Gastrodia elata Blume (Orchidaceae) is a holomycotrophic perennial plant living on a symbiotic mycorrhizal fungus Armillaria mellea at a vegetative growth stage of its life cycle [1-3]. The steamed and dried rhizome of G. elata, known as "tian ma" in Chines e, is an important traditional Chinese herbal medicine that has been used for the treatment of various neuralgic and nervous disorders (such as vertigo, blackout, headaches, migraine, epilepsy, neuralgia, and paralysis) for over 2000 years [1-4]. This herbal medicine has health benefits enhancing strength and virility and improving memory and blood circulation [4]. The fresh and prepared rhizomes of G. elata are used also in Chinese cuisines. Although this plant has become a wildly endangered species in China, pharmaceutical and catering demands are completely fulfilled by agricultural cultivation, which is benefited from a great achievement of scientific researches on symbiotic associations with fungi engaging in seed germination and vegetative growth since the late of 1950 [1-3]. Previous pharmacological and chemical studies mainly focusing on ethanol or methanol extracts of the herbal medicine showed that p-hydroxybenzyl analogues were the main active constituents [5-10]. To be consistent with a traditional and practical application of the herbal medicines by decocting with water, we conducted investigation of an aqueous extract of G. elata rhizomes as part of a program to systematically study the chemical diversity of traditional Chinese medicines and their biological effects [11-31]. In our previous papers [32-38], 50 compounds were reported from polar fractions of the extract, along with preliminary bioassays of those isolates and pharmacological functions of a minor component N6-(p-hydroxybenzyl) adenosine (NHBA). Herein, reported is the isolation and structure elucidation of an unprecedented 9, 90-neolignan possessing a novel carbon skeleton modified by two p-hydroxybenzyl at C-7, named gastradefurphenol (1) (Fig. 1) from a less polar fraction of the extract. In addition, free rotational restriction of C-7 -C-7'' and C-7 -C-7''' bonds and transformational equilibrium between two molecular states of 1 in acetone-d6 were observed and discussed by further analysis of the NMR spectroscopic data in CD3OD.
|
Download:
|
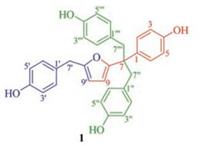
| Fig. 1. Structure of 1. | |
2. Results and discussion
Compound 1 was obtained as yellowish amorphous powder. Its IR spectrum showed absorptions due to hydroxyl (3373 cm-1) and aromatic ring (1612 and 1514 cm-1) functionalities. The molecular formula of 1 was determined as C32H28O5 by HR-ESIMS at m/z 515.1831 (calcd. for C32H28O5Na, 515.1829) and NMR spectroscopic data (Table 1). The 1H NMR spectrum of 1 in acetone-d6 showed resonances attributable to two inequivalent para-substituted phenyls at δH 6.70 (d, 2H, J = 8.4 Hz, H-3/5), 6.75 (d, 2H, J = 8.4 Hz, H-3'/5'), 6.93 (d, 2H, J = 8.4 Hz, H-2/6), and 7.07 (d, 2H, J = 8.4 Hz, H-2'/6'); two equivalent para-substituted phenyls at δH 6.49 (d, 4H, J = 8.4 Hz, H-2''/2'''/6''/6''') and 6.53 (d, 4H, J = 8.4 Hz, H-3''/3'''/5''/5'''); and a disubstituted furan ring at δH 5.98 (d, 1H, J = 3.6 Hz, H-9) and 5.94 (d, 1H, J = 3.6 Hz, H-9'). In addition, the spectrum showed resonances assignable to three isolated methylene at δH 3.82 (s, 2H, H-7'), 3.13 (d, 2H, J = 13.8 Hz, H-7''a/7'''a), and 3.10 (d, 2H, J = 13.8 Hz, H-7''b/7'''b), as well as signals due to four phenolic hydroxyls at δH 8.17 (s, 1H, OH-4), 8.15 (s, 1H, OH-4'), and 8.02 (s, 2H, OH-4''/4'''). The 13C NMR and DEPT spectra displayed 32 carbon resonances (Table 1) corresponding to the above units and an additional quaternary carbon at δC 50.3 (C-7). As compared with those of the previously isolated compounds from "tian ma" [32, 34, 35, 37], these spectroscopic data indicate that 1 possesses an unusual structure consisting of one furan ring and four p-hydroxybenzyl units, which was further constructed by 2D NMR data analysis.
