Phytochemicals have been successfully used for the development of biologically functional molecules because of their potential group arrays and novel scaffold architectures [1-4]. Phytoalexin is an important part of plant defense system against diseases. In 1940, Müller and Börger first proposed the concept of phytoalexin, which is used to describe the phenomenone that potato could produce the chemical molecules to resist bacteria further damage after invaded by Phytophthora infestans [5]. Although the amounts of phytoalexin in plants are not very high, they show a special activity to a variety of diseases. More and more studies have indicated that the phytoalexin plays an important role in resisting the invasion of bacterium, fungus, nematodes and insects [6]. In recent years, research on the biological activity, structure-activity relationship and action mechanism of the phytoalexin are developing rapidly. Therefore, it becomes an important research direction for the development of new pesticide using the phytoalexin as the guide structure.
A number of plants including Musaceae, Strelitziaceae, Pontederiaceae and Haemodoraceae produce phytoalexins containing phenalenone (PN) structural component [7-10]. These natural PNs or their modified structures showed fungicidal [10, 11], malariacidal [12], radical scavenging capacity [13] and antiprotozoal activity [14]. PN derivatives have also been researched as dental drugs to control oral key bacteria [15]. Recently Dirk Hölscher et al. reported that some PN analogues are the main defensive phytoalexins of banana plants attacked by nematodes [16].
With a view to investigate the insecticidal effect of phenalenone analogues on cowpea aphids and armyworms, herein, we reported the synthesis of a series of phenalenone derivatives and their insecticidal activities against cowpea aphids and armyworms.
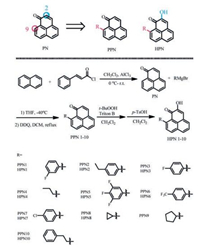
2. Results and discussion 2.1. Molecular designHolscher et al. [16] reported 9-phenyl-phenalenonles (PPNs) or 2-hydroxyl-9-phenylphenalenones (HPNs) have the rapid and long-lasting nematicide activity to burrowing nematode Radopholus similis [14]. The substituents on the core PN have some influence on the activity level. Compounds with fluorine are possible to improve the biological activities due to its simulation effect, electronic effect, blocking effect and penetration effect [17]. Based on the above descriptions, we prepared nineteen substituted compounds at 9-position and 2-position by attaching different aryl groups or non-aryl groups. The synthetic sequences for the target compounds are depicted in Scheme 1.
|
Download:
|
| Scheme 1. Molecular design of phenalenone derivatives and their synthetic routes. | |
2.2. Synthesis
The PN was prepared by reaction of naphthalene and cinnamoyl chloride via Friedel-Crafts reaction and aromatization through elimination of benzene [18]. PPNs were synthesized by slow adding PN to Grignard reagents in tetrahydrofuran at -40 ℃ and then in refluxing dichloromethane catalyzed by 2, 3-dichloro-5, 6-dicyano-1, 4-benzoquinone (DDQ). Epoxidation of PPNs and acid treatment of the intermediary epoxide facilitated the adding of 2-hydroxyl group to generate HPNs [19]. Thorresponding mechanism was shown in Scheme 2. Based on our experiments, a Michael addition of the Grignard reagents and PN was prefer to happen. And the addition products were mainly determined by the steric effect. We got major products PPNs and little byproducts 1. The byproducts 2 from neucleophilic addition of carbonyl were seldom found. Another method to synthesize PPNs is bioisosterism. But bioisosterism could limit the variety of substitutes at 9-positiion.So we chose the method of Michael addition and then epoxidation to synthesize PPNs and HPNs.

|
Download:
|
| Scheme 2. The mechanism of the synthesis of PPNs and the buproducts. | |
The methods to prepare Grignard reagents are very stable, but still have some limitations. It was difficult to prepare Grignard reagents with nitro benzene and other strong electron-withdrawing substituented benzene, which limited the introduction of types of substituents. When the substituents were aryl groups and naphthenic groups, the yields were high reaching about 70%. But when the substituents were alkanes, the byproducts increased. For compounds PPNs, due to the steric effect of aromatic, we obtained compounds substituted at 9-position instead of 1-positiion or 3-positiion. All the yields were low in the synthesis of 2 hydroxyl substituted compounds.
