b RCMI Cancer Research Center, Xavier University of Louisiana, New Orleans 70125, USA;
c Department of Chemistry, Xavier University of Louisiana, New Orleans 70125, USA;
d Department of Nuclear Medicine Affiliated Hospital, Luzhou Medical College, Luzhou 646000, China
The construction of nitrogen-containing heterocycles catalyzed by transition metal complexes is a fascinating methodology and has been applied to the synthesis of many natural products and biologically active compounds [1-10]. Copper complexes are especially attractive catalysts due to their low cost, readily availability and versatile reactivity [11-34]. In our continuing effort to explore the application of transition metal as catalyst in synthetic methodology for discovering bioactive molecules [37-40]. We investigated the amidation reactions of arenes and found that [Cu(CH3CN)4]ClO4 can synergistically catalyze the intramolecular functionalization of arenes to afford the corresponding 1, 4-benzoxazin-3-ones in moderate to high yield by C—H bond activation and concomitant amidation.
To the best of our knowledge, the construction of C—N bonds by oxidative amidation via insertion into C—H bonds with transition metal complexes is a significant approach [11-19]. Insertion requires activated C—N bonds (e.g., allylic or benzylic C—N bonds), and insertion and coupling with inactive benzene C—N bonds remain a formidable task. Although a few of studies have demonstrated that the intramolecular catalytic amidation reactions of C—H bonds are feasible [20-34], the intramolecular catalytic reactions of oxidatively constructing the C-N bonds between N—H and inactive C—N bonds have rarely been studied. Only a few studies have identified such a biphenyl compound with an amide, an aniline or a sulfonamide as potential nitrogen sources and substrates for this kind reactions to afford five-membered nitrogen-containing heterocyclic products or six-membered N-MeO, N-phenyl and N-alkyl substituted nitrogen-containing heterocyclic products, and the reaction conditions are harsh and the catalysts are expensive and not easily available [20-24].
2H-1, 4-Benzoxazin-3-(4H)-one derivatives are now known to possess useful biological and medicinal activities [36, 37]. The reported synthetic methods of 2H-1, 4-benzoxazin-3-(4H)-ones are all using 2-aminophenols, substituted 2-nitrophenols, or 2-halophenols as precursors [35-37], their disadvantage is that only a limited number of substrates can be employed or the reaction conditions are not mild. With respect to these considerations, we are thinking to develop a more convenient and novel method through arene oxidation and C-N bond formation to produce 2H-1, 4-benzoxazin-3-(4H)-ones. In this paper, we describe a mild and efficient synthesis of 2H-1, 4-benzoxazin-3-(4H)-one derivatives by the oxidative intramolecular amidation between inactive C—N and N—H functionalization from N-(1, 3-diphenyl-1H-1, 2, 4-triazol-5-yl)-2-phenoxyacetamides as a nitrogen source (Scheme 1).

|
Download:
|
| Scheme1. Synthetic procedure for benzo[1, 4]oxazin-3-one derivatives with a selective oxidative amidation. | |
2. Results and discussion 2.1. Chemical synthesis
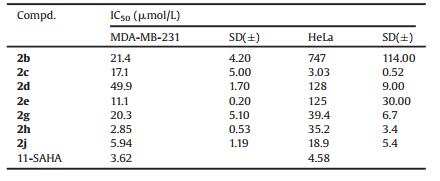
N-(1, 3-Diphenyl-1H-1, 2, 4-triazol-5-yl)-2-phenoxyacetamide (1a) was chosen as a model substrate for optimizing the reaction conditions. Firstly, oxidants and catalysts were investigated to develop a mild and environmentally friendly methodology to accomplish the intramolecular cyclization reactions. The effects of the tested oxidants and catalysts are listed in Table 1.
