It is known that compounds containing fluorine and fluorinated moieties play an important role in a variety of applied chemistry areas, such as pharmaceutical chemistry, agrochemical chemistry, and material science [1-3]. Introducing fluorine atom or fluoroalkyl group into molecules of drug candidates and synthesis of new drug candidates from highly reactive fluorinated building blocks are frequently used strategies in drug discovery [4, 5]. Many fluorine-containing compounds also exhibit a broad spectrum of bioactivities in the field of pesticides, such as herbicidal [6], insecticidal [7], acaricidal, and fungicidal [8] activities. For example, the commercial pesticides Etoxazole, Picoxystrobin, Flazasulfuron, Triflumuron, Vaniliprole [9] and Flubendiamide each contain the fluorinated groups within their structures.
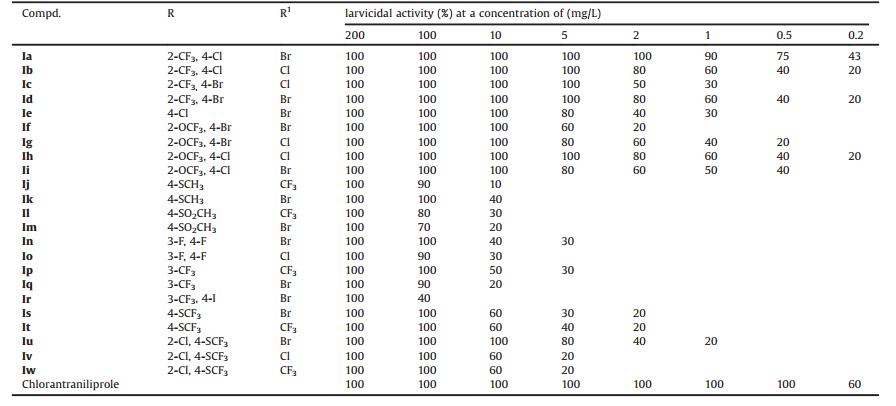
In the current agrochemical markets of China, some conventional insecticides such as methamidophos, dicofol, monocrotophos, fipronil etc. have been prohibited due to their toxicity to mammals or damage to the proliferation of bees. To overcome the serious ecological problems, the discovery of new potent insecticides with novel modes of action is an important task for effective pest management. Anthranilic diamides insecticides (Fig. 1) were discovered by DuPont as highly potent activators of the insect ryanodine receptors (RyRs) [10]. They can evoke massive calcium release from intracellular stores, disrupt calcium homeostasis, and cause the final death of insects [11]. Since the discovery of anthranilic diamides, some structural modifications have been reported. Most of modifications were related to the aliphatic amide moiety [12, 13], the amide bridge moiety [14, 15] and the Npyridylpyrazole moiety [16, 17]. Nevertheless, the modification of the methyl, chlorine or cyano substituents on the phenyl ring part was seldom reported [18, 19]. Considering that fluorine atom was a good hydrogen bond acceptor, the introduction of fluorinated groups to certain insecticide might increase the affinity of the parent compound to its receptors. On the basis of the above viewpoints, a series of novel anthranilic diamide derivatives containing various fluorinated moieties in the phenyl part were designed and synthesized. Their insecticidal activities against oriental armyworm and diamondback moth were tested accordingly. The preliminary structure-activity relationship (SAR) was also summarized.
|
Download:
|
| Fig. 1. Design of the title compounds. | |
2. Results and discussion 2.1. Synthesis and spectra characterization
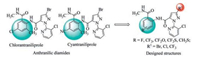
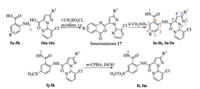
As shown in Scheme 1, target compounds Ia-Ik and In-Iw were synthesized referring to the previous literature in moderate to good yields [20]. Diamides Ij and Ik were oxidized with m-CPBA to give the target compounds Il and Im. In the 1H NMR spectra, the active proton signals of the amide moieties Ph-NH-CO-and Ph-CONH-were observed at δ 10.53-13.24 and 8.45-9.37 in DMSO-d6, respectively. In the 19F NMR spectra, the signals of fluorine atoms in CF3, CF3O, and CF3S groups appeared as singlet at -62.18 to -58.27 ppm, -56.78 to -56.75 ppm and -42.34 to -41.49 ppm, respectively.