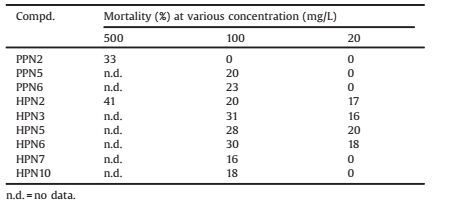
2.3. Antiaphid and antiarmyworm activitiesA total of nineteen phenalenone-based compounds were investigated for their activities against cowpea aphids and armyworms, the results were summarized in Table 1 and other results were shown in the supporting information. Unfortunately, all the synthesized compounds had no obvious antiarmyworm activities.
|
|
Table 1 Activities against Aphis craccivora. |
Due to the poor aqueous solubility, the starting testing concentration of most compounds was 100 mg/L. All the compounds had no insecticidal activities against armyworm at 100 mg/ L. Compounds PPN2, PPN5 and PPN6 had lower insecticidal activity against aphids and the others did not show any insecticidal activities. Compounds HPN2, HPN3, HPN5, HPN6, HPN7 and HPN10 had an insecticidal mortality of 16%-41%. The compounds with substituted halogens at the para-position of the benzene had a little bit better insecticidal activity with 16%-31% mortality at 100 mg/L. Compounds HPNs had slightly better insecticidal activities than PPNs. In general, compounds had potential activities against aphids when the substituents were aromatic ring at 9-position. However, compounds with fluorine did not show a good insecticidal activity as respected. When the substituents were cyclanes or alkanes, the compounds lost antiaphid activities.
3. ConclusionScreening of a collection of modified phenalen-1-one based structures has led to the identification of several new and potent insecticidal agents. Neither compound showed signs of antiarmyworm activities at concentration of 100 mg/L. While some compounds exhibited moderate antiaphid activities. Introduction of aryl at 9-position could improve the antiaphid activities. Introduction of hydroxyl at 2-position would enhance the antiaphid activities.
4. Experimental 4.1. Synthesis1H NMR and 13C NMR spectra were recorded on BrukerAM-400 (1H at 400 MHz, 13C at 100 MHz) spectrometer with CDCl3, D2O or DMSO-d6 as the solvent and TMS as the internal standard. High resolution electron mass spectra (ESI-TOF) were performed on a Micromass LC-TOF spectrometer. Chemical shifts are reported in δ (parts per million) values. Analytical thin-layer chromatography (TLC) was carried out on precoated plates (silica gel 60 F254), and spots were visualized with ultraviolet (UV) light.
General procedure for compounds PN: Alchlor (30 mmol) and naphthalene (10 mmol) were added to 10 mL anhydrous dichloromethane, the mixture was cooled to room temperature. Cinnamoyl chloride (10 mmol) was added in succession under 0 ℃, and the mixture was refluxed for 3 h. Then the reaction mixture was cooled with iced hydrochloric acid and filtered. The filtrate was extracted with dichloromethane. The solids were repeatedly boiled with hot dichloromethane and filtered until the filtrate became colorless. All the organic extracts were combined, dried over anhydrous sodium sulfate, and subjected to a rotary evaporator to give a yellow solid. The crude product was further purified by column chromatography (petroleum ether: ethyl acetate = 10:1). Yield 68% [20].
General procedure for the synthesis of PPN1-10: PN (0.004 mol) was dissolved in anhydrous tetrahydrofuran (10 mL). The solution was added dropwise to the appropriate Grignard reagent (0.006 mol, 1 mol/L in tetrahydrofuran, prepared from respective bromide and magnesium) via a syringe at -40 ℃. After stirring for 20 min, the reaction was moved to ice-bath and quenched with saturated aqueous solution of ammonium chloride. The aqueous phase was extracted with dichloromethane and the combined organic layer was washed with brine, dried over anhydrous sodium sulfate. The solvent was evaporated and the residue was dissolved in dichloromethane and treated with DDQ (0.004 mol). The reaction was refluxed for 3 h, filtered to move the precipitate. The crude product was further purified by column chromatography (petrol ether: ethyl acetate = 5:1) [21].