|
|
Table 1 Effect of catalysts and oxidants on the amidation reaction.a |
The results of entries 1-8 in Table 1 show that PhI(OAc)2, PhI (CF3CO2)2, and PhI = O (Table 1, entries 1, 2, and 3) are active oxidants for the oxidative amidation of 1a to form 2a with Cu (CF3SO3)2 catalyst. Next, using PhI(CF3CO2)2 as oxidant, both Cu-ophenanthroline and Cu[(CH3CN)4]ClO4 (Table 1, entries 17 and 18) display as the best catalysts to transform 1a to 2a by C—N bond formation. Cu[(CH3CN)4]ClO4 is much less expensive than Cu-ophenanthroline, so we chosed Cu[(CH3CN)4]ClO4 as catalyst and PhI(CF3CO2)2 as oxidant in further condition optimization for the intramolecular amidation reaction. From the entry 19, we can find that the yield reaches to 66% from 60% (entry 18) by increasing the Cu[(CH3CN)4] ClO4 loading from 10 to 20% molar equivalent to 1a. However, the yield maintains 61% when the catalyst was loaded to 25% molar of 1a (entry 20). Thus, 4-(1, 3-diphenyl-1H-1, 2, 4-triazol-5-yl)-2H-benzo[b][1, 4]oxazin-3 (4H)-one (2a) was obtained in the best yield of 66% when using N-(1, 3-diphenyl-1H-1, 2, 4-triazol-5-yl)-2-phenoxyacetamide (1a) as the substrate at room temperature with a molar ratio of substrate/PhI(CF3CO2)2/Cu [(CH3CN)4] ClO4 of 1:2.5:0.2. The solvent effects of this reaction was also explored. Among CH2Cl2, ClCH2CH2Cl, CH3CN, CHCl3, THF, and DMF, halogenated hydrocarbon solvents provided good yields under identical experimental conditions, and the best one was CH2Cl2 which provided the highest yield (Table 1, entry 19). Finally, to test the effect of temperature on the yield, identical experimental conditions were utilized while varying the temperature from 0 ℃ to 60 ℃. The optimal yield and shortest reaction time was achieved at room temperature. Therefore, the optimized conditions for this intramolecular oxidative amidation were as follows: substrate, 1a (1.0 equiv.); oxidant, PhI(CF3CO2)2 (2.5 equiv.); catalyst, Cu[(CH3CN)4]ClO4 (20 mol%); solvent, CH2Cl2; and temperature, room temperature.
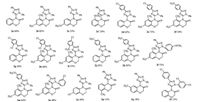
We further investigated the scope of this method and performed intramolecular oxidative amidation reactions using different arenes under the above optimized conditions. As showed in Fig. 1, various substrates were subjected to intramolecular oxidative amidation, affording corresponding N-1H-1, 2, 4-triazol-2H-1, 4-benzoxazin-3-(4H)-ones in moderate to good yields. It is worth mentioning that, our substrates have three aromatic rings (Scheme 1), which bear different substituent.
|
Download:
|
| Fig. 1. Intramolecular oxidative amidation catalyzed by Cu[(CH3CN)4] ClO4. All reactions were performed at room temperature, CH2Cl2 as the solvent for 4-10 h with a molar ratio of substrate oxidant/catalyst of 1:2.5:0.2. The reaction was conducted at 0 ℃ for 5 h. (2c and 2c'). | |
Interestingly, only the cyclization products on ring C were obtained in the amidation reaction, no cyclizationproducts on ring B were found (Scheme 1). The reason of chemoselective formation of N-1H-1, 2, 4-triazol-2H-1, 4-benzoxazin-3-(4H)-ones (2a-2r) from N-(1, 3-diphenyl-1H-1, 2, 4-triazol-5-yl)-2-phenoxy-acetamide derivative-s may be that the amidation reaction favors the formation of more stable N-1H-1, 2, 4-triazol-2H-1, 4-benzoxazin-3-(4H)-ones which could be enolated to fully conjugated structure. In Fig. 1, the substrate with methoxyl in the meta-position of ring C (1c) gave two corresponding six-membered heterocyclic products 2c and 2c', the ratio of 2c and 2c' reflects the regioselectivity difference in the reaction which arises from steric and electronic effect in the formation of products. At the same time, we notice that the reaction of the methoxy group substituent in the para-position gave only corresponding five-membered triazole-spirodienone product in 94% yield (Scheme 2).

