b School of Pharmaceutical Sciences, Jilin University, Changchun 130012, China
Type 2 diabetes mellitus (DM2) is a metabolic disorder that characterized by hyperglycemia involving in dysfunction of insulin and relative insulin deficiency. Diabetic patients usually suffer from complications, such as nephropathy, neuropathy, cataract formation, retinopathy and cardiovascular disease [1-3]. Furthermore, the long-term secondary complications are the main cause of morbidity and mortality [4]. The unifying pathophysiological mechanism that underlies diabetic complications mainly involved inoxidative stress, increased flux of polyol pathway, enhanced formation of advanced glycation end products (AGEs) [5-7]. Growing evidence indicated that the above-mentioned pathways were novel therapeutic targets for the treatment of diabetic complications [8, 9].
High glucose ambience in DM2 leads to activation of polyolpathway and consequent accumulation of sorbitol that generated from glucose by aldose reductase (AR). AR, the key ratelimiting enzyme in the polyol pathway, mediated hyperglycemiainduced oxidative stress and overexpressed in DM2 [8]. AGEs are the products of non-enzymatic glycation and oxidation of proteins and lipids that formed both extracellularly from glucose and intracellularly from various dicarbonyls, and their formation accelerates in hyperglycemia [9]. They could generate reactive oxygen species andactivate inflammatory signaling cascades via AGE-specific receptor which linking to diabetic complications. Necarboxymethyl-lysine (CML) is a stable AGE that forms on lysyl side-chains in the presence of glucose that served as an indicator of AGE concentration in urine [10]. Additionally, previous literatures have indicated that amino acid metabolism plays a potential important role in the pathogenesis of diabetes, and amino acid profiles could be applied in diabetes risk assessment [11].
Radix Scutellariae, one of the most valuable herbal medicines, has been used for centuries in the treatment of DM2 and its complications [12, 13]. Pharmacological studies have shown that Radix Scutellariae possesses many beneficial activities with respect to anticancer, antiviral, antibacterial, anti-inflammation, cleaning away heat, detoxifying toxicosis and decreasing blood pressures [14-16]. Baicalin and baicalein, as the main ingredients of Radix Scutellariae, were found to be effective in stimulating insulin secretion, β-cellproliferation and preserving renal function [17-19]. Moreover, our previous studies demonstrated that the main flavonoids from Radix Scutellariae were AR inhibitors [20].
Given the potential role of Radix Scutellariaein preventing DM2 and diabetic complications, we measured urinary sorbitol, CML and several amino acids as indicators of poly pathway, AGEs and amino acids to evaluateits therapeutic effect on DM2 in the current study. The levels of sorbitol, CML and several amino acids were measured by LC-MS/MS quantitative analysis combined with multivariate statistical analysis for evaluating the metabolic response of Radix Scutellariaeon DM2 rats. Our results may help to better understand the endogenous variation and effects contributed by Radix Scutellariaein treatment of type 2 diabetic complications.
2. Results and discussion 2.1. Biochemical parametersAs shown in Table 1, long-term treatment with Radix Scutellariae could partially reverse fasting blood glucose, serum total cholesterol (TC) and triglyceride (TG) levels caused by diabetes. Oxidative stress was induced in rats of model group with a significant reduction of serum SOD activities, and the content increasing of serum malondialdehyde (MDA) compared with normal rats (P < 0.05). Treatment of Radix Scutellariae could mitigate the oxidative stress in diabetic rats, along with regulated serum SOD activities and MDA contents. These biochemical parameters indicated the therapeutic effect of Radix Scutellariae on DM2 rats.