9-(4-Ethylphenyl)-1H-phenalen-1-one (PPN2): Yield, 71.3%; mp. 101.3-103.2 ℃; 1H NMR (400 MHz, CDCl3): δ 8.13 (d, 1H, J = 8.3), 8.01 (d, 1H, J = 7.9), 7.75 (d, 1H, J = 6.9), 7.66 (d, 1H, J = 9.7), 7.60 (d, 1H, J = 2.3), 7.58 (dd, 1H, J = 5.3, 2.7), 7.34-7.27 (m, 4H), 6.59 (d, 1H, J = 9.7), 2.74 (q, 2H, J = 7.6), 1.32 (t, 3H, J = 7.6); 13C NMR (100 MHz, CDCl3): δ 185.88, 147.91, 143.18, 140.34, 140.09, 133.71, 131.83, 131.74, 131.67, 131.37, 130.60, 128.49, 128.46, 127.96, 127.83, 126.28, 126.10, 28.70, 15.41. HRMS (EI +) calcd. for C21H16O (M +): 284.1201; found: 284.1198.
9-(3, 4, 5-Trifluorophenyl)-1H-phenalen-1-one (PPN5): Yield, 48.2%; mp. 204.7-206.9 ℃; 1H NMR (400 MHz, CDCl3): δ 8.20 (d, 1H, J = 8.3), 8.06 (d, 1H, J = 8.2), 7.82 (d, 1H, J = 6.9), 7.72 (d, 1H, J = 9.7), 7.69-7.63 (m, 1H), 7.51 (d, 1H, J = 8.3), 7.00-6.90 (m, 2H), 6.60 (d, 1H, J = 9.7); 13C NMR (100 MHz, CDCl3): δ 185.41, 153.57-151.87 (m), 150.09-149.80 (m), 143.85, 140.88, 139.65-138.31 (m), 134.14, 132.21, 131.99, 131.90, 130.62, 130.22, 128.50, 128.17, 127.02, 126.12, 112.27 (dd, JCF = 16.2, 5.7 Hz). HRMS (EI+) calcd. for C19H9F3O (M+): 310.0605; found: 310.0604.
9-(4-(Trifluoromethyl)phenyl)-1H-phenalen-1-one (PPN6): Yield, 73.5;mp. 191.3-193.5 ℃; 1H NMR (400 MHz, CDCl3): δ 8.21 (d, 1H, J = 8.3), 8.07 (d, 1H, J = 8.1), 7.81 (d, 1H, J = 6.9), 7.71 (dd, 3H, J = 8.9, 3.2), 7.65 (dd, 1H, J = 8.1, 7.2), 7.54 (d, 1H, J = 8.3), 7.46 (d, 2H, J = 8.0), 6.59 (d, 1H, J = 9.7); 13C NMR (100 MHz, CDCl3): δ 185.65, 146.82, 145.84, 140.83, 134.06, 132.08, 131.88, 131.83, 130.92, 130.24, 129.18 (q, JCF = 32.5), 128.51, 128.24, 126.83, 126.09, 125.25 (q, JCF = 3.7), 124.42 (q, JCF = 270.4); 19F (376 MHz, CDCl3): δ -62.35 (s, 3F). HRMS (EI+) calcd. for C20H11F3O (M+): 324.0762; found: 324.0753. (The analytical data of other target compounds were shown in the Supporting information).
General procedure for the synthesis of HPN1-8: PPN (0.002 mol) was dissolved in anhydrous dichloromethane and cooled to 0 ℃, then treated with Triton B (0.003 mmol, CH3OH) and t-butylhydroperoxide (0.003 mmol) for five hours. The reaction was quenched with saturated aqueous solution NH4Cl, the organic phase was separated, dried and evaporated. The residue was dissolved in dichloromethane and p-toluenesulfonic acid (0.002 mmol). The progress was monitored by TLC. The final product was purified by column chromatography (petrol ether: ethyl acetate = 6:1), affording deep-yellow solids [22].
2-Hydroxy-9-(4-ethylphenyl)-1H-phenalen-1-one (HPN2): Yield, 29.6%; mp. 132.7-133.9 ℃; 1H NMR (400 MHz, CDCl3): δ 8.22 (d, 1H, J = 8.2), 7.94 (d, 1H, J = 8.2), 7.74 (d, 1H, J = 7.0), 7.60 (t, 2H, J = 7.0), 7.33 (s, 4H), 7.13 (s, 1H), 4.73 (s, 1H, -OH), 2.78 (q, 2H, J = 7.2), 1.34 (t, 3H, J = 7.6, ); 13C NMR (100 MHz, CDCl3): δ 180.29, 149.77, 149.35, 143.63, 139.56, 135.60, 131.42, 130.70, 129.77, 128.79, 127.91, 127.77, 126.90, 125.06, 123.59, 112.67, 28.68, 15.26. HRMS (EI+) calcd. for C21H16O2 (M+): 300.1150; found: 300.1146.
