Download:
|
| Fig. 4. Representative fluorescent labeling studies of HEPG2, HEP3B, and GES-1Cells. HEP3 B cells express only Ley, HEPG2 cells express only sLex, GES-1 cells do not express either antigen. (A) negative controls; (B) Compound 6c is used in these cells at a certain concentration. λex = 405 nm, fluorescence collection range: 410-510 nm. | |
3. Conclusion
Several new bisboronic acids 6(a-f) as the fluorescent sensors towards mono-/oligosaccharides were designed and synthesized. The results of the fluorescent binding experiments showed that compounds 6d and 6e had strong binding affinities with the glucose and fructose. Compound 6c with a hexamethylene linker could recognize sLex selectivity and stain the HEPG2 cells at 1 μmol/L obviously. However, the selectivity still needs improvements, and many showed certainly level of promiscuity in binding.
4. ExperimentalMelting points were measured in an open capillary on a SGW X-4 melting point apparatus and are uncorrected. Element analysis was performed using a Heraeus (CHNO, rapid) elemental analyzer. 1H NMR spectra were recorded on a RT-NMR Bruker AVANCE 600 M spectrometer at 600 MHz for 1H, using tetramethylsilane (Me4Si) or solvent as an internal standard. NMR chemical shifts were expressed in ppm and coupling constants in Hz. The following multiplicity abbreviations are used: (s) singlet, (d) doublet, (t) triplet, (q) quartet, (m) multiplet, and (br) broad. ESIMS was determined using an Agilent G6300 ion Trap mass spectrometer, and signals were recorded in m/z. Absorption spectra were recorded on a Shimadzu UV-3600 spectrophotometer. Emission spectra were recorded on a Hitachi F-7000 fluorescence spectrophotometer. Fluorescent labeling studies were taken with FV-1000 laser scanning confocal fluorescence microscope (Olympus). The silica gel (300-400 mesh) for flash column chromatography was from Qingdao Marine Chemical (China). Lewis sugars were purchased from Sigma. Other reagents were from commercial sources.
4.1. General procedures for the synthesis of compounds 4(a-f)To the solution of compound 2 (0.5 g, 2.42 mmol) in MeOH (5 mL) and THF (5 mL) was added the aqueous solution of α, ω-diaminoalkane 3 (0.5 equiv., 1.21 mmol). The resulting mixture was stirred at roomtemperature under nitrogen for 12 h. Then sodium borohydride (3.3 equiv., 8.0 mmol) was added and the mixture subsequently stirred for another 30 min. After solvent evaporation, the resulting solid was dissolved in the mixture of ethyl acetate (100 mL) and water (50 mL). The organic phase was separated and dried over MgSO4. Solvent evaporation gave a crude product, which was purified on a silica gel column, eluting with ethyl acetate/dichloromethane(1/6-15), to give compound 4 as a yellow solid (80-89%).
N1, N3-Bis(anthracen-9-ylmethyl)propane-1, 3-diamine (4a): Yellow solid, yield 81.2%, mp 103-104 ℃; 1H NMR (CDCl3, 600 MHz): δ 8.40 (s, 2H), 8.31 (d, 4H, J = 6.0 Hz), 8.00 (d, 4H, J = 6.0 Hz), 7.48-7.43 (m, 8H), 4.71 (s, 4H), 2.99 (t, 4H, J = 6.0 Hz), 1.89-1.86 (m, 2H). MS (ESI) m/z: 455.4([M+H]+). Anal. calcd. for C33H30N2: C, 87.19; H, 6.65; N, 6.16. Found: C, 87.02; H, 6.62; N, 6.19.
N1, N5-Bis(anthracen-9-ylmethyl)pentane-1, 5-diamine (4b): Yellow solid, yield 81.0%, mp 100-101 ℃; 1H NMR (CDCl3, 600 MHz): δ 8.40 (s, 2H), 8.32 (d, 4H, J = 6.0 Hz), 8.01 (d, 4H, J = 6.0 Hz), 7.52 (t, 4H, J = 6.0 Hz), 7.45 (t, 4H, J = 6.0 Hz), 4.71 (s, 4H), 2.87 (t, 4H, J = 6.0 Hz), 1.63-1.58 (m, 4H), 1.45-1.40 (m, 2H). MS (ESI) m/z: 483.59 ([M+H]+). Anal. calcd. for C35H34N2: C, 87.10; H, 7.10; N, 5.80. Found: C, 87.01; H, 7.03; N, 5.91.
