b SIMM-CUHK Joint Research Laboratory for Promoting Globalization of Traditional Chinese Medicines between Shanghai Institute of Materia Medica, Chinese Academy of Sciences and The Chinese University of Hong Kong, China;
c School of Biomedical Sciences, The Chinese University of Hong Kong, Hong Kong, China;
d School of Life Science and Technology, ShanghaiTech University, Shanghai 201203, China
Species of the Ericaceae family are world-widely utilized for ornamental as well as medicinal use. Some of those species are used as analgesic and insecticide in traditional medicine [1]. Previous research reported that grayanotoxins, a type of diterpenoids exclusive in Ericaceae family, are the bioactive constituents of Ericaceae plants [2, 3].
Rhododendron molle G. Don is one of the most well-known poisonous plants of the Ericaceae family. Distributing widely in China, its flowers have long been recorded as narcotic, anodyne and insecticidal agents in ancient monographs [4]. Phytochemical studies of R. molle revealed the existence of grayanotoxins, the characteristic diterpenoids of the Ericaceae family. Over fifty grayanane diterpenoids [5-9] and four novel modified skeletons [10-13] have been reported from this plant in recent years.
Diterpenoids are a class of natural products that has been wellknown for its highly diverse structures and strong bioactivities, such as antitumor, antiinflammatory and neurotoxicity [14-16]. With the passion of searching for more interesting diterpenoids with diversified structures, a detailed chemical investigation of R. molle was carried out, resulting in the isolation of three unprecedented diterpenoid dimers named birhodomolleins A-C (1-3, Fig. 1). Herein, we present the isolation and structural elucidation of compounds 1-3.

|
Download:
|
| Fig. 1. Chemical structures of birhodomolleins A–C (1–3). | |
2. Results and discussion
The flowers of R. molle were air-dried, ground and then extracted with 95% ethanol at room temperature. The extract was partitioned with petroleum ether, CH2Cl2, and EtOAc, successively. The CH2Cl2 extract was separated with repeated column chromatography to afford compounds 1-3.
Compound 1 was obtained as amorphous powder. The quasimolecular ion peak at m/z 901 [M+HCOOH-H]- and an isotopic ion at m/z 903 [M+2+HCOOH-H]- with ca. 30% intensity suggested the existence of a chlorine atom. The HRESIMS of 1 recording the quasi-molecular ion peak at m/z 879.4271 [M+Na]+ (calcd. 879.4274), together with its 13C NMR data, established a molecular formula of C44H69O14Cl, corresponding to ten degrees of unsaturation. The IR absorption band at 3442 cm-1 indicated the presence of hydroxyl groups.
The 1H NMR data of 1 (Table 1) displayed signals for eight singlet methyls (δH 0.98, 1.16, 1.16, 1.22, 1.28, 1.29, 1.41, 1.41), two acetyl methyls (δH 2.07, 2.11) and eight oxygenated or chlorinated methines (δH 3.72, 3.91, 4.18, 4.30, 4.49, 4.53, 5.25, 5.33). Apart from four carbons ascribed to two acetyl groups (δC 21.5, 21.7, 172.1, 172.8), 40 carbon resonances were observed in the 13C NMR (DEPT) data (Table 1), including eight methyls, eight methylenes, fourteen methines (seven oxygenated at δC 78.3, 78.8, 79.2, 85.6, 88.9, 89.1, 89.7) and ten quaternary carbons (six oxygenated at δC 77.9, 79.6, 80.5, 81.0, 84.7, 85.8). In comparison to a known grayanane diterpene rhodomollein XI3, which contained four methyls, four methylenes, seven methines (four oxygenated) and five quaternary carbons (three oxygenated), the above data suggested the presence of two grayanane diterpenoid moieties in the molecule.