2-Hydroxy-9-(4-fluorophenyl)-1H-phenalen-1-one (HPN3): Yield, 22.45; mp. 185.1-186.5 ℃; 1H NMR (400 MHz, CDCl3): δ 8.23 (d, 1H, J = 8.2), 7.94 (d, 1H, J = 8.1), 7.74 (d, 1H, J = 7.0), 7.64-7.59 (m, 1H), 7.57 (d, 1H, J = 8.2), 7.38-7.32 (m, 2H), 7.22-7.15 (m, 2H), 7.13 (s, 1H), 7.01 (s, 1H, -OH); 13C NMR (100 MHz, CDCl3): δ 180.31, 162.45 (d, JCF = 246.9), 149.77, 147.91, 138.09 (d, JCF = 3.5), 135.75, 131.63, 131.16, 130.93, 129.85, 129.73 (d, JCF = 8.1), 128.79, 127.14, 124.99, 123.67, 115.32 (d, JCF = 21.6), 112.91; 19F (376 MHz, CDCl3) δ -114.42 to -114.49 (m, 1F). HRMS (EI+) calcd. for C19H11FO2 (M+): 290.0743; found: 290.0742.
2-Hydroxy-9-(3, 4, 5-trifluorophenyl)-1H-phenalen-1-one (HPN5): Yield, 34.5%; mp. 201.5-203.3 ℃; 1H NMR (400 MHz, CDCl3): δ 8.27 (d, 1H, J = 8.2), 7.96 (d, 1H, J = 8.1), 7.77 (d, 1H, J = 7.0), 7.68-7.61 (m, 1H), 7.54 (d, 1H, J = 8.2), 7.15 (s, 1H), 7.02-6.95 (m, 2H), 6.94 (s, 1H, -OH); 13C NMR (100 MHz, CDCl3): δ 180.06, 152.58-152.02 (m), 150.14-149.70 (m), 149.61, 145.01, 138.18-137.95 (m), 135.98, 131.99, 131.31, 130.22, 129.92, 128.86, 127.62, 124.83, 123.72, 113.24, 112.42 (dd, JCF = 16.3, 5.9); 19F (376 MHz, CDCl3): δ -134.42 to -134.63 (m, 2F), -161.90 to -162.04 (m, 1F). HRMS (EI+) calcd. for C19H9F3O2 (M+): 326.0555; found: 326.0554.
2-Hydroxy-9-(4-(trifluoromethyl)phenyl)-1H-phenalen-1-one (HPN6): Yield, 30.0%; mp. 223.9-225.3 ℃; 1H NMR (400 MHz, CDCl3): δ 8.28 (d, 1H, J = 8.2), 7.97 (d, 1H, J = 8.2), 7.81-7.71 (m, 3H), 7.67-7.61 (m, 1H), 7.56 (d, 1H, J = 8.2), 7.49 (d, 2H, J = 8.2), 7.15 (s, 1H), 6.95 (s, 1H, -OH); 13C NMR (100 MHz, CDCl3): δ 180.27, 149.69, 147.07, 146.13, 135.89, 131.86, 131.14, 130.54, 129.66 (q, JCF = 32.5), 128.30, 127.42, 125.64, 125.26 (q, JCF = 3.7), 124.90, 124.29 (q, JCF = 270.4), 123.66, 122.94, 113.18; 19F (376 MHz, CDCl3): δ -62.36 (s, 3F). HRMS (EI+) calcd. for C20H11F3O2(M+): 340.0711; found: 340.0706.
2-Hydroxy-9-(4-chlorophenyl)-1H-phenalen-1-one (HPN7): Yield, 33.3%; mp. 211.2-212.8 ℃; 1H NMR (400 MHz, CDCl3): δ 8.24 (d, J = 8.2, 1H), 7.95 (d, J = 8.2, 1H), 7.75 (d, J = 7.1, 1H), 7.62 (t, J = 7.7, 1H), 7.56 (d, J = 8.2, 1H), 7.47 (d, J = 8.3, 2H), 7.32 (d, 2H, J = 8.3), 7.14 (s, 1H), 6.99 (s, 1H, -OH); 13C NMR (100 MHz, CDCl3): δ 180.29, 149.74, 147.58, 140.70, 135.81, 133.73, 131.70, 130.99, 130.89, 129.86, 129.36, 128.82, 128.54, 127.22, 124.96, 123.62, 112.98. HRMS (EI+) calcd. for C20H1135ClO2 (M+): 306.0448; found: 306.0466; HRMS (EI+) calcd. for C20H1137ClO2 (M+): 308.0418; found: 308.0418.