N1, N6-Bis(anthracen-9-ylmethyl)hexane-1, 6-diamine (4c): Yellow solid, yield 83.5%, mp 118-119 ℃; 1H NMR (CDCl3, 600 MHz): δ 8.40 (s, 2H), 8.34 (d, 4H, J = 6.0 Hz), 8.00 (d, 4H, J = 6.0 Hz), 7.53 (t, 4H, J = 6.0 Hz), 7.46 (t, 4H, J = 6.0 Hz), 4.72 (s, 4H), 2.85 (t, 4H, J = 6.0 Hz), 1.60-1.57 (m, 4H), 1.38-1.34 (m, 4H). MS (ESI) m/z: 498.1 ([M+H]+). Anal. calcd. for C36H36N2: C, 87.05; H, 7.31; N, 5.64. Found: C, 86.89; H, 7.23; N, 5.81.
N1, N7-Bis(anthracen-9-ylmethyl)heptane-1, 7-diamine (4d): Yellow solid, yield 83.4%, mp 62-63 ℃; 1H NMR (CDCl3, 600 MHz): δ 8.40 (s, 2H), 8.34 (d, 4H, J = 6.0 Hz), 8.00 (d, 4H, J = 6.0 Hz), 7.54 (t, 4H, J = 7.2 Hz), 7.46 (t, 4H, J = 6.6 Hz), 4.73 (s, 4H), 2.85 (t, 4H, J = 7.2 Hz), 1.59-1.55 (m, 4H), 1.34-1.32 (m, 6H). MS (ESI) m/z: 511.8([M+H]+). Anal. calcd. for C37H38N2: C, 87.02; H, 7.50; N, 5.49. Found: C, 86.81; H, 7.22; N, 5.77.
N1, N8-Bis(anthracen-9-ylmethyl)octane-1, 8-diamine (4e): Yellow solid, yield 83.8%, mp 55-56 ℃; 1H NMR (CDCl3, 600 MHz): δ 8.44 (s, 2H), 8.26 (d, 4H, J = 6.0 Hz), 8.00 (d, 4H, J = 7.2 Hz), 7.56 (t, 4H, J = 7.8 Hz), 7.46 (t, 4H, J = 7.2 Hz), 4.78 (s, 4H), 2.86 (t, 4H, J = 7.2 Hz), 1.58-1.55 (m, 4H), 1.28-1.25 (m, 8H). MS (ESI) m/z: 525.6 ([M+H]+). Anal. calcd. for C38H40N2: C, 86.98; H, 7.68; N, 5.34. Found: C, 86.74; H, 7.34; N, 5.69.
N, N'-(1, 4-Phenylenebis(methylene))bis(1-(anthracen-9-yl) methanamine) (4f): Yellow solid, yield 89.1%, mp 107-108 ℃; 1H NMR (CDCl3, 600 MHz): δ 8.40 (s, 2H), 8.25 (d, 4H, J = 9.0 Hz), 8.00 (d, 4H, J = 8.4 Hz), 7.50-7.43 (m, 12H), 4.72 (s, 2H), 4.07 (s, 2H). MS (ESI) m/z: 517.90 ([M+H]+). Anal. calcd. for C38H32N2: C, 88.34; H, 6.24; N, 5.42. Found: C, 88.23; H, 6.11; N, 5.79.
4.2. General procedures for the synthesis of compounds 6(a-f)The diamine compound 4 (0.20 mmol)was dissolved in 10 mL dry DMF; then compound 5 (136 mg, 0.48 mmol), DIPEA (62 mg, 0.48 mmol) and potassium iodide (2 mg) were added. The reaction mixture was stirred under nitrogenat 75 ℃ for 12 h before filtration. The filtrate was evaporated in vacuo. The residue was dissolved in CH2Cl2 and 10% aqueous solution of sodium bicarbonate (55 mL), and the mixture was stirred at room temperature for 1 h. The organic phase was separated and washed with water (2 × 30 mL) and dried over MgSO4. After removal of the solvent, the crude product was purified on the Al2O3 column chromatography (DCM/MeOH, 100:1, v/v), and a yellow powder was obtained after recrystallization in the mixture of DCM and MeOH.