|
Download:
|
| Scheme2. The reaction results of p-methoxy substituted aromatic ether derivatives as substrates. | |
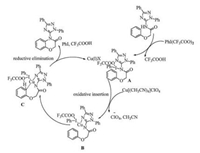
On the basis of the mechanisms proposed originally for the amidation by Antonchick [30], Zhao [41] and Himo [42], we proposed a mechanism for oxidative amidation reactions of arenes with N-triazoleamides (Scheme 3). Initially ditrifluoroacetoxyiodobenzene reacts with the amide to give intermediate A, which is then transformed into intermediate B through an oxidative insertion of N—I bond by copper(Ⅱ). The reductive elimination of intermediate C releases copper (Ⅰ) salt, trifluoroacetic acid and iodobenzene, leading to final product 4-(2, 5-diphenyl-2H-1, 2, 4-triazol-3-yl)-2H benzo[b] [1, 4] oxazin-3(4H)-one.
|
Download:
|
| Scheme 3. Proposed mechanism for the oxidative amidation of arenes. | |
2.2. Biological testing
Some of benzo[1, 4]oxazin-3-one derivatives were examined for in vitro cytotoxic activities against human breast cancer cell line MDA-MB-231, and cervical cancer cell line HeLa. Table 2 lists the IC50 values obtained with these compounds. Most compounds showed moderate antiproliferative effects. They displayed IC50 values in the micromolar concentration range against MDA-MB-231 and HeLa cells. These biological data indicate that the benzo [1, 4]oxazin-3-ones can be useful templates upon which to develop potential small molecule anti-cancer agents. Further investigation of the scope, mechanism and synthetic application of the intramolecular oxidative amidation is currently underway.
|
|
Table 2 Antiproliferation activity against cancer cell lines of some compounds. |
3. Conclusion
In summary, we developed a novel protocol for the synthesis of 2H-1, 4-benzoxazin-3-(4H)-one derivatives. This method efficiently performs the intramolecular oxidative amidation of arenes to install C—N bonds catalyzed by Cu[(CH3CN)4]ClO4 under mild conditions. The approach enables the synthesis of various products from a wide range of aromatic ethers in moderate to good yields utilizing simple and inexpensive reagents and catalysts under mild conditions and shows good chemo-and regioselectivity. Preliminary cytotoxicity assays in two metastatic cancer cell lines suggests that benzo[1, 4]oxazin-3-ones can be useful templates upon which to develop potential small molecule anticancer agents.
4. Experimental 4.1. General of synthesisAll reactions were performed by using standard Schlenk techniques under nitrogen and argon atmosphere. Flash column chromatography was performed on silica gel. 1H NMR and 13C NMR spectra were recorded on Varian Mercury 400 MHz spectrometers. High-resolution mass spectral (HRMS) analyses were carried out using a MALDI-TOF MS instrument.
A flame-dried round-bottom flask was charged, under nitrogen atmosphere, with the substrate (0.25 mmol, 1.0 equiv.), PhI (CF3CO2)2 (0.625 mmol, 2.5 equiv.), Cu[(CH3CN)4]ClO4 (0.05 mmol, 20 mol%), and distilled dichloromethane (10 mL). The resulting mixture was stirred at room temperature for 5 h. Then the mixture was washed with NaOH (5%), brine and dried over MgSO4. The filtrate was purified by flash column chromatography (PE/EtOAc, 3:1) on silica gel.
4.2. Antiproliferative assaysHuman breast adenocarcinoma (MDA-MB-231) and human cervix carcinoma (HeLa) cells were grown in DMEM medium supplemented with 115 units/mL of penicillin G, 115 mg/mL of streptomycin, and 10% fetal bovine serum (all from Life Technologies, Grand Island, NY). Cells were seeded in 96-well plates (5 ×103 cells/well) containing 50 mL growth medium for 24 h. After medium removal, 100 mL fresh medium containing individual analog compounds at different concentrations was added to each well and incubated at 37 ℃ for 72 h. After 24 h of culture, the cells were supplemented with 50 mL of analog compounds dissolved in DMSO (less than 0.25% in each preparation). After 72 h of incubation, 20 mL of resazurin was added for 2 h before recording fluorescence at 560 nm (excitation) and 590 nm (emission) using a Victor microtiter plate fluorimeter (Perkin-Elmer, USA). The IC50 was defined as the compound concentration required to inhibit cell proliferation by 50%, in comparison with cells treated with the maximum amount of DMSO (0.25%) and considered as 100% viability.