2-Hydroxy-9-hydrocinnamyl-1H-phenalen-1-one (HPN10): Yield, 66.4%; mp. 67.9-69.8 ℃; 1H NMR (400 MHz, CDCl3): δ 8.08 (d, 1H, J = 8.3), 8.02 (d, 1H, J = 8.1), 7.80 (d, 1H, J = 6.9), 7.76 (d, 1H, J = 9.7), 7.60 (dd, 1H, J = 7.9, 7.3), 7.44-7.37 (m, 2H), 7.32 (dd, 2H, J = 10.3, 4.8), 7.21 (t, 1H, J = 7.3), 6.78 (d, 1H, J = 9.6), 3.73-3.69 (m, 2H), 3.05-2.93 (m, 2H). HRMS (EI+) calcd. for C21H16O (M+): 284.1201; found:284.1202.
The analytical data of other target compounds were shown in the Supporting information.
4.2. Antiaphid and antiarmyworm activitiesAll bioassays were performed on representative test organisms raised in the laboratory. Each experiment was repeated three times at 25 ±1 ℃ according to the statistical requirements. Mortality rates were evaluated on the basis of a percentage scale of 0 to 100, 0 means no activity while 100 means total kill. If the mortality rates of the blank control were less than 5%, the results could be directly used. If the mortality rates were more than 5% and less than 20%, the results should be corrected by V = ((X-Y)/X) × 100 (V = value of corrected mortality; X = livability of the blank control; Y = livability of the treat) [23].
AcknowledgmentsThis work was financial supported by National Natural Science Foundation of China (Nos. 21472046, 21372079) and Science and Technology Commission of Shanghai Municipality (No. 16391902300).
Appendix A. Supplementary dataSupplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.cclet.2017.04.003.
| [1] | B. Vieira da Silva, J.C.M. Barreira, M.B.P.P. Oliveira. Natural phytochemicals and probiotics as bioactive ingredients for functional foods:extraction:biochemistry and protected-delivery technologies. Trends Food Sci. Technol. 50(2016)144–158. DOI:10.1016/j.tifs.2015.12.007 |
| [2] | M.H. Chen, X.J. Chen, M. Wang, L.G. Lin, Y.T. Wang. Ophiopogon japonicus-a phytochemical. ethnomedicinal and pharmacological review. J. Ethnopharmacol. 181(2016)193–213. DOI:10.1016/j.jep.2016.01.037 |
| [3] | X.L. Wang, X.X. Di, T. Shen, et al., New phenolic compounds from the leaves of Artocarpus heterophyllus. Chin. Chem. Lett 28(2017)37–40. DOI:10.1016/j.cclet.2016.06.024 |
| [4] | Y. Zhang, Y.B. Liu, Y. Li, et al., Phenolic constituents from the roots of Alangium Chinense. Chin. Chem. Lett 28(2017)32–36. DOI:10.1016/j.cclet.2016.05.012 |
| [5] | K. O. Müeller, H. Börger, Experimentelle Untersuchungen über die Phytophthora-Resistenz der Kartoffel, Berlin, Arbeiten aus der biolgiscen Bundensanstalt fur Land und Forstwirtscaft, vol. 231940, pp. 189-231. |
| [6] | J. Enkerli, G. Bhatt, S.F. Covert. Maackiain detoxification contributes to the virulence of nectria haematococca MP Ⅵ on chickpea. Mol. Plant Microbe Interact. 11(1998)317–326. DOI:10.1094/MPMI.1998.11.4.317 |
| [7] | F. Otálvaro, F. Echeverri, W. Quiñones, F. Torres, B. Schneider. Correlation between phenylphenalenone phytoalexins and phytopathological properties in Musa and the role of a dihydrophenylphenalene triol. Molecules 7(2002)331–340. DOI:10.3390/70300331 |
| [8] | F. Otálvaro, J. Nanclares, L.E. Vasquez. Phenalenone-type compounds from Musa acuminata var. "Yangambi km 5" (AAA) and their activity against Mycosphaerella fijiensi. J. Nat. Prod. 70(2007)887–890. DOI:10.1021/np070091e |