|
|
Table 1 Biochemical parameters of rats in different groups after treated for 16 weeks. |
2.2. Optimization of experimental conditions
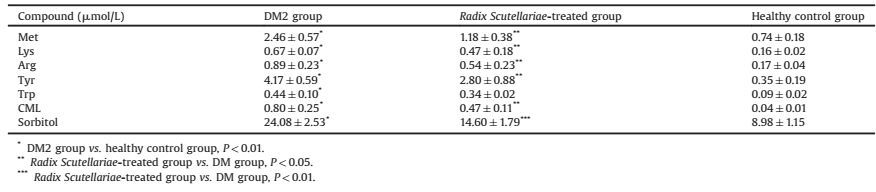
Prior to the LC-MS/MS analysis, the IntelliStart function was applied to optimize the cone voltages and the collision energies. The appropriate instrumental parameters were obtained through infusing each standard solution into the mass spectrometer via a syringe pump at a rate of 20 μL/min respectively. In this research, the most abundant ions of analytes obtained in positive mode had higher sensitivity and intensity to permit quantitative measurement than the ions in negative mode. In this case we chose the quantitative ionpairs inpositive mode. To collect sufficient data for the integrative research, two precursor/product ion transitions of each analyte were selected to create MS/MS approach. MS/MS spectra of these analytes were presented in Supporting information Fig. S1. The highest sensitive ion transition was chosen for the quantitative analysis (Fig. 1), while another one was elected as a qualifier for the confirmatory analysis. The ion transitions with appropriate instrumental parameters for the MRM detection are shown in Supporting information Table S1.

|
Download:
|
| Fig. 1. The MRM chromatograms of the quantitative ion transitions of each analyte. | |
2.3. Validation of LC-MS/MS method
Prior to the analysis of real samples, the suitability of the approach was properly verified to ensure the obtained results were reliable. Method validation was implemented via evaluating performance characteristics including linearity, accuracy, precision, matrix effects, and limit of quantification (LOQ). A series of samples with different concentration levels were utilized for the construction of calibration curves. LOQ was used to describe the lowest concentrations of an analyte that can be reliably measured byan analytical procedure. In cases where noise was present in the chromatogram of the analyte, the signal-to-noise ratio (S/N) of LOQ was 10. The intra-day and inter-day precision and accuracy were investigated at three levels. Relative error (RE) and relative standard deviation (RSD) were used to estimate the accuracy and precision, respectively. The suitability of the precision and accuracy was assessed by the following criteria: The RSD should not exceed 15% and the accuracy should be within 15% of the actual values. Additionally, the recoveries of analytes were also determined at three levels.
Peak area ratios (analyte/internal standard) of the urine samples were utilized for the construction of linear calibration curves (Table S2 in Supporting information) using LC-MS/MS method. The final concentrations of internal standard were kept constant at 1 μmol/L. The standard curves of analytes were not forced through the origin and presented excellent linearity with correlation coefficients higher than 0.9900. The precision and accuracy results are shown in Table S3 (Supporting information), which demonstrated that the method possessed sufficiently precise and of good accuracy. Moreover, the recovery of each analyte was 85%-115% and RSD were less than 15%. These results met the requirements of an analytical assay.
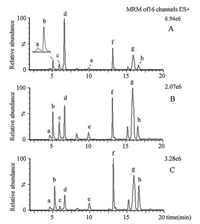
2.4. Multivariate analysisThe urine samples of healthy control group, DM2 model group and Radix Scutellariae-treated group were determined using the proposed LC-MS/MS method. The results of the quantitative analysis were summarized in Table 2. Including sorbitol, Met, Lys, Arg, Tyr, Trp and CML, the seven target analyteswere related to the polyol pathway, amino acid protein glycation reaction. The MRM chromatograms of analytes detected in urine of different groups were shown in Fig. 2. The significance between groups was expressed by using Student's t-test. By comparison with healthy control group, the level of seven analytes in DM group increased significantly (P < 0.01). As shown in Table 2, the concentration of sorbitol, Met, Lys, Arg, Tyr and CML were down regulated significantly after Radix Scutellariae treatment. As to the Trp content, there is no significant difference between two groups but a decline trend.
|
Download:
|
| Fig. 2. The MRM chromatograms of analytes detected in urine of DM2 model group (A), Radix Scutellariae-treated group (B) and healthy control group (C). The detected analytes were presented as follows: Lys (a), Arg (b), CML (c), Sorbitol (d), Met (e), Tyr (f), Trp (g), N, N-Phe (h). | |
|
|
Table 2 The concentrations of analytes in the urinary samples of healthy control group, DM group and Radix Scutellariae-treated group. |
Multivariate profile-wide predictive models were performed using HCA and PLS-DA by the software package SPSS 18.0 and SIMAC-P 11.5, respectively. HCA is a statistical method to build relatively homogeneous clusters of cases according to the measured characteristics, which could be expressed as a tree or dendrogram, where each step in the clustering process was illustrated by a join of the tree. In our experiment, HCA was performed using between-groups linkage and squared Euclidean distance. The generated dendrogram was presented in Fig. 3. It showed a clear separation of different classes, forming three groups at a relative distance between 4.0 and 6.0, yet there were two groups when the relative distance higher than 6.0. These results indicated that Radix Scutellariae could affect the metabolism of diabetic complications, such as the polyol pathway and formation of advanced glycation end-products.