|
Download:
|
| Scheme1. Synthetic route of title compounds Ia-Iw. | |
2.2. Structure-activity relationship (SAR)
Larvicidal activity against oriental armyworm: The larvicidal activities of target compounds Ia-Iw and chlorantraniliprole against oriental armyworm were summarized in Table 1. The biological assay indicated that most of the title compounds had significant larvicidal activities against oriental armyworm. For example, Ia, Ib, Id, Ih and Ii exhibited 90%, 60%, 60%, 60% and 50% lethal rates at 1.0 mg/L, respectively. In particular, Ia showed 43% larvicidal activity against oriental armyworm at 0.2 mg/L, which was slightly lower than that of the commercial chlorantraniliprole (0.2 mg/L, 60%).
|
|
Table 1 Insecticidal activities of compounds Ia-Iw and chlorantraniliprole against oriental armyworm. |
To explore the effect of the substituent groups in the orthoposition of the amide moiety on the larvicidal activities, compounds Ia (R = 2-CF3, 4-Cl), Ie (R = 2-H, 4-Cl), and Ii (R = 2-OCF3, 4-Cl) were tested for their insecticidal activities. The results indicated that the sequence of the larvicidal activity was CF3 (Ia) > CF3O (Ii) > H (Ie). The R1 groups in the pyrazole ring had little influence on the larvicidal activity. The sequence of the larvicidal activity was generally Br > Cl (Ia > Ib, Id > Ic, In > Io, Iu > Iv). On the other hand, the larvicidal activities of compounds with CF3 group at the C-3' position were generally near to those of the brominated pyrazole diamides (Ij, Ik, Il, Im, Ip, Iq, It, Is). However, the brominated pyrazole diamides Iu showed higher insecticidal activity than that of the corresponding trifluoromethyl-substituted diamide Iw. In addition, when R1 was fixed as Br, the bioactivity of compounds Ie, Ik, Im, Is revealed the trend of 4-Cl > 4-SCF3 > 4-SCH3, 4-SO2CH3. These observations clearly showed that the substituent Cl at the C-4 position of benzene ring was a key pharmacophore for the maintenance of insecticidal activity. Compounds In-Ir with the fluorinated substituents at the C-3 position exhibited very low activities at 10 mg/L. These results indicated that getting rid of the substituent groups at the C-2 position of phenyl ring had a negative effect on the larvicidal activity. The LC50 value of Ia was 0.2241 mg/L, higher than that of chlorantraniliprole (0.0664 mg/L, Table 2), which was in accordance with the corresponding results in Table 1.
|
|
Table 2 LC50 values of compound Ia and chlorantraniliprole against oriental armyworm. |
Larvicidal activity against diamondback moth: Some representative compounds with moderate to high larvicidal activities against oriental armyworm were further investigated. From Table 3, it was found that the tested compounds generally showed moderate to excellent insecticidal activities against diamondback moth. Especially, If exhibited 100% and 40% larvicidal activities at 1 and 0.1 mg/L, respectively. It was worthy of note that Iu showed a 70% lethal rate at 0.01 mg/L against diamondback moth, slightly higher than that of chlorantraniliprole (65%, 0.01 mg/L). These results revealed that the substituents at the C-2 and C-4 positions of the phenyl ring part had an important influence on the bioactivities. The LC50 value of Iu was 0.0053 mg/L, lower than that of chlorantraniliprole (0.0334 mg/L, Table 4), which indicated that Iu was more effective than commercial chlorantraniliprole against diamondback moth.