|
|
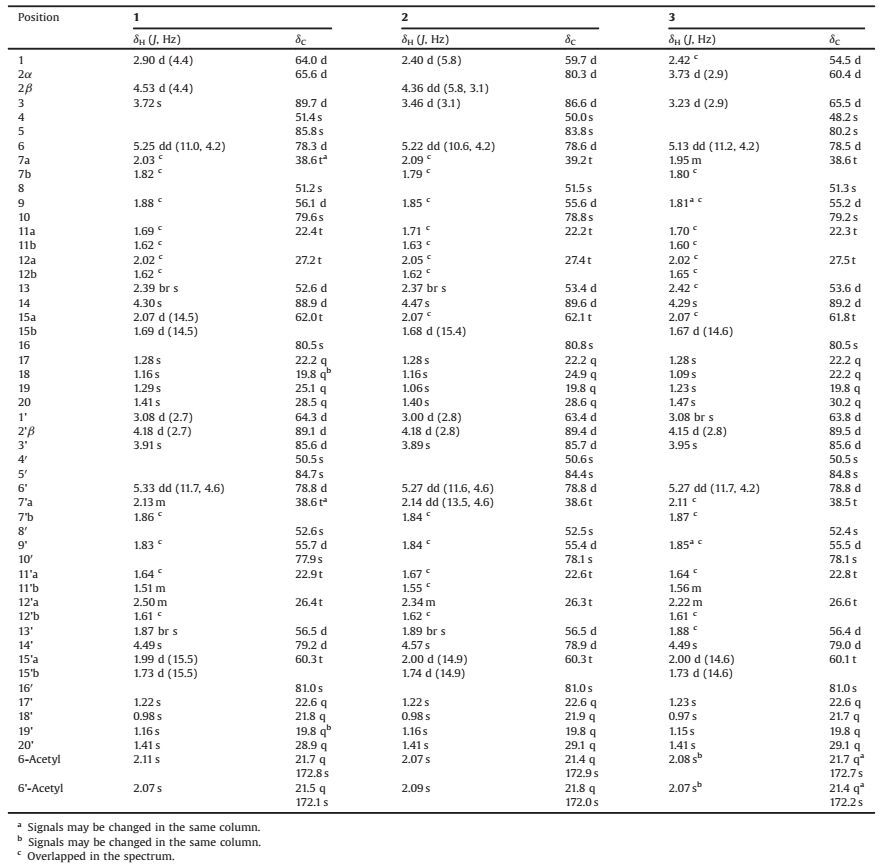
Table 1 1H and 13C NMR data for compounds 1–3, measured at 500 MHz (1H) and 125 MHz (13C) in methanol-d4. |
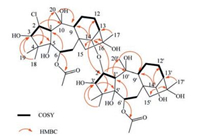
The 1H-1H COSY correlations of 1 (Fig. 2) revealed the presence of the following spin systems: Two --CH--CH--CH(OH)--, two --CH(OAc)--CH2-, and two --CH--CH2--CH2--CH--, which were further identified as two sets of characteristic fragments of --C(1) H--C(2) H--C(3) H-, --C(6) H--C(7) H2-and --C(9) H--C(11) H2--C (12) H2--C(13) H--in a common grayanane skeleton. These structural features supported the speculation of two grayanane moieties. These two moieties could be distinguished through the HMBC experiment (Fig. 2). Correlations from H-2 to C-4 and C-10, from H-3 to C-5, from H-6 to C-1 and the carbonyl carbon of one acetyl group (δC 172.8), from H-14 to C-9, C-12, C-15 and C-16, from H3-18(19) to C-3, from H-1 to C-20, from H3-20 to C-9, and from H3-17 to C-13 identified moiety A as a grayanane fragment, as drawn. Correlations from H-2' to C-4' and C-10', from H-3' to C-5', from H-6' to C-1' and the other carbonyl carbon of one acetyl group (δC 172.1), from H-14' to C-9', C-12', C-15' and C-16', from H3-18'(19') to C-3', from H-1' to C-20', from H3-20' to C-9', and from H3-17' to C-13' constructed the other fragment, moiety B, similar with moiety A. Thus, both moieties were elucidated. The two proposed structural segments contributed to 10° of unsaturation, which suggested that the linkage between two moieties should be a single bond.
|
Download:
|
| Fig. 2. Selected COSY (—), and HMBC correlations (H→C) of 1. | |
Further analyses revealed that the spectroscopic data of the respective moiety (Table 1) closely resembled those of rhodomollein XI3 except for three obvious differences. The chemical shift of C-2 of moiety A was observed at a higher field (δC 65.6, CH) compared with that of rhodomollein XI (δC 80.7, CH), suggesting C (2) H could be a chlorinated methine rather than an oxygenated one. In addition, C-14 of moiety A (δC 88.9, CH) and C-2' of moiety B (δC 89.1, CH) were found to resonate at lower field compared with those of rhodomollein XI (δC 80.7 and 78.9, respectively), revealing that the oxygenated C-14 and C-2' might be the connecting positions of the two moieties. HMBC correlations from H-14 to C-2' and from H-2' to C-14 further confirmed the existence of a C--O--C linkage between two moieties. Further HSQC, HMBC, and ROESY studies enabled full assignments of the 1H NMR and 13C NMR spectra of 1 (see Fig. S4-S6 in Supporting information).