AcknowledgmentsThis work was financially supported by the National Natural Science FoundationofChina (No.21072131), and SichuanUniversity--Lu Zhou Strategic Cooperation Projects (No. 2013CDLZ-S18) (Ling He) and the NIH RCMI program at Xavier University of Louisiana (No. 2G12MD007595-07) (G. Wang).
| [1] | B. Achari, S.B. Mandal, P.K. Dutta, C. Chowdhury. Perspectives on 1, 4-benzodioxins, 1, 4-benzoxazines and their 2, 3-dihydro derivatives. Synlett 14(2004)2449–2467. |
| [2] | J. Ilas, P.S. Anderluh, M.S. Dolenc, D. Kikelj. Recent advances in the synthesis of 2H-1, 4-benzoxazin-3-(4H)-ones and 3, 4-dihydro-2H-1, 4-benzoxazines. Tetrahedron 61(2005)7325–7348. DOI:10.1016/j.tet.2005.05.037 |
| [3] | J. Ilas, Z. Jakopin, T. Borstnar, M. Stegnar, D. Kikelj. 3, 4-dihydro-2H-1, 4-benzoxazine derivatives combining thrombin inhibitory and glycoprotein Ⅱb/Ⅲa receptor antagonistic activity as a novel class of antithrmbotic compounds with dual function. J. Med. Chem. 51(2008)5617–5629. DOI:10.1021/jm8003448 |
| [4] | M. Zhong, T.R. Gadek, M. Bui, et al., Discovery and development of potent LFA-1/ICAM-1 antagonist SAR 1118 as an ophthalmic solution for treating dry eye. ACS Med. Chem. Lett. 3(2012)203–206. DOI:10.1021/ml2002482 |
| [5] | P.J. Rybczynski, R.E. Zeck, J. Dudash, et al., Benzoxazinones as PPARγ agonists agonists. 2. SAR of the amide substituent and in vivo results in a type 2 diabetes model. J. Med. Chem. 47(2004)196–209. DOI:10.1021/jm0301888 |
| [6] | A.G. Sams, M. Hentzer, G.K. Mikkelsen, et al., Discovery of N-{1-[3-(3-Oxo-2, 3-dihydrobenzo[1, 4] oxazin-4-yl)propyl]piperidin-4-yl}-2-phenylacetamide (Lu AE51090):an allosteric muscarinic M1 receptor agonist with unprecedented selectivity and procognitive potentia. J. Med. Chem. 53(2010)6386–6397. DOI:10.1021/jm100697g |
| [7] | X.H. Du, M. Lizarzaburu, S. Turcotte, et al., Optimization of triazoles as novel and potent nonphlorizin SGLT2 inhibitors. Bioorg. Med. Chem. Lett. 21(2011)3774–3779. DOI:10.1016/j.bmcl.2011.04.053 |
| [8] | Z.T. Piao, L.P. Guan, L.M. Zhao, H.M. Piao, Z.S. Quan. Synthesis of novel 7-benzylamino-2H-1, 4-benzoxazin-3(4H)-ones as anticonvulsant agents. Eur. J. Med. Chem. 43(2008)1216–1221. DOI:10.1016/j.ejmech.2007.08.006 |
| [9] | S.M. Bromidge, B. Bertani, M. Borriello, et al., 6--[2-(4-Aryl-1-piperazinyl) ethyl]-2H-1, 4-benzoxazin-3(4H)-ones:dual-acting 5-HT1 receptor antagonists and serotonin reuptake inhibitors. Bioorg. Med. Chem. Lett. 18(2008)5653–5656. DOI:10.1016/j.bmcl.2008.08.084 |
| [10] | S.M. Bromidge, B. Bertani, M. Borriello, et al., 8-[2-(4-Aryl-1-piperazinyl) ethyl]-2H-1, 4-benzoxazin-3(4H)-ones:dual-acting 5-HT1 receptor antagonists and serotonin reuptake inhibitors-Part Ⅱ. Bioorg. Med. Chem. Lett. 19(2009)2338–2342. DOI:10.1016/j.bmcl.2009.02.056 |
| [11] | J.L. Liang, S.X. Yuan, J.S. Huang, W.Y. Yu, C.M. Che. Highly diastereo-and enantioselective intramolecular amidation of saturated C—N bonds catalyzed by ruthenium porphyrins. Angew. Chem. Int. Ed. 41(2002)3645–3648. DOI:10.1002/1521-3773(20021004)41:19<3645::AID-ANIE3645>3.0.CO;2-F |
| [12] | C.G. Espino, J. Du Bois. A rh-catalyzed C—N insertion reaction for the oxidative conversion of carbamates to oxazolidinones. Angew. Chem. Int. Ed. 40(2001)598–600. DOI:10.1002/1521-3773(20010202)40:3<>1.0.CO;2-A |