|
|
Table 1 NMR spectroscopic data for compound 1.a |
The proton and proton-bearing carbon resonances in the NMR spectra of 1 were unambiguously assigned by homonuclear vicinal coupling cross-peaks in the 1H-1H COSY spectrum, in combination with one-bond heteronuclear correlations in the HSQC spectrum. In the HMBC spectrum, the two-and three-bond heteronuclear correlations (Fig. 2) from H-2/6 to C-3/5, C-4, and C-7; from H-3/5 to C-1 and C-4, and from OH-4 to C-4 and C-3/5, in combination with their chemical shifts and quaternary nature of C-7 (Table 1), demonstrated that there was a 7, 7-disubstituted 4-hydroxybenzyl unit in 1. The HMBC correlations from H-2'/6' to C-3'/5', C-4', and C-7'; from H-3'/5' to C-1' and C-4'; from H2-7' to C-1' and C-2'/6'; and from OH-4' to C-4' and C-3'/5' confirmed the presence of a 4'-hydroxybenzyl. Similarly, the HMBC correlations from H-2''/2'''/6''/ 6''' to C-3''/3'''/5''/5''', C-4''/4''', and C-7''/7'''; from H-3''/3'''/5''/5''' to C-1''/1''' and C-4''/4'''; from H2-7''/7''' to C-1''/1''' and C-2''/2'''/ 6''/6'''; and from OH-4''/4''' to C-4''/4''' and C-3''/3'''/5''/5''' verified the occurrence of the two equivalent p-hydroxybenzyl (e.g. 4''-and 4'''-hydroxybenzyls). In addition, the HMBC correlations of both C-1 and C-7 with H2-7''/7''' revealed that the two equivalent p-hydroxybenzyl located at the quaternary C-7 of the 4-hydroxybenzyl, which was further supported by the overlapped correlations of H-7''/C-7''' and H-7'''/C-7''. Meanwhile, the presence of the disubstituted furan ring was supported by the 1H-1H COSY cross-peaks of H-9/H-9' and the HMBC correlations of H-9/C-8, C-8', and C-9' in the HMBC spectrum, together with chemical shifts of these proton and carbon resonances and the coupling constant value 3JH-9, H-9' (3.6 Hz). The HMBC correlations of C-8 with H2-7''/ 7''' indicated that the two equivalent p-hydroxybenzyl connected via the quaternary C-7 to C-8 of the furan ring in 1. Moreover, the HMBC correlations of C-8' and C-9' with H2-7' unequivocally located the 4'-hydroxybenzyl at C-8' of the furan ring. Therefore, the structure of compound 1 was determined as shown and named gastradefurphenol. According to the IUPAC suggested nomenclature of neolignans [39], this compound is systematically named 4, 4'-dihydroxy-7, 7-bis(p-hydroxybenzyl)-8, 8'-epoxy-9, 9'-neoligna-8, 8'-diene.

|
Download:
|
| Fig. 2. Main 1H-1H COSY (thick lines) and bond HMBC correlations (red arrows, from 1H to 13C) of 1. | |
Interestingly and notably, the 1H NMR spectrum of 1 in acetone-d6 showed a singlet for H2-7', an overlapped AB coupling system for H2-7''/7''', and vicinal coupling doublets for aromatic protons of the p-hydroxybenzyl (Table 1 and Figs. S7 and S8 in Supporting information), as assigned unambiguously by 2D NMR spectroscopic data analysis. In addition, in the 13C NMR spectrum, for the symmetric p-hydroxybenzyl the carbons attaching to and closing to the methylenes (C-1, C-1', C-1''/1''', C-2/6, C-2'/6', C-2''/6'', and C-2'''/6''') appeared as the normal singlets. However, the carbons attaching to and closing to the phenolic hydroxyls appeared as paired resonances with almost identical intensities and chemical shift differences in ΔδC 0.10 (15 Hz) for C-4, C-4', and C-4''/4''' and 0.09 (13.5 Hz) for C-3/5, C-3'/5', C-3''/5'', and C-3'''/5''' (Table 1 and Figs. S9-S11 in Supporting information). Because 1 is achiral the AB coupling system of H2-7''/7''' and the paired carbon resonances are abnormal. The AB coupling system of H2-7''/7''' demonstrated that the germinal protons at C-7''/7''' were chemically inequivalent. This could be explained from that free rotation of the C-7-C-7'' and C-7-C-7''' bonds was restricted by intramolecular interaction of four bulky groups at C-7 of 1 in the acetone-d6 solution, because there is no chiral carbon or double bond to induce inequivalence of the germinal protons at C-7''/C-7''' in the structure. However, the paired resonances of C-4, C-4', C-4''/4''', C-3/5, C-3'/5', C-3''/5'', and C-3'''/5''' must be due to the presence of two chemically inequivalent states of the molecule since these spin nuclei are away from the crowded center C-7 and toward outside of the molecule. Accordingly, we speculate that there is transformational equilibrium between two molecular states in the acetone-d6 solution of 1. In order to explore a solvent effect on the chemical inequivalence of the germinal protons and the carbon nuclei in solution state, the NMR spectra of 1 in CD3OD were acquired (Figs. S16-S20 in Supporting information). As compared, the AB system and the paired carbon resonances in acetone-d6 were replaced by a singlet for the germinal protons and corresponding unpaired carbon resonances in CD3OD, respectively. This revealed that free rotational restriction of the C-7-C-7'' and C-7-C-7''' bonds in 1 as well as transformational equilibrium between two molecular states were solvent-dependent and that the restriction and equilibrium only existed in the aprotic solvent acetone-d6 instead in the protic solvent CD3OD. Therefore, intermolecular interactions between the solute molecules and between the solute and solvent molecules play potent roles in both restriction of the bond rotations and equilibrium of the molecular states. Although details of the solvent-dependent equilibration were not further investigated due to limitation of the sample amount, in the acetone-d6 the paired carbon resonances of C-4, C-4', C-4''/4''', C-3/ 5, C-3'/5', C-3''/5'', and C-3'''/5''' in the symmetric p-hydroxybenzyl demonstrated that the phenolic hydroxyls in 1 must involve in the intermolecular interactions.
Gastradefurphenol (1) represents the first 9, 90-neolignan with the furan ring motif formed between the C3 units and with the novel carbon skeleton modified at C-7 by two p-hydroxybenzyl though the only natural product of 9, 9'-neolignan category, e.g. 4, 4'-dimethoxy-9, 9'-neoligna-7, 7'-diene (ocimin) was reported from an essential oil of Ocimum americanum seeds [40, 41]. Based on the unique structure feature consisting of two p-hydroxyphenylpropene (C6C3) units and two p-hydroxybenzyl, the biosynthetic precursors of 1 are traced to p-hydroxyphenylpropene derivatives, such as (+)-(2S)-2-hydroxy-3-(p-hydroxyphenyl)propyl aldehyde (2) and 1-(p-hydroxyphenyl)propan-1, 2-dione (3). A plausible biosynthetic pathway for 1 is postulated in Scheme 1. An enzyme-catalyzed Aldol addition of 2 and 3, followed by sequential or simultaneous intramolecular nucleophilic addition and dehydration, would generate a key intermediate 4. The intermediate undergoes condensation with two molecules of p-cresol (5) to afford 1. The plausible biosynthetic pathway was supported by concomitant isolation of the acid form of 2, as well as of compound 3 and the β-D-glucoside of 5 from the extract in this study. These biosynthetic precursors would be generated from biosynthetic and/or metabolic processes of L-tyrosine [42, 43], which is supported by the occurrence of L-tyrosine in the rhizome of G. elata [44].

|
Download:
|
| Scheme 1. The plausible biosynthetic pathway of 1. | |
3. Conclusion
A novel neolignan, named gastradefurphenol (1) possessing an unprecedented 7, 7-bis(benzyl)-9, 9'-neolignane skeleton, was isolated as the minor constituent from the aqueous extract of the G. elata rhizomes. Although biological activity of 1 was not assayed due to limitation of the sample amount, the unique structure adds a new skeletal entity to the neolignan natural products, and provides a selectable framework for synthesis and biological evaluation in future. In particular, the plausible biosynthetic pathway provides a strategy for studies of biomimetic and total synthesis to obtain enough amount of the sample for biological evaluation, as well as for biosynthesis of the diverse benzylcontaining metabolites in this orchid plant. Solvent-dependent equilibrium of intermolecular interactions as indicated by the 13C NMR spectroscopic data of 1 also deserve detailed investigations. The result in this study continuously reveals that the chemical constituents of holomycotrophic G. elata have characteristic structure features modified by and derived from p-hydroxybenzyl, indicating that production of these metabolites is predominated by the metabolic process of L-tyrosine. However, a literature survey showed that the symbiotic mycorrhizal fungus Armillaria mellea mainly produced antimicrobial and cytotoxic aryl esters of protoilludane-type sesquiterpenoids without p-hydroxybenzyl [45-47]. This, together with identification of various p-hydroxybenzyl analogues and p-hydroxybenzyl-substituted metabolites, including p-hydroxybenzyl-substituted amino acids, glutathione derivatives, and nucleosides [32, 34, 35, 37], suggests the presence of independent and unique biosynthetic pathways and associated enzymes to accumulate the diverse p-hydroxybenzyl-substituted natural products in the holomycotrophic plant.
4. Experimental 4.1. GeneralThe UV spectrum was recorded on a V-650 spectrometer (JASCO). The IR spectrum was recorded on a Nicolet 5700 FT-IR Microscope spectrometer (FT-IR Microscope Transmission) (Thermo Electron Corporation, Madison, WI, USA). 1D-and 2DNMR spectra were obtained at 600 MHz for 1H and 150 MHz for 13C, respectively, on a SYS 600 MHz (Varian Associates Inc., Palo Alto, CA, USA) spectrometer, with in acetone-d6 as a solvent and solvent peaks as references. ESIMS and HR-ESIMS data were obtained on Agilent 1100 Series LC-MSD-Trap-SL and Agilent 6520 AccurateMass Q-TOFL CMS spectrometers (Agilent Technologies, Ltd., Santa Clara, CA, USA), respectively. Column chromatography (CC) was performed with macroporous adsorbent resin (HPD-100, Cangzhou Bon Absorber Technology Co. Ltd., Cangzhou, China), MCI gel (CHP 20P, 75-150 μm) (Mitsubishi Chemical Corporation, Tokyo, Japan), silica gel (200-300 mesh, Qingdao Marine Chemical Inc., China), and Sephadex LH-20 (Pharmacia Biotech AB, Uppsala, Sweden). HPLC separation was performed on an instrument equipped with an Agilent ChemStation for LC system, an Agilent 1100 pump, and an Agilent 1100 single-wavelength absorbance detector (Agilent Technologies, Ltd.), using a Shiseido column (250 mm × 10 mm, i.d.) packed with C8 reversed phase silica gel (5 μm) (Shiseido Co., Ltd, Tokyo, Japan). TLC was carried out with glass precoated silica gel GF254 plates (Qingdao Marine Chemical Inc.). Spots were visualized under UV light or by spraying with 5% H2SO4 in EtOH followed by heating. Unless otherwise noted, all chemicals were purchased from commercially available sources and were used without further purification.
4.2. Plant materialSee Ref. [34].
4.3. Extraction and isolationFor extraction and preliminary fractionation of the extract, see refs 34 and 35. Fractions C4 and C5 were merged together on the basis of TLC analysis to give a mixture C4 + 5 (6.8 g), which was subjected to CC over silica gel (200-300 mesh), eluted with a gradient of increasing acetone (0-100%) in petroleum ether, to yield C4 + 5-1-C4 + 5-18. Subfraction C4 + 5-13 (199 mg) was separated by CC over Sephadex LH-20 (petroleum ether-CH2Cl2-MeOH, 5:5:1, v/v/v) to yield C4 + 5-13-1-C4 + 5-13-3, of which C4 + 5-13-2 (60 mg) was further isolated by CC over silica gel, with elution of CH2Cl2-MeOH (30:1, v/v), to afford C4 + 5-13-2-1-C4 + 5-13-2-3. Purification of C4 + 5-13-2-2 (6.5 mg) by RP-HPLC (C8 column, 70% MeOH in H2O) obtained 1 (2.0 mg, tR = 16.1 min, 0.000004%).
Gastradefurphenol (1): Yellowish amorphous powder; UV (MeOH) λmax (log ε): 203 (2.85), 222 (2.61), 279 (1.94) nm; IR vmax 3373, 3027, 2925, 2854, 1679, 1612, 1514, 1446, 1370, 1209, 1149, 1107, 965, 834, 801, 726, 557, 537 cm-1; 1H NMR (acetone-d6, 600 MHz) data, see Table 1; 13C NMR (acetone-d6, 150 MHz) data, see Table 1; (+)-ESIMS m/z 515 [M + Na]+, 531 [M + K]+; (+)-HRESIMS m/z 515.1831 (calcd. for C32H28NaO5, 515.1829).
AcknowledgmentFinancial support from the National Natural Science Foundation of China (Nos. 81502942 and 81630094) is acknowledged.