The relative configuration of 1 was inferred from the ROESY experiment. The cross-peaks of H-2/H3-20, H-2'/H3-20', H-6/H-14, H-1/H-14, H-6'/H-14' and H-1'/H-14' suggested the same configuration of two respective moiety as rhodomollein XI (Fig. 3). Accordingly, the structure of 1 was established as α-chlorine-14'β-hydroxy-14β, 2'α-oxo-di-(6β-acetoxyl-3β, 5β, 10α, 16α-tetrahydroxygrayanane) and named birhodomollein A (Fig. 1). Birhodomollein A represents the first dimeric grayanane type diterpenoid with a rare chloro-substitution.

|
Download:
|
| Fig. 3. Key ROESY correlations (H↔H) of 1. | |
Compound 2 was obtained as amorphous powder. The HRESIMS, combined with the 13C NMR spectrum of 2, gave a molecular formula of C44H70O15 with 10° of unsaturation on the basis of the quasi-molecular ion at m/z 837.4644 [M-H]- (calcd. 837.4636). The IR data indicated the presence of hydroxyl groups (3431 cm-1).
Detailed analysis of 1D and 2D NMR data (Table 1) revealed a high similarity between the structures of 1 and 2 except for the difference observed for C-2. Compared with 1, the carbon resonance of C-2 low-field shifted to δC 80.3 from δC 65.6, which, combined with its molecular formula, suggested that an oxygen atom instead of a chlorine atom was substituted at C-2. The relative configuration of 2 was deduced to be the same as that of 1 due to the highly resembled NMR data. The configuration was further proven by the ROESY cross-peaks of H-2/H3-20, H-2'/H3-20', H-6/ H-1, H-1/H-14, H-6'/H-1' and H-1'/H-14' (see Fig. S13 in Supporting information). Thus, the structure of 2 was fully established as 2α, 14'β-dihydroxy-14β, 2'α-oxo-di-(6β-acetoxyl-3β, 5β, 10α, 16α-tetrahydroxygrayanane) and named birhodomollein B (Fig. 1).
The molecular formula of compound 3 was established as C44H68O14 according to the quasi-molecular ion at m/z 843.4539 [M+Na]+ (cal. 843.4507) in the HRESIMS, indicating 11° of unsaturation. Compared with 2, the molecular formula of 3 was one more degree of unsaturation and a H2O unit less.
A detailed analysis of the 1H and 13C NMR data of 3 (Table 1) revealed a high similarity between the structures of 2 and 3, suggesting again a dimeric diterpene comprising two grayanane moieties. The major differences were observed for C-1, C-2 and C-3, which resonated at higher filed for 3 (δC 54.5, 60.4, 65.5) compared with those for 2 (δC 59.7, 80.3, 86.6). These data were very similar to those of rhodojaponin Ⅱ [17], suggesting that 3 could also contain a 2, 3-epoxy fragment. Similarly, the relative configuration of 3 was deduced by ROESY correlations of H-6/H-1, H-1/H-14, H-2'/H3-20', H-6'/H-1'and H-1'/H-14' (see Fig. S20 in the Supporting information). Accordingly, the structure of 3 was established as 2β, 3β-epoxy-3'β, 14'β-dihydroxy-14β, 2'α-oxo-di-(6β-acetoxyl-5β, 10α, 16α-trihydroxygrayanane) and named birhodomollein C (Fig. 1).
3. ConclusionBihodomolleins A-C represent a novel class of compounds dimerized from two grayanane type units through an oxygen bridge. They are the first examples of dimeric diterpenes from the Ericaceae family although diversified monomeric diterpenoids have been reported from the ericaceous plants. Bihodomollein A features a chlorine-substitution which is rarely discovered from plants. These findings expand our knowledge of the chemical diversity in natural products.