|
|
Table 3 Insecticidal activities of compounds Ia-Ig, Ii, Iu, Iw and chlorantraniliprole against diamondback moth. |
|
|
Table 4 LC50 values of compound Iu and chlorantraniliprole against diamondback moth. |
3. Conclusion
In summary, 23 novel fluorinated anthranilic diamide analogues were designed, synthesized, and evaluated against oriental armyworm and diamondback moth for their insecticidal activities. The preliminary bioassay indicated that some of them exhibited favorable larvicidal activities. In particular, Ia showed 43% larvicidal activity against oriental armywarm at 0.2 mg/L, with the LC50 value of 0.2241 mg/L. Moreover, Iu displayed 70% larvicidal activity against diamondback moth at 0.01 mg/L, higher than that of chlorantraniliprole. The present work demonstrated that compounds Ia and Iu could be used as novel lead structures for the development of new insecticides.
4. Experimental 4.1. Synthetic proceduresCommercial chlorantraniliprole was synthesized according to procedures in the literature [20]. N-Pyridylpyrazole carboxylic acids 16a-16c were synthesized from the starting materials 3-chloro-2-hydrazinylpyridine [21] and 2-acetylfuran [14] referring to our previous work. Detailed synthetic procedures and spectral data for title compounds Ia-Iw and intermediates 5a-5k were given in the Supporting information.
4.2. Biological assayAll biological assays were performed on test organisms reared in a greenhouse. The bioassay was replicated at 25±1 ℃ according to statistical requirements. Assessments were made on a dead/ alive basis, and mortality rates were corrected by applying Abbott's formula. Error of the experiments was 5%. LC50 values were calculated by probit analysis [16]. The larvicidal activity of title compounds Ia-Iw against oriental armyworm (M. separata) was tested according to the leaf-dip method using the reported procedure [21]. The larvicidal activity of some representative title compounds against diamondback moth (P. xylostella) was tested according to the reported leaf-dip method [14]. For comparative purposes, commercial chlorantraniliprole was tested as positive control under the same conditions.
AcknowledgmentsThis work was financially supported by the National Natural Science Foundation of China (No. 21372133), 973 Program (No. 2010CB126106), and "111" Project of Ministry of Education of China (No. B06005).
Appendix A. Supplementary dataSupplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.cclet.2017.01.019.
| [1] | J. Wang, M. Sa'nchez-Rosello', J.L. Acenña, et al., Fluorine in pharmaceutical industry:fluorine-containing drugs introduced to the market in the last decade (2001-2011). Chem. Rev. 114(2014)2432–2506. DOI:10.1021/cr4002879 |
| [2] | M.B. Kanani, M.P. Patel. Synthesis of N-arylquinolone derivatives bearing 2-thiophenoxyquinolines and their antimicrobial evaluation. Chin. Chem. Lett. 25(2014)1073–1076. DOI:10.1016/j.cclet.2014.04.002 |
| [3] | F. Giornal, S. Pazenok, L. Rodefeld, et al., Synthesis of diversely fluorinated pyrazoles as novel active agrochemical ingredients. J. Fluorine Chem. 152(2013)2–11. DOI:10.1016/j.jfluchem.2012.11.008 |
| [4] | S. Purser, P.R. Moore, S. Swallow, V. Gouverneur. Fluorine in medicinal chemistry. Chem. Soc. Rev. 37(2008)320–330. DOI:10.1039/B610213C |
| [5] | W.K. Hagmann. The many roles for fluorine in medicinal chemistry. J. Med. Chem. 51(2008)4359–4369. DOI:10.1021/jm800219f |