|
Download:
|
| Fig. 3. The obtained dendrogram using average linkage, rescaled distance cluster combine of HCA (1-7: healthy control group; 8-13: RS-treated group; 14-19: DM2 model group). | |
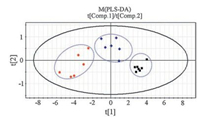
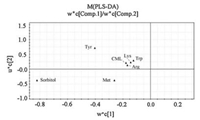
PLS-DA, a supervised chemometrics method based on PLS, explains the maximum separation between defined class samples in the data (X) using a Y matrix that represents an orthogonal unit vector for each class [21]. The quantitative data of analytes in urine samples were processed by PLS-DA method. Variable scaling is an integral part of multivariate analysis as it regulates the relative importance of each variable in the subsequent mode [22]. The Q2 value of PLS-DA model was 0.674 by the two first principal components (PCs), while the value of R2X and R2Y was 0.955 and 0.769, respectively. These results indicated that the statistical model was credible because it possessed good goodness of fit and goodness of prediction. Here, the differences between groups were presented by score plot and loading plot. The score plot (Fig. 4) indicated that an obvious separation of different classes had been achieved, which was consistent with the result of HCA. The loading plot (Fig. 5) demonstrated that sorbitol and Tyr played important roles in the class separation.
|
Download:
|
| Fig. 4. Scores plot of two principal components of PLS-DA model (■: healthy control group; ◆: Radix Scutellariae-treated group; ●: DM2 model group). | |
|
Download:
|
| Fig. 5. Loadings plot of two principal components of PLS-DA model. | |
2.5. Quantitative analysis of urinary endogenous markers
To develop a suitable and robust LC method, in the preliminary experiment several experimental parameters were investigated, including mobile phase, column temperature and gradient elution program. In order to obtain the stable chromatographic resolution conditions, other parameters such as injection volume and column temperature were also investigated. The optimal separation of each analyte was achieved using a gradient elution as described in the experimental section.
It is generally known that diabetes mellitus is characterized by high blood glucose, which could stimulate the polyol pathway in diabetic patients. In the polyol pathway, aldose reductase catalyzes the reduction of glucose to sorbitol in the presence of NADPH [23]. Yet sorbitol does not readily diffuse across cell membranes which results in its intracellular accumulation in insulin-resistant tissues such as small blood vessels, nerves, lens, retina and kidney. Consequently, those cases ultimately lead to chronic complications of diabetes, which is the reason why the amount of urinary sorbitol increased by comparing DM group with healthy control group. Moreover, our previous study has shown the main flavonoids from Radix Scutellariae were aldose reductase inhibitors, which maybe the main reason leading to down regulation of sorbitol in Radix Scutellariae treated group [20]. In addition, endogenous formation of AGEs is known to contribute to the progression of pathogenesis in conditions associated with diabetic complications [24]. CML, a common AGE, was determined in the present study. The result revealed that urinary CML increased significantly in DM subjects versus healthy controls, and the concentration of urinary CML residues decreased in Radix Scutellariae-treated subjects, with respect to DM rats. The changes of CML residue in this study suggested that the diabetic state is associated with significantly increased damage to protein due to glycation [25]. In summary, the changes found in urinary excretion of diabetic rats with Radix Scutellariae treatment were consistent with reducing the protein damage and the development of polyol pathway, which was in agreement with our previous study in vitro [26].
The pathophysiology of DM2 involves insulin resistance and impaired β-cell function. Dysregulated amino acids metabolism reported in DM2 was closely related to β-cell function and insulin resistance [27-29], and amino acid profiles could be applied in diabetes risk assessment [11, 30]. For instance, in arginine metabolism pathway, nitric oxide (NO) is the down product of arginine. Endothelial NO production was thought to be the pivotal roles in the pathogenesis of cardiovascular disease in DM2 [31]. Of note, insulin could modulate the activation of arginine-NO pathway which was impaired in DM2 [32]. And insulin resistance was suggested to attribute to the development of cardiovascular disease in DM2 through this way. In this study, significantly upregulated levels of urinary amino acids observed in DM group could be from long-term insulin resistance and damage of β-cell function. The declined level of urinary amino acids in Radix Scutellariae treated group can partly reflect the anti-diabetic effect of Radix Scutellariae. It is reasonable to assume that Radix Scutellariae mediated regulation of amino acids resulting in a beneficial effect in preventing diabetes-associated complications.