| [13] | M.M. Diaz-Requejo, T.R. Belderrain, M.C. Nicasio, S. Trofimenko, P.J. Pérez. Cyclohexane and benzene amination by catalytic nitrene insertion into C—H bonds with the copper-homoscorpionate catalyst TpBr3CuNCMe. J. Am. Chem. Soc. 125(2003)12078–12079. DOI:10.1021/ja037072l |
| [14] | D.S. Albone, P.S. Aujla, P.C. Taylor, S. Challenger, A.M. Derrick. A simple copper catalyst for both aziridination of alkenes and amination of activated hydrocarbons with chloramine-t trihydrate. J. Org. Chem. 63(1998)9596–9571. |
| [15] | M.R. Fructos, S. Trofimenko, M.M. Diaz-Requejo, P.J. Pérez. Facile amine formation by intermolecular catalytic amidation of carbon-hydrogen bonds. J. Am. Chem. Soc. 128(2006)11784–11791. DOI:10.1021/ja0627850 |
| [16] | Y. Cui, C. He. Efficient aziridination of olefins catalyzed by a unique disilver (Ⅰ) compound. J. Am. Chem. Soc. 125(2003)16202–16203. DOI:10.1021/ja038668b |
| [17] | Z.G. Li, D.A. Capretto, R. Rahaman, C. He. Silver-catalyzed intermolecular amination of C—N groups. Angew. Chem. Int. Ed. 46(2007)5184–5186. DOI:10.1002/(ISSN)1521-3773 |
| [18] | H.M.L. Davies, J.R. Manning. Catalytic C—N functionalization by metal carbenoid and nitrenoid insertion. Nature 451(2008)417–424. DOI:10.1038/nature06485 |
| [19] | M.M. Díaz-Requejo, P.J. Pérez. Coinage Metal Catalyzed C—N bond Functionalization of Hydrocarbons. Chem. Rev. 108(2008)3379–3394. DOI:10.1021/cr078364y |
| [20] | Y. Kikugawa, A. Nagashima, T. Sakamoto, E. Miyazawa, M. Shiiya. Intramolecular cyclization with nitreniumions generated by treatment of N-acylaminophthalimides with hypervalent iodine compounds:formation of lactams and spirofused lactams. J. Org. Chem. 68(2003)6739–6744. DOI:10.1021/jo0347009 |
| [21] | F. Péron, C. Fossey, T. Cailly, F. Fabis. N-tosylcarboxamide as a transformable directing group for Pd-catalyzed C—N ortho-arylation. Org. Lett. 14(2012)1827–1829. DOI:10.1021/ol3004244 |
| [22] | E.T. Nadres, O. Daugulis. Heterocycle synthesis via direct C—N/N—H coupling. J. Am. Chem. Soc. 134(2012)7–10. DOI:10.1021/ja210959p |
| [23] | W. Zhou, Y. Liu, Y.P. Yang, G.J. Deng. Copper-catalyzed intramolecular direct amination of sp2 C—N bonds for the synthesis of N-aryl acridones. Chem. Commun. 48(2012)10678–10680. DOI:10.1039/c2cc35425j |
| [24] | J. Karthikeyan, C.H. Cheng. Synthesis of phenanthridinones from Nmethoxybenzamides and arenes by multiple palladium-catalyzed C—N activation steps at room temperature. Angew. Chem. Int. Ed. 50(2011)9880–9883. DOI:10.1002/anie.v50.42 |
| [25] | G.W. Wang, T.T. Yuan, D.D. Li. One-pot formation of C-C and C-N bonds through palladium-catalyzed dual C—N activation:synthesis of phenanthridinones. Angew. Chem. Int. Ed. 50(2011)1380–1383. DOI:10.1002/anie.v50.6 |
| [26] | T.S. Mei, X.S. Wang, J.Q. Yu. Pd (Ⅱ)-catalyzed amination of C—N bonds using single-electron or two-electron oxidants. J. Am. Chem. Soc. 131(2009)10806–10807. DOI:10.1021/ja904709b |
| [27] | M.S. Christodoulou, K.M. Kasiotis, N. Fokialakis, I. Tellitu, S.A. Haroutounian. PIFA-mediated synthesis of novel pyrazoloquinolin-4-ones as potential ligands for the estrogen receptor. Tetrahedron Lett. 49(2008)7100–7102. DOI:10.1016/j.tetlet.2008.09.098 |