4. Experimental 4.1. GeneralThe optical rotations were obtained on a Perkin-Elmer 341 polarimeter. IR spectra were recorded with a Nicolet Magna FTIR-750 spectrometer. Analytical HPLC and ESIMS spectra were performed on a Waters 2695 instrument with a 2998 PAD coupled with a Waters Acquity ELSD and a Waters 3100 SQDMS detector. All HPLC analyses were carried out on a Waters Sunfire® RP C-18 column, 3.5 μm, 4.6 mm × 100 mm eluted with a gradient of CH3CN-H2O (5% to 95%) with 0.1% CH3COOH. HRESIMS spectra were recorded on a Waters Q-Tof Ultima mass detector. 1H, 13C, and 2D NMR spectra were recorded using an AVANCE Ⅲ 500 instrument. Chemical shifts are reported in ppm (δ) with solvent (methanol-d4) signals used as internal standards and coupling constants (J) in hertz. Silica gel for flash chromatography was produced by Qingdao Marine Chemical Industrials. MCI gel CHP20P (75-150 μm, Mitsubishi Chemical Industries, Japan), and Sephadex LH-20 (Pharmacia Biotech AB, Uppsala, Sweden) were used for column chromatography (CC). TLC was carried out on precoated silica gel 60 F254 aluminium sheets (Merck, Germany) and the TLC spots were viewed at 254 nm and visualized by 5% sulfuric acid in alcohol containing 10 mg/mL vanillin.
4.2. Plant materialThe flowers of R. molle were collected in Jinxiu county, Guangxi province of China in 2010, and identified by Associate Professor JinGui Shen from Shanghai Institute of Materia Medica. A voucher specimen (No. 20100811) was deposited at the herbarium of Shanghai Institute of Materia Medica, Chinese Academy of Sciences.
4.3. Extraction and isolationThe air-dried flowers of R. molle (5 kg) were ground into powder and extracted with 95% ethanol (3 × 25 L) at room temperature for three times (72 h each). Evaporation of the combined percolates under reduced pressure yielded a dark brown crude extract (400 g). The extract was then suspended in water and partitioned with petroleum ether, CH2Cl2, and EtOAc, successively, yielding the fractions of PE (90 g), CH2Cl2 (90 g) and EtOAc (30 g). The CH2Cl2 extract was applied to an MCI gel column (eluted with MeOH in water 50%, 60%, 70%, 80% and 95%, successively) to afford 9 fractions (CA-CI) on the basis of TLC analysis. Fraction CD was subjected to a Sephadex LH-20 column (eluted with MeOH) to give subfractions CD1-CD3. CD1 was chromatographed on a silica gel column (300-400 mesh) eluted with petroleum ether-acetone 1:2 to afford 1 (13 mg) and 3 (9 mg). Fraction CF was subjected to a Sephadex LH-20 column (eluted with MeOH) to give subfractions CF1-CF4. CF1 was separated by a silica gel column (200-300 mesh, eluted with petroleum ether-acetone 3:1) to afford subfraction CF1a. Subfractoin CF1a was further purified by Sephadex LH-20 to yield 2 (13 mg).
Birhodomollein A (1): Amorphous powder, [α]D20 -21.8 (c 0.06, MeOH). IR (KBr) vmax 3442, 2922, 1714, 1631, 1261, 1099, 1043 cm-1; 1H NMR and 13C NMR data (in methanol-d4), see Table 1; ESIMS m/z 901 [M+HCOOH-H]-, HR-ESIMS m/z 879.4271 [M+Na]+ (calcd. for C44H69O14ClNa 879.4274).
Birhodomollein B (2): Amorphous powder, [α]D20 -18.0 (c 0.05, MeOH). IR (KBr) vmax 3431, 2927, 1714, 1647, 1261, 1101, 1045 cm-1; 1H NMR and 13C NMR data (in methanol-d4), see Table 1; ESIMS m/z 883 [M+HCOOH-H]-, HR-ESIMS m/z 837.4644 [M-H]- (calcd. for C44H69O15 837.4636).
Birhodomollein C (3): Amorphous powder, [α]D20 -20.4 (c 0.05, MeOH). IR (KBr) vmax 3446, 2922, 1712, 1623, 1376, 1263, 1043 cm-1; 1H NMR and 13C NMR data (in methanol-d4), see Table 1; ESIMS m/z 865 [M+HCOOH-H]-, HR-ESIMS m/z 843.4539 [M+Na]+ (calcd. for C44H68O14Na 843.4507).
AcknowledgmentsWe are thankful to the financial support of the National Science & Technology Major Project "Key New Drug Creation and Manufacturing Program" (Nos. 2012ZX09301001-001, 2015ZX09103002). Our thanks are also given to the National Natural Science Fundation of China (Nos. 81302657, 81473112, 81573305).