3. ConclusionIn the present work, a quantitative analysis method based on LC-MS/MS combining with multivariate statistical analysis have been used to investigate the therapeutic effect of Radix Scutellariae on type 2 diabetic complications. With PLS-DA and HCA analysis, a clear separation has been achieved among normal control group, DM2 group and Radix Scutellariae-treated group after 16 weeks treatment. According to the quantitative results of seven analytes involved in polyol pathway, AGEs and amino acids metabolism, Radix Scutellariae should have the pharmacological effect on preventing or delaying the onset and progression of diabetes and its complications. Our research results could provide a reference for clinical studies in the prevention and treatment of diabetic complications.
4. Experimental 4.1. Reagents and chemicalsCML was obtained from Toronto Research Chemicals Inc. (Canada). L-lysine (Lys), L-arginine (Arg), L-tryptophan (Trp), L-methionine (Met), L-tyrosine (Tyr), N, N-dimethylphenylalanine (N, N-Phe), sorbitol, and Streptozotocin (STZ) were purchased from Sigma Chem. Co., (St. Louis, MO, USA). Acetic acid and acetonitrile of HPLC grade were supplied by Tedia Company, Inc. (USA) and Fisher Scientific (Fair Lawn, NJ, USA), respectively. Deionized water was prepared using a Milli-Q water purification apparatus (Bedford, MA, USA). All other chemicals and reagents were of analytical grade. TG kits, TC kits, SOD kits, MDA kits, were obtained from Nanjing Jiancheng Bioengineering Institute (Nanjing, China).
4.2. Preparation of Radix Scutellariae extractRadix Scutellariae was purchased from Tongrentang Pharmacy (Changchun, China) and authenticated by Professor Shumin Wang (Changchun University of Traditional Chinese Medicine, China). The powdered Radix Scutellariae which was selected by 100 mesh sieve was weighed accurately, and then the powder was refluxed once with ethanol: water (70:30, v/v) at a sample-to-solvent ratio 1:6 (w/v) for 40 min. Afterward, the Radix Scutellariaeresidue was refluxed twice again with water at a sample-to-solvent ratio 1:6 (w/v) for 1.5 h. The filtrates were combined, filtered, concentrated decompression until without alcohol odor. Then, the concentrated solution was diluted with water and lyophilized to obtain extract powder which was stored in plastic bags at room temperature.
4.3. Animal experimentTwenty four 4-week-old male Wistar rats were purchased from Experimental Animal Center of Jilin University (China). All rats were kept in a barrier system with regulated temperature (15-25 ℃) and humidity (40%-80%) and on a 12/12-h light-dark cycle. Sixteen rats were fed with high-sucrose and high-fat chow (10% lard, 0.4% cholesterol, 3% raw egg yolk, 20% sucrose and 66.6% standard rat chow) as the diabetic rat model group and the other eight rats were fed with standard rat chow as the healthy control group. After a 10-week feeding, the rats fed with high-sucrose and high-fat chow were injected intraperitoneal with STZ freshly prepared in citrate buffer (0.1 mol/L, pH 4.5) at a single dosage of 35 mg/kg body weight (BW), while the healthy control group was injected with citrate buffer. Then, the tail-blood glucose level was measured by One Touch Ultra Meter (Lifescan Inc., CA, USA) after a week. Rats presenting blood glucose values higher than 16.7 mmol/L were defined as diabetic [33]. Twelve DM2 rats were randomly divided into two groups. One group was treated with Radix Scutellariae extract 3 g/kg BW once a day by gastric irrigation, while the other was administered with water in parallel. In addition, the healthy control group was also administered with water by gastric irrigation. During the experimental period, body weights and fasting blood glucose were recorded weekly. After sixteen-week continuous treatment, 24-h urine samples were collected from fasting rats and kept at -80 ℃ until analysis. At the end of sixteen-weeks, blood was collected and placed on ice for 30 min and then centrifuged at 3000 rmp for 10 min at 4 ℃ to get the serum sample. The serum samples were kept at -80 ℃ for later biochemical analysis. All the animal studies were performed according to institutional guidelines for the care and use of laboratory animals, and protocols were approved by the Animal Research Ethics Committee of Chinese Academy of Sciences.