N, N-Bis(2-boronophenyl)methyl-N, N-9-anthracenylmethyl-1, 3-propanediamine (6a): Yellow solid, yield 81.2%, mp 218-220 ℃; 1H NMR (CDCl3, 600 MHz): δ 8.32 (s, 2H), 7.89-7.85 (m, 10H), 7.34-7.27 (m, 8H), 7.23-7.21 (m, 6H), 4.27 (s, 4H), 3.62 (s, 4H), 2.11 (t, 4H, J = 6.0 Hz), 1.65 (s, 2H). MS (ESI) m/z: 783.7 ([M +3MeOH-2H2O+H]+). Anal. calcd. for C47H44B2N2O4: C, 78.13; H, 6.14; N, 3.88. Found: C, 78.01; H, 6.03; N, 3.92.
N, N-Bis(2-boronophenyl)methyl-N, N-9-anthracenylmethyl-1, 5-pentanediamine (6b): Yellow solid, yield 82.7%, mp 179-180 ℃; 1H NMR (CDCl3, 600 MHz): δ 8.36 (s, 2H), 8.02-7.97 (m, 6H), 7.93 (d, 4H, J = 6.0 Hz), 7.40-7.37 (m, 12H), 7.31(s, 2H), 4.38 (s, 4H), 3.74 (s, 4H), 2.32 (s, 4H), 1.28 (s, 4H), 0.68-0.63 (m, 2H). MS (ESI) m/z: 765.5 ([M+MeOH-H2O+H]+). Anal. calcd. for C49H48B2N2O4: C, 78.41; H, 6.45; N, 3.73. Found: C, 78.21; H, 6.32; N, 3.81.
N, N-Bis(2-boronophenyl)methyl-N, N-9-anthracenylmethyl-1, 6-hexanediamine (6c): Yellow solid, yield 83.5%, mp 111-112 ℃; 1H NMR (CDCl3, 600 MHz): δ 8.31 (s, 2H), 7.99-7.98 (m, 4H), 7.89-7.66 (m, 6H), 7.35-7.34 (m, 8H), 7.31-7.30 (s, 6H), 4.40 (s, 4H), 3.78 (s, 4H), 2.30 (t, 4H, J = 6.0 Hz), 0.84-0.81 (t, 4H, J = 6.0 Hz), 0.65 (t, 4H, J = 6.0 Hz). MS (ESI) m/z: 833.5 ([M+2MeOH-H2O+ Na]+). Anal. calcd. for C50H50B2N2O4: C, 78.55; H, 6.59; N, 3.66. Found: C, 78.39; H, 6.41; N, 3.72
N, N-Bis(2-boronophenyl)methyl-N, N-9-anthracenylmethyl-1, 7-heptanediamine (6d): Yellow solid, yield 83.8%, mp 105-106 ℃; 1H NMR (CDCl3, 600 MHz): δ 8.34 (s, 2H), 8.09 (brs, 4H), 7.98 (brs, 2H), 7.92 (d, 8H, J = 9.0 Hz), 7.41-7.36 (m, 14H), 4.48 (s, 4H), 3.87 (s, 4H), 2.41 (s, 4H), 1.32 (s, 4H), 0.79-0.77 (m, 2H), 0.70 (brs, 4H). MS (ESI) m/z: 858.9 ([M+3MeOH-3H2O+K]+). Anal. calcd. for C51H52B2N2O4:C, 78.67; H, 6.73; N, 3.60. Found: C, 78.15; H, 6.23; N, 3.36.
N, N-Bis(2-boronophenyl)methyl-N, N-9-anthracenylmethyl-1, 8-octanediamine (6e): Yellow solid, yield 83.8%, mp 97-98 ℃; 1H NMR (CDCl3, 600 MHz): δ 8.32 (s, 2H), 8.05 (br. s, 4H), 7.90-7.88 (m, 6H), 7.37-7.33 (m, 14 H), 4.45 (s, 4H), 3.85 (s, 4H), 2.41 (s, 4H), 1.36 (s, 4H), 0.79-0.75 (m, 8H). MS (ESI) m/z: 815.7 ([M+Na]+). Anal. calcd. for C51H52B2N2O4: C, 78.80; H, 6.87; N, 3.53. Found: C, 78.35; H, 6.41; N, 3.71.
N, N-Bis(2-boronophenyl)methyl-N, N-9-anthracenylmethyl-1, 4-phenylenediamine (6f): Yellow solid, yield 83.9%, mp 178-179 ℃; 1H NMR (CDCl3, 600 MHz): δ 8.33 (s, 2H), 8.08 (d, 4H, J = 6.0 Hz), 7.90-7.87 (m, 4H), 7.72 (s, 2H), 7.36 (t, 8H, J = 6.0 Hz), 7.26-7.22 (m, 6H), 7.12 (s, 4H), 4.52 (s, 4H), 3.76 (s, 4H), 3.61 (s, 4H). MS (ESI) m/z: 845.8 ([M+3MeOH-2H2O+1H]+). Anal. calcd. for C52H46B2N2O4: C, 79.61; H, 5.91; N, 3.57. Found: C, 79.43; H, 5.78; N, 3.69.