| [9] | J.C.d. Río, J. Jiménez-Barbero, M.I. Chávez, A. Gutiérrez. Phenylphenalenone type compounds from the leaf fibers of Abaca (Musa textilis). J. Agric. Food Chem. 54(2006)8744–8748. DOI:10.1021/jf061781b |
| [10] | C. Flors, S. Ninell. Light and singlet oxygen in plant defense against pathogens:Phototoxic phenalenone phytoalexins. Acc. Chem. Res. 39(2006)293–300. DOI:10.1021/ar0402863 |
| [11] | W. Hidalgo, L. Duque, J. Saez. Structure-activity relationship in the interaction of substituted perinaphthenones with Mycosphaerella fijiensis. J. Agric. Food Chem. 57(2009)7417–7421. DOI:10.1021/jf901052e |
| [12] | D. Gutierrez, N. Flores, T. Abad-Grillo, G. McNaughton-Smith. Evaluation of substituted phenalenone analogues as antiplasmodial agents. Exp Parasitol. 135(2013)456–458. DOI:10.1016/j.exppara.2013.08.008 |
| [13] | L. Duque, C. Zapata, B. Rojano, B. Schneider, F. Otálvaro. Radical scavenging capacity of 2. 4-dihydroxy-9-phenyl-1H-phenalen-1-one:a functional group exclusion approach. Org. Lett. 15(2013)3542–3545. DOI:10.1021/ol400384z |
| [14] | L.I. Rosquete, M.G. Cabrera-Serra, J.E. Pinero. Synthesis and in vitro antiprotozoal evaluation of substituted phenalenone analogues. Bioorg. Med. Chem. 18(2010)4530–4534. DOI:10.1016/j.bmc.2010.04.062 |
| [15] | A. Spath, et al., Improving photodynamic inactivation of bacteria in dentistry:highly effective and fast killing of oral key pathogens with novel tooth-colored type-Ⅱ photosensitizers. J. Med. Chem. 57(2014)5157–5168. DOI:10.1021/jm4019492 |
| [16] | D. Holscher, S. Dhakshinamoorthy, T. Alexandrov. Phenalenone-type phytoalexins mediate resistance of banana plants (Musa spp) to the burrowing nematode Radopholus similis. Proc. Natl. Acad. Sci. 111(2014)105–110. DOI:10.1073/pnas.1314168110 |
| [17] | E.P. Gillis, K.J. Eastman, M.D. Hill, D.J. Donnelly, N.A. Meanwell. Applications of fluorine in medicinal chemistry. J. Med. Chem. 58(2015)8315–8359. DOI:10.1021/acs.jmedchem.5b00258 |
| [18] | C.F. Koelsch, J.A. Anthes. Studies in the perinaphthene1 series. IV2. some attempts to synthesize 9-phenyl-perinaphthanone-7. J. Org. Chem. 6(1941)558–565. DOI:10.1021/jo01204a009 |
| [19] | L. Duque, C. Zapata, B. Rojano, B. Schneider, F. Otálvaro. Radical scavenging capacity of 2, 4-dihydroxy-9-phenyl-1H-phenalen-1-one:a functional group exclusion approach. Org. Lett. 15(2013)3542–3545. DOI:10.1021/ol400384z |
| [20] | Y Y. -Li, B. Yan, F X. -Qiao. Visible light excitation and near-infrared luminescence of organo-lanthanide hybrids with mesoporous silica through 9-hydroxyphenalen-1-one linkage. Microporous Mesoporous Mater. 169(2013)60–66. DOI:10.1016/j.micromeso.2012.09.042 |
| [21] | L. Duque, C. Zapata, B. Rojano, B. Schneider, F. Otálvaro. Radical scavenging capacity of 2, 4-dihydroxy-9-phenyl-1H-phenalen-1-one:a functional group exclusion approach. Org. Lett. 15(2013)3542–3545. DOI:10.1021/ol400384z |
| [22] | W. Quinones, G. Escobar, F. Echeverri, et al., Synthesis and antifungal activity of Musa phytoalexins and structural analogs. Molecules 5(2000)974–980. DOI:10.3390/50700974 |
| [23] | W.S. Abbott. A method of computing the effectiveness of an insecticide. J. Econ. Entomol. 18(1925)265–267. |
 2017, Vol. 28
2017, Vol. 28