4.4. Sample preparationThe urine samples were thawed at 4 ℃ and centrifuged at 10000 rpm for 10 min. Prior to analysis, the samples were filtered through a 0.22 μm membrane filter and diluted with water (1: 9, v/ v). Then, N, N-Phe, the internal standard, was added to the urine samples with a final concentration of 1 μmol/L. The obtained samples were used for analysis by LC-MS/MS method.
4.5. Biochemical analysesTo evaluate the therapeutic effect of Radix Scutellariae on DM2 rats, several diabetic related biochemical parameters were tested. Serum TG and TC were measured enzymatically using commercial kits. Anti-oxidative stress parameters, including activity of SOD and the concentration of malondialdehyde (MDA), were measured.
4.6. LC-MS/MS analysesLC-MS/MS analyses were performed using an Acquity-Ultra Performance LC system (Waters, Milford, MA, USA) combined with Xevo TQ mass spectrometer (Waters, Milford, MA, USA). LC system equipped with a binary solvent delivery system, an auto-sampler and column heater. The chromatographic separation was achieved using a Venusil ASB C18 HPLC column (250 mm × 4.6 mm, 5 μm, Agela Technologies) with a binary mobile phase composed of eluent A (0.12% (v/v) acetic acid in water) and eluent B (33% acetonitrile containing 0.9% acetic acid aqueous) pumped at a flow rate of 0.5 mL/min. The temperature of column heater was kept constant at 25 ℃. The gradient elution program was started with 0% B and maintained for 2.5 min, then increased linearly to 17% B in 5 min, then to 33% B over 3 min, further to 100% A in the next 0.5 min and maintained this gradient for 8.5 min. Additionally, the injection volume was 3 μL considering the sensitivity of the instrument.
The mass spectrometer equipped with electrospray ionization (ESI) ion source was operated in the multiple reaction monitoring (MRM) mode for the accurate quantitative analysis in positive mode. The direct flow-injection mode was applied to optimize the cone voltages and the collision energies. The ion source temperature and desolvation temperature were maintained at 150 and 350 -C, respectively. The spray voltage was set at 3.0 kV. Nitrogen was supplied as the cone gas and desolvation gas at flows of 60 and 800 L/h, respectively, while argon was used as collision gas at a pressure of 0.1 MPa and at a flow of 0.15 mL/min.
4.7. Data processingThe LC-MS/MS data were acquired via MassLynx4.1 software (Waters), and the MassLynx4.1 with TargetLynx was applied to data processing. Multivariate statistical analyses were performed using SIMCA-P software (version 11.5, Umetrics AB, Umeå, Sweden) and SPSS software (version 18.0, SPSS Inc., USA).
AcknowledgmentsThis work was supported by the National Natural Science Foundation of China (No. 81373952, 81473537) and the the Jilin province science and technology development projects (No. 20150311039YY).
Appendix A. Supplementary dataSupplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.cclet.2016.12.039.