| [6] | L.Y. Zhang, B.L. Wang, Y.Z. Zhan, et al., Synthesis and biological activities of some fluorine-and piperazine-containing 1, 2, 4-triazole thione derivatives. Chin. Chem. Lett. 27(2016)163–167. DOI:10.1016/j.cclet.2015.09.015 |
| [7] | A.Y. Guan, Y.K. Qin, J.F. Wang, B. Li. Synthesis and insecticidal activity of novel dihalopropene derivatives containing benzoxazole moiety:a structureactivity relationship study. J. Fluorine Chem. 156(2013)120–123. DOI:10.1016/j.jfluchem.2013.09.003 |
| [8] | L. Li, M. Li, H.W. Chi, et al., Discovery of flufenoxystrobin:novel fluorinecontaining strobilurin fungicide and acaricide. J. Fluorine Chem. 185(2016)173–180. DOI:10.1016/j.jfluchem.2016.03.013 |
| [9] | J. M. Huang, H. M. , Ayad, P. R. Timmons, Pesticidal 1-aryl-5-(substituted alkylideneimino) pyrazoles, US 5360910. |
| [10] | G.P. Lahm, T.P. Selby, J.H. Freudenberger, et al., Insecticidal anthranilic diamides:a new class of potent ryanodine receptor activators. Bioorg. Med. Chem. Lett. 15(2005)4898–4906. DOI:10.1016/j.bmcl.2005.08.034 |
| [11] | D. Cordova, E.A. Benner, M.D. Sacher, et al., Anthranilic diamides:a new class of insecticides with a novel mode of action ryanodine receptor activation. Pesticides Biochem. Physiol. 84(2006)196–214. DOI:10.1016/j.pestbp.2005.07.005 |
| [12] | Y.H. Li, H.J. Zhu, K. Chen, et al., Synthesis insecticidal activity, and structureactivity relationship (SAR) of anthranilic diamides analogs containing oxadiazole rings. Org. Biomol. Chem. 11(2013)3979–3988. DOI:10.1039/c3ob40345a |
| [13] | M.Z. Mao, Y.X. Li, Y.Y. Zhou, et al., Synthesis and insecticidal evaluation of novel N-Pyridylpyrazolecarboxamides containing an amino acid methyl ester and their analogues. J. Agric. Food Chem. 62(2014)1536–1542. DOI:10.1021/jf500003d |
| [14] | J.F. Zhang, J.Y. Xu, B.L. Wang, et al., Synthesis and insecticidal activities of novel anthranilic diamides containing acylthiourea and acylurea. J. Agric. Food Chem. 60(2012)7565–7572. DOI:10.1021/jf302446c |
| [15] | B.L. Wang, H.W. Zhu, Y. Ma, et al., Synthesis insecticidal activities, and SAR studies of novel pyridylpyrazole acid derivatives based on amide bridge modification of anthranilic diamide insecticides. J. Agric. Food Chem. 61(2013)5483–5493. DOI:10.1021/jf4012467 |
| [16] | J.B. Liu, Y.X. Li, X.L. Zhang, et al., Novel anthranilic diamide scaffolds containing N-substituted phenylpyrazole as potential ryanodine receptor activators. J. Agric. Food Chem. 64(2016)3697–3704. DOI:10.1021/acs.jafc.6b00380 |
| [17] | Q. Feng, Z.L. Liu, L.X. Xiong, et al., Synthesis and insecticidal activities of novel anthranilic diamides containing modified N-pyridylpyrazoles. J. Agric. Food Chem. 58(2010)12327–12336. DOI:10.1021/jf102842r |
| [18] | B. Li, H. F. Wu, H. B. Yu, H. B. Yang, A preparation method of phenylcarboxamides, WO 2009121288. |
| [19] | E. G. Taylor, Method for preparing fused oxazinones from Ortho-amino aromatic carboxylic acid and a carboxylic acid in the presence of a sulfonyl chloride and pyridine, WO 2004011447. |
| [20] | G.P. Lahm, T.M. Stevenson, T.P. Selby, et al., RynaxypyrTM:a new insecticidal anthranilic diamide that acts as a potent and selective ryanodine receptor activator. Bioorg. Med. Chem. Lett. 17(2007)6274–6279. DOI:10.1016/j.bmcl.2007.09.012 |
| [21] | W.L. Dong, J.Y. Xu, L.X. Xiong, X.H. Liu, Z.N. Li. Synthesis, structure and biological activities of some novel anthranilic acid esters containing Npyridylpyrazole. Chin. J. Chem. 27(2009)579–586. DOI:10.1002/cjoc.v27:3 |
 2017, Vol. 28
2017, Vol. 28