4.3. Fluorescent binding experimentsThe fluorescent binding experiments of the bisboronic acids 6 (a-d) with several mono-/oligo-saccharides were conducted in a mixture of MeOH and 0.1 mol/L phosphate buffer (pH 7.4) (3:2, v/ v). The sensors 6(a-d) concentration was fixed at 1.0 × 10-5 mol/L or 1.0 × 10-6 mol/L, while the concentrations of the mono/ oligosaccharide were gradually increased.
4.4. Cell cultureHEP3B cells were maintained in MEM with 10% FBS(GIBCO) and 1% NEAA; HEPG2 cells were maintained in MEM with 10% FBS (GIBCO) and 1% NEAA; GES-1 cells were maintained in DMEM with 10% FBS(GIBCO).
4.4.1. Fluorescent labeling studiesSix-well plates were seeded with 8 × 105 cells per well and incubated at 37 ℃ and 5% CO2 for 48 h. The media was removed and cells were washed twice with PBS. The cells were fixed with 1.5 mL of 3:2 methanol/PBS and incubated 20 min at 4 ℃. After incubation, the methanol/PBS solution was removed and cells were washed twice with PBS.
Bisboronic acid compounds were resuspended in 3:2 methanol/ PBS and added to wells at 0.5-10 μmol/L concentrations. One well was incubated only in methanol/PBS without compound as a negative control. The plates were then incubated in darkness at 4 ℃ for 45 min. Plates were examined with phase contrast microscopy followed by fluorescent microscopy (blue cube wavelengths).
4.4.2. ImagingPhase contrast and fluorescence overlay images were taken with FV-1000 laser scanning confocal fluorescence microscope by the process imaging software Olympus Fluoview FV1000 (emission wavelength: 405 nm).
AcknowledgmentsThis work was supported by the National Natural Science Foundation of China (NSFC) (No. 21372060), Hebei Province Natural Science Fund for Distinguished Young Scholars (No. B2015201005).
Appendix A. Supplementary dataSupplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.cclet.2017.02.013.
The 1H NMR spectra of compounds 6(a-f) are available.
| [1] | A.P. de Silva, H.Q.N. Gunaratne, T. Gunnlaugsson, et al., Signaling recognition events with fluorescent sensors and switches. Chem. Rev. 97(1997)1515–1566. DOI:10.1021/cr960386p |
| [2] | L. Pu. Fluorescence of organic molecules in chiral recognition. Chem. Rev. 104(2004)1687–1716. DOI:10.1021/cr030052h |
| [3] | T. D. James, S. Shinkai, Artificial receptors as chemosensors for carbohydrates, in: S. Penadés (Ed. ), Host-Guest Chemistry, Springer, Berlin Heidelberg, 2002, pp. 159-200. |
| [4] | W. Wang, X.M. Gao, B.H. Wang. Boronic acid-based sensors. Curr. Org. Chem. 6(2002)1285–1317. DOI:10.2174/1385272023373446 |
| [5] | S. Striegler. Selective carbohydrate recognition by synthetic receptors in aqueous solution. Curr. Org. Chem. 7(2003)81–102. DOI:10.2174/1385272033373201 |
| [6] | T.D. James, K.R.A.S. Sandanayake, S. Shinkai. Chiral discrimination of monosaccharides using a fluorescent molecular sensor. Nature 374(1995)345–347. DOI:10.1038/374345a0 |
| [7] | D.K. Scrafton, J.E. Taylor, M.F. Mahon, J.S. Fossey, T.D. James. Click-fluors:modular fluorescent saccharide sensors based on a 12, 3-triazole ring. J. Org. Chem. 73(2008)2871–2874. DOI:10.1021/jo702584u |
| [8] | X. Wu, Z. Li, X.X. Chen, et al., Selective sensing of saccharides using simple boronic acids and their aggregates. Chem. Soc. Rev. 42(2013)8032–8048. DOI:10.1039/c3cs60148j |
| [9] | C.F. Dai, A. Sagwal, Y.F. Cheng, et al., Carbohydrate biomarker recognition using synthetic lectin mimics. Pure Appl. Chem. 84(2012)2479–2498. |