| [1] | D. Porte Jr, M.W. Schwartz. Diabetes complications-why is glucose potentially toxic. Science (1996)272–699. |
| [2] | I.M. Stratton, A.I. Adler, H.A.W. Neil, et al., Association of glycaemia with macrovascular and microvascular complications of type 2 diabetes (UKPDS 35):prospective observational study. BMJ 321(2000)405–412. DOI:10.1136/bmj.321.7258.405 |
| [3] | R. Ramasamy, I.J. Goldberg. Aldose reductase and cardiovascular diseases, creating human-like diabetic complications in an experimental model. Circ. Res. 106(2010)1449–1458. DOI:10.1161/CIRCRESAHA.109.213447 |
| [4] | M. Brownlee. Biochemistry and molecular cell biology of diabetic complications. Nature 414(2001)813–820. DOI:10.1038/414813a |
| [5] | A.L.Y. Ton, J.M. Forbes, M.E. Cooper. AGE, RAGE, and ROS in diabetic nephropathy. Semin. Nephrol. 27(2007)130–143. DOI:10.1016/j.semnephrol.2007.01.006 |
| [6] | S.S.M. Chung, E.C.M. Ho, K.S.L. Lam, S.K. Chung. Contribution of polyol pathway todiabetes-induced oxidative stress. J. Am. Soc. Nephrol. 14(2003)S233–S236. DOI:10.1097/01.ASN.0000077408.15865.06 |
| [7] | T. Inoguchi, T. Sonta, H. Tsubouchi, et al., Protein kinase C-dependent increase in reactive oxygen species (ROS) production in vascular tissues of diabetes:role of vascular NAD(P)H oxidase. J. Am. Soc. Nephrol. 14(2003)S227–S232. |
| [8] | R. Maccari, R. Ottanà. Targeting aldose reductase for the treatment of diabetes complications and inflammatory diseases:new insights and future directions. J. Med. Chem. 58(2015)2047–2067. DOI:10.1021/jm500907a |
| [9] | P.M. Magalhães, H.J. Appell, J.A. Duarte. Involvement of advanced glycation end products in the pathogenesis of diabetic complications:the protective role of regular physical activity. Eur. Rev. Aging Phys. Act. 5(2008)17–29. DOI:10.1007/s11556-008-0032-7 |
| [10] | S. Choudhuri, D. Dutta, A. Sen, et al., Role of N-ε-carboxy methyl lysine, advanced glycation end products and reactive oxygen species for the development of nonproliferative and proliferative retinopathy in type 2 diabetes mellitus. Mol. Vis. 19(2013)100–113. |
| [11] | C.Y. Wang, H.B. Zhu, Z.F. Pi, et al., Classification of type 2 diabetes rats based on urine amino acids metabolic profiling by liquid chromatography coupled with tandem mass spectrometry. J. Chromatogr. B 935(2013)26–31. DOI:10.1016/j.jchromb.2013.07.016 |
| [12] | K.H. Song, S.H. Lee, B.Y. Kim, A.Y. Park, J.Y. Kim. Extracts of Scutellaria baicalensis reduced body weight and blood triglyceride in db/db Mice. Phytother. Res. 27(2013)244–250. DOI:10.1002/ptr.v27.2 |
| [13] | K.S. Suh, Y.H. Nam, Y.M. Ahn, et al., Effect of scutellariae radix extract on the high glucose-induced apoptosis in cultured vascular endothelial cells. Biol. Pharm. Bull. 26(2003)1629–1632. DOI:10.1248/bpb.26.1629 |
| [14] | D.Y. Zhang, J. Wu, F. Ye, et al., Inhibition of cancer cell proliferation and prostaglandin E2 synthesis by Scutellaria baicalensis. Cancer Res. 63(2003)4037–4043. |
| [15] | H.B. Li, Y. Jiang, F. Chen. Separation methods used for Scutellaria baicalensis active components. J. Chromatogr. B 812(2004)277–290. DOI:10.1016/S1570-0232(04)00545-8 |
| [16] | Z.Y. Zhu, L.A. Zhao, X.F. Liu, et al., Comparative pharmacokinetics of baicalin and wogonoside by liquid chromatography-mass spectrometry after oral administration of Xiaochaihu Tang and Radix scutellariae extract to rats. J. Chromatogr. B 878(2010)2184–2190. DOI:10.1016/j.jchromb.2010.06.021 |
| [17] | A. Ahad, M. Mujeeb, H. Ahsan, W.A. Siddiqui. Prophylactic effect of baicalein against renal dysfunction in type 2 diabetic rats. Biochimie 106(2014)101–110. DOI:10.1016/j.biochi.2014.08.006 |