Appendix A. Supplementary dataSupplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.cclet.2017.02.020.
| [1] | Editorial Committee of Flora of China, Flora of China 14, Science Press or Missouri Botanical Garden Press, Beijing or St. Louis, 2005, pp. 242-517. |
| [2] | Y. Li, Y.B. Liu, J.J. Zhang, et al., Antinociceptive grayanoids from the roots of Rhododendron molle. J. Nat. Prod. 78(2015)2887–2895. DOI:10.1021/acs.jnatprod.5b00456 |
| [3] | H.P. Zhang, L.Q. Wang, G.W. Qin. Grayanane diterpenoids from the leaves of Craiobiodendron yunnanense. Bioorg. Med. Chem. 13(2005)5289–5298. DOI:10.1016/j.bmc.2005.06.006 |
| [4] | Editorial Committee of Flora of China, Flora of China (Zhongguo zhiwuzhi), vol. 57, Science Press, Beijng, 1994, pp. 367-369(in Chinese). |
| [5] | C.J. Li, L.Q. Wang, S.N. Chen, G.W. Qin. Diterpenoids from the fruits of Rhododendron molle. J. Nat. Prod. 63(2000)1214–1217. DOI:10.1021/np000009e |
| [6] | S.N. Chen, H.P. Zhang, L.Q. Wang, G.H. Bao, G.W. Qin. Diterpenoids from the flowers of Rhododendron molle. J. Nat. Prod. 67(2004)1903–1906. DOI:10.1021/np040012o |
| [7] | G.H. Bao, L.Q. Wang, K.F. Cheng, G.W. Qin. One new diterpene glucoside from the roots of Rhododendron molle. Chin. Chem. Lett. 13(2002)955–956. |
| [8] | G.H. Bao, L.Q. Wang, K.F. Cheng, et al., Diterpenoid and phenolic glycosides from the roots of Rhododendron molle. Planta Med. 69(2003)434–439. DOI:10.1055/s-2003-39716 |
| [9] | G.H. Zhong, M.Y. Hu, X.Y. Wei, et al., Grayanane diterpenoids from the flowers of Rhododendron molle with cytotoxic activity against a Spodoptera frugiperda cell line. J. Nat. Prod. 68(2005)924–926. DOI:10.1021/np049645t |
| [10] | S.J. Wang, S. Lin, C.G. Zhu, et al., Highly acylated diterpenoids with a new 3, 4-secograyanane skeleton from the flower buds of Rhododendron molle. Org. Lett. 12(2010)1560–1563. DOI:10.1021/ol1002797 |
| [11] | Y. Li, Y.B. Liu, J.J. Zhang, et al., Mollolide A a diterpenoid with a new 1, 10:2, 3-disecograyanane skeleton from the roots of Rhododendron molle. Org. Lett. 15(2013)3074–3077. DOI:10.1021/ol401254e |
| [12] | S.Z. Zhou, S. Yao, C.P. Tang, et al., Diterpenoids from the flowers of Rhododendron molle. J. Nat. Prod. 77(2014)1185–1192. DOI:10.1021/np500074q |
| [13] | Y. Li, Y.B. Liu, Y.L. Liu, et al., Mollanol A, a diterpenoid with a new C-nor-Dhomograyanane skeleton from the fruits of Rhododendron molle. Org. Lett. 16(2014)4320–4323. DOI:10.1021/ol5020653 |
| [14] | M. Zhang, J.M. Liu, J.L. Zhao, et al., Two new diterpenoids from the endophytic fungus Trichoderma sp.Xy24 isolated from mangrove plant Xylocarpus granatum. Chin. Chem. Lett. 27(2016)957–960. DOI:10.1016/j.cclet.2016.02.008 |
| [15] | Z.Y. Wu, H.Z. Li, W.G. Wang, et al., Lyonin A a new 9, 10-Secograyanotoxin from Lyonia ovalifolia. Chem. Biodivers. 8(2011)1182–1187. DOI:10.1002/cbdv.v8.6 |
| [16] | T. Li, B. Wang, Voogd N.J. de, et al., Two new diterpene alkaloids from the South China Sea Sponge Agelas aff. Nemoechinata. Chin. Chem. Lett. 27(2016)1048–1051. DOI:10.1016/j.cclet.2016.05.017 |
| [17] | T. Ohta, H. Hikino. Carbon-13 NMR spectra of ericaceous toxins. Org. Mag. Res. 12(1979)445–449. DOI:10.1002/(ISSN)1097-458X |
 2017, Vol. 28
2017, Vol. 28