| [10] | H.B. El-Serag, A.C. Mason. Rising incidence of hepatocellular carcinoma in the United States. N. Engl. J. Med. 340(1999)745–750. DOI:10.1056/NEJM199903113401001 |
| [11] | E.K. Nakakura, M.A. Choti. Managementof hepatocellular carcinoma. Oncology 14(2000)1085–1098. |
| [12] | A.S. Culf, H.M. Yin, S. Monro, et al., A spectroscopic study of substituted anthranilic acids as sensitive environmental probes for detecting cancer cells. Bioorg. Med. Chem. 24(2016)929–937. DOI:10.1016/j.bmc.2015.12.044 |
| [13] | N. Husseinzadeh. Status of tumor markers in epithelial ovarian cancer has there been any progress? A review. Gynecol. Oncol. 120(2011)152–157. DOI:10.1016/j.ygyno.2010.09.002 |
| [14] | S. Jin, Y.F. Cheng, S. Reid, M.Y. Li, B.H. Wang. Carbohydrate recognition by boronolectins small Molecules, and lectins. Med. Res. Rev. 30(2010)171–257. |
| [15] | S. Craig. Synthesis and evaluation of aryl boronic acids as fluorescent artificial receptors for biological carbohydrates. Bioorg. Chem. 40(2012)137–142. DOI:10.1016/j.bioorg.2011.11.003 |
| [16] | M.H. Chang, C.N. Chang. Synthesis of three fluorescent boronic acid sensors for tumor marker Sialyl Lewis X in cancer diagnosis. Tetrahedron Lett. 55(2014)4437–4441. DOI:10.1016/j.tetlet.2014.05.112 |
| [17] | X.M. Gao, M.Y. Zhu, H.Y. Fan, et al., A fluorescent bisboronic acid compound that selectively labels cells expressing oligosaccharide Lewis X. Bioorg. Med. Chem. Lett. 25(2015)2501–2504. DOI:10.1016/j.bmcl.2015.04.069 |
| [18] | W.Q. Yang, S.H. Gao, X.M. Gao, et al., Diboronic acids as fluorescent probes for cells expressing sialyl Lewis X. Bioorg. Med. Chem. Lett. 12(2002)2175–2177. DOI:10.1016/S0960-894X(02)00339-6 |
| [19] | Y. Chu, D.Z. Wang, K. Wang, et al., Fluorescent conjugate of sLex-selective bisboronic acid for imaging application. Bioorg. Med. Chem. Lett. 23(2013)6307–6309. DOI:10.1016/j.bmcl.2013.09.063 |
| [20] | T.D. James, K.R.A.S. Sandanayake, R. Iguchi, S. Shinkai. Novel saccharide-photoinduced electron transfer sensors based on the interaction of boronic acid and amine. J. Am. Chem. Soc. 117(1995)8982–8987. DOI:10.1021/ja00140a013 |
| [21] | C.F. Dai, L.H. Cazares, L.F. Wang, et al., Using boronolectin in MALDI-MS imaging for the histological analysis of cancer tissue expressing the sialyl Lewis X antigen. Chem. Commun. 47(2011)10338–10340. DOI:10.1039/c1cc11814e |
| [22] | R.W. Sweger, A.W. Czarnik. Rapid intermolecular electron transfer specific to the Zwitterionic form of N-(9-(10-Hydroxyanthryl))piperidine (HAP). J. Am. Chem. Soc. 113(1991)1523–1530. DOI:10.1021/ja00005a009 |
| [23] | L. Aoki, T. Sakaki, S. Shinkai. A new metal sensory system based on intramolecular fluorescence quenching on the ionophoric calix[4] arene ring. J. Chem. Soc. Chem. Commun. (1992)730–732. |
| [24] | W.J. Ni, G. Kaur, G. Springsteen, B.H. Wang, S. Franzen. Regulating the fluorescence intensity of an anthracene boronic acid system:a B-N bond or a hydrolysis mechanism. Bioorg. Chem. 32(2004)571–581. DOI:10.1016/j.bioorg.2004.06.004 |
| [25] | J.Z. Zhao, M.G. Davidson, M.F. Mahon, G. Kociok-Köhn, T.D. James. An enantioselective fluorescent sensor for sugar acids. J. Am. Chem. Soc. 126(2004)16179–16186. DOI:10.1021/ja046289s |
| [26] | S. Arimori, M.D. Phillips, T.D. James. Probing disaccharide selectivity with modular fluorescent sensors. Tetrahedron Lett. 45(2004)1539–1542. DOI:10.1016/j.tetlet.2003.12.013 |
 2017, Vol. 28
2017, Vol. 28