| [18] | S.M. Park, S.M. Hong, S.R. Sung, J.E. Lee, D.Y. Kwon. Extracts of Rehmanniae radix, Ginseng radix and Scutellariae radix improve glucose-stimulated insulin secretionand β-cell proliferation through IRS2 induction. Genes Nutr. 2(2008)347–351. DOI:10.1007/s12263-007-0065-y |
| [19] | H.M. El-Bassossy, N.A. Hassan, M.F. Mahmoud, A. Fahmy. Baicalein protects against hypertension associated with diabetes:effect on vascular reactivity and stiffness. Phytomedicine 21(2014)1742–1745. DOI:10.1016/j.phymed.2014.08.012 |
| [20] | G.Y. Hou, R.X. Zhang, Z.F. Pi, et al., A new method for screeningaldose reductase inhibitors using ultrahigh performance liquid chromatography-tandem mass spectrometry. Anal. Methods 6(2014)7681–7688. DOI:10.1039/C4AY00857J |
| [21] | M. Pérez-Enciso, M. Tenenhaus. Prediction of clinical outcomewith microarray data:a partial least squares discriminant analysis (PLS-DA) approach. Hum. Genet. 112(2003)581–592. |
| [22] | F.F. Hsu, J. Turk. Characterization of phosphatidylethanolamine as a lithiated adduct by triple quadrupole tandem mass spectrometry with electrospray ionization. J. Mass Spectrom. 35(2000)596–606. |
| [23] | D.K. Wilson, K.M. Bohren, K.H. Gabbay, F.A. Quiocho. An unlikely sugar substrate site in the 1.65A structure of the human aldose reductase holoenzyme implicated in diabetic complications. Science 257(1992)81–84. DOI:10.1126/science.1621098 |
| [24] | S.Y. Goh, M.E. Cooper. The role of advanced glycation end products in progression and complications of diabetes. J. Clin. Endocrinol. Metab. 93(2008)1143–1152. DOI:10.1210/jc.2007-1817 |
| [25] | N. Ahmed, R. Babaei-Jadidi, S.K. Howell, P.J. Beisswenger, P.J. Thornalley. Degradation products of proteins damaged by glycation, oxidation and nitration in clinical type 1 diabetes. Diabetologia 48(2005)1590–1603. DOI:10.1007/s00125-005-1810-7 |
| [26] | G.Y. Hou, L. Wang, S. Liu, F.R. Song, Z.Q. Liu. Inhibitory effect of eleven herbal extracts on advanced glycation end-products formation and aldose reductase activity. Chin. Chem. Lett. 25(2014)1039–1043. DOI:10.1016/j.cclet.2014.04.029 |
| [27] | P.M. Piatti, L.D. Monti, G. Valsecchi, et al., Long-term oral L-arginine administration improves peripheral and hepatic insulin sensitivity in type 2 diabetic patients. Diabetes Care 24(2001)875–880. DOI:10.2337/diacare.24.5.875 |
| [28] | V.L. Malloy, R.A. Krajcik, S.J. Bailey, et al., Methionine restriction decreases visceral fat mass and preserves insulin action in aging male Fischer 344 rats independent of energy restriction. Aging Cell 5(2006)305–314. DOI:10.1111/ace.2006.5.issue-4 |
| [29] | S.J. Mihalik, S.F. Michaliszyn, las Heras J. de, et al., Metabolomic profiling of fatty acid and amino acid metabolism in youth with obesity and type 2 diabetes evidence for enhanced mitochondrial oxidation. Diabetes Care 35(2012)605–611. DOI:10.2337/DC11-1577 |
| [30] | Y. Qu, R.H. Slocum, J. Fu, et al., Quantitative amino acid analysis using a Beckman system gold HPLC 126AA analyzer. Clin. Chim. Acta 312(2001)153–162. DOI:10.1016/S0009-8981(01)00615-5 |
| [31] | J.R. Petrie, S. Ueda, D.J. Webb, H.L. Elliott, J.M.C. Connel. Endothelial nitric oxide production and insulin sensitivity-Aphysiological link with implications for pathogenesis of cardiovascular disease. Circulation 93(1996)1331–1333. DOI:10.1161/01.CIR.93.7.1331 |
| [32] | N.W. Rajapakse, A.L. Chong, W.Z. Zhang, D.M. Kaye. Insulin-mediated activation of the L-arginine nitric oxide pathway in man, and its impairment in diabetes. PLoS One 8(2013)e61840. DOI:10.1371/journal.pone.0061840 |
| [33] | P.J. Tuitoek, S. Ziari, A.T. Tsin, R.V. Rajotte. Streptozotocin-induced diabetes in rats is associated with impaired metabolic availability of vitamin A (retinol). Br. J. Nutr. 75(1996)615–622. DOI:10.1079/BJN19960164 |
 2017, Vol. 28
2017, Vol. 28