| [28] | J.V. Prata, D.S. Clemente, S. Prabhakar, A.M. Lobo, I. Mourato, P.S. Branco. Intramolecular addition of acyldiazenecarboxylates onto double bonds in the synthesis of heterocycles. J. Chem. Soc. Perkin Trans. 1(2002)513–528. |
| [29] | S.H. Cho, J. Yoon, S. Chang. Intramolecular oxidative C—N bond formation for the synthesis of carbazoles:comparison of reactivity between the copper-catalyzed and metal-free conditions. J. Am. Chem. Soc. 133(2011)5996–6005. DOI:10.1021/ja111652v |
| [30] | R. Antonchick, K. Samanta. Organocatalytic, oxidative, intramolecular C—H bond amination and metal-free cross-amination of unactivated arenes at ambient temperature. Angew. Chem. Int. Ed. 50(2011)8605–8608. DOI:10.1002/anie.201102984 |
| [31] | P.C. Huang, K. Parthasarathy, C.H. Cheng. Copper-catalyzed intramolecular oxidative C—N functionalization and C-N formation of 2-aminobenzophenones:unusual pseudo-1, 2-shiftof the substituenton the Aryl Ring. Chemistry 19(2013)460–464. DOI:10.1002/chem.201203859 |
| [32] | S.R. Chemler. Copper's contribution to amination catalysis. Science 341(2013)624–626. DOI:10.1126/science.1237175 |
| [33] | A.A. Kantak, S. Potavathri, R.A. Barham, R.A. Romano, B. Deboef. Metal-free intermolecular oxidative C—N bond formation via tandem C—N and N—H bond fun-ctionalization. J. Am. Chem. Soc. 133(2011)19960–19965. DOI:10.1021/ja2087085 |
| [34] | S. Serna, I. Tellitu, E. Domínguez, I. Moreno, R. SanMartin. Iodine(Ⅲ)-mediated aromatic amidation vs olefin amidohydroxylation. The amide N-substituent makes the difference. Tetrahedron 60(2004)6533–6539. DOI:10.1016/j.tet.2004.06.007 |
| [35] | E.G. Feng, H. Huang, Y. Zhou, D.J. Ye, H.L. Jiang, H. Liu. Copper(Ⅰ)-catalyzed onepot synthesis of 2H-1, 4-Benzoxazin-3-(4H)-ones from o-halophenols and 2-chloroacetamides. J. Org. Chem. 74(2009)2846–2849. DOI:10.1021/jo802818s |
| [36] | D.B. Chen, G.D. Shen, W.L. Bao. An efficient cascade synthesis of various 2H-1, 4-benzoxazin-3-(4H)-ones from o-halophenols and 2-halo-amides catalyzed by CuI. Org. Biomol. Chem. 7(2009)4067–4073. DOI:10.1039/b906210f |
| [37] | L. He, J. Yu, J. Zhang, X.-Q. Yu. α-amidation of cyclic ethers catalyzed by simple copper salt and a mild and efficient preparation method for α, ϖ-aminoalcohols. Org. Lett. 9(2007)2277–2280. DOI:10.1021/ol070537i |
| [38] | Y.X. Deng, J.P. Xie, W.W. Zhang, P. Yin, J. Yu, L. He. Oxidative amidation of aromatic ethers catalyzed by rhodium acetate. Chemistry 18(2012)1077–1082. DOI:10.1002/chem.201102303 |
| [39] | N. Liu, B.P. Yin, Y. Chen, Y. Deng, L. He. Catalyzed imidation of tertiaryamines by simple copper salts. Eur. J. Org. Chem. 2009(2009)2059–2062. DOI:10.1002/ejoc.v2009:13 |
| [40] | N. Liu, P. Yin, Y. Chen, Y. Deng, L. He. Preparation of α-sulfonylethanone oximes from oxidized hydroxylamine. Eur. J. Org. Chem. (2012)2711–2714. |
| [41] | X.W. Liu, Y.M. Zhang, L. Wang, H. Fu, Y.Y. Jiang, Y.F. Zhao. General and efficient copper-catalyzed amidation of saturated C—N bonds using Nhalosuccinimides as the oxidants. J. Org. Chem. 73(2008)6207–6212. DOI:10.1021/jo800624m |
| [42] | S. Santoro, R.Z. Liao, F. Himo. Theoretical study of mechanism and selectivity of copper-catalyzed C—N bond amidation of indoles. J. Org. Chem. 76(2011)9246–9252. DOI:10.1021/jo201447e |
 2017, Vol. 28
2017, Vol. 28