Appendix A. Supplementary dataSupplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.cclet.2017.03.028.
| [1] | J.T. Xu, S.X. Guo. Retrospect on the research of the cultivation of Gastrodia elata Bl, a rare traditional Chinese medicine. Chin. Med. J. 113(2000)686–692. |
| [2] | H. Liu, Y. Luo, H. Liu. Studies of mycorrhizal fungi of Chinese orchids and their role in orchid conservation in China-a review. Bot. Rev. 76(2010)241–262. DOI:10.1007/s12229-010-9045-9 |
| [3] | E.J. Park, W.Y. Lee, J.K. Ahn. In vitro propagation of myco-hetertrophic Gastrodia elata. Hort. Environ. Biotechnol. 53(2012)415–420. DOI:10.1007/s13580-012-0046-y |
| [4] | Jiangsu New Medical College, Dictionary of Traditional Chinese Medicine, Shanghai Science and Technology Publishing House, Shanghai, 1977, pp. 315-317 |
| [5] | J. Zhou, X.Y. Pu, Y.B. Yang. Phenolic constituents of fresh Gastrodia elata Blume. Chin. Sin. Bull. 18(1981)1118–1120. |
| [6] | E.J. Shin, W.K. Whang, S. Kim, et al., Parishin C attenuates phencyclidineinduced schizophrenia-like psychosis in mice:involvements of 5-HT1A receptor. J. Pharmacol. Sci. 113(2010)404–408. DOI:10.1254/jphs.10040SC |
| [7] | N.K. Huang, Y. Chern, J.M. Fang, et al., Neuroprotective principles from Gastrodia elata. J. Nat. Prod. 70(2007)571–574. DOI:10.1021/np0605182 |
| [8] | K.Y. Kam, S.J. Yu, N. Jeong, et al., p-Hydroxybenzyl alcohol prevents brain injury and behavioral impairment by activating Nrf2 PDI, and neurotrophic factor genes in a rat model of brain ischemia. Mol. Cells 31(2011)209–215. DOI:10.1007/s10059-011-0028-4 |
| [9] | X. Zhao, Y. Zou, H. Xu, et al., Gastrodin protect primary cultured rat hippocampal neurons against amyloid-beta peptide-induced neurotoxicity via ERK1/2-Nrf2 pathway. Brain Res. 1482(2012)13–21. DOI:10.1016/j.brainres.2012.09.010 |
| [10] | B. W. Kim, S. Koppula, J. W. Kim, et al. , Modulation of LPS-stimulated neuroinflammation in BV-2 microglia by Gastrodia elata: 4-Hydroxybenzyl alcohol is the bioactive candidate, J. Ethnopharmacol. 139(2012) 549-557 and references cited therein. |
| [11] | W.D. Xu, Y. Tian, Q.L. Guo, Y.C. Yang, J.G. Shi. Secoeuphoractin, a minor diterpenoid with a new skeleton from Euphorbia micractina. Chin. Chem. Lett. 25(2014)1531–1534. DOI:10.1016/j.cclet.2014.09.012 |
| [12] | Y. Tian, Q. Guo, W. Xu, et al., A minor diterpenoid with a new 6/5/7/3 fused-ring skeleton from Euphorbia micractina. Org. Lett. 16(2014)3950–3953. DOI:10.1021/ol501760h |
| [13] | W.X. Song, Y.C. Yang, J.G. Shi. Two new β-hydroxy amino acid-coupled secoiridoids from the flower buds of Lonicera japonica:isolation, structure elucidation, semisynthesis, and biological activities. Chin. Chem. Lett. 25(2014)1215–1219. DOI:10.1016/j.cclet.2014.05.037 |
| [14] | Z.B. Jiang, W.X. Song, J.G. Shi. Two new 1-(6'-O-acyl-β-D-glucopyranosyl) pyridinium-3-carboxylates from the flower buds of Lonicera japonica. Chin. Chem. Lett. 26(2015)69–72. DOI:10.1016/j.cclet.2014.10.011 |
| [15] | Y. Yu, Z. Jiang, W. Song, et al., Glucosylated caffeoylquinic acid derivatives from the flower buds of Lonicera japonica. Acta Pharm. Sin. B 5(2015)210–214. DOI:10.1016/j.apsb.2015.01.012 |
| [16] | W.X. Song, Q.L. Guo, Y.C. Yang, J.G. Shi. Two homosecoiridoids from the flower buds of Lonicera japonica. Chin. Chem. Lett. 26(2015)517–521. DOI:10.1016/j.cclet.2014.11.035 |
| [17] | Y.F. Liu, M.H. Chen, X.L. Wang, et al., Antiviral enantiomers of a bisindole alkaloid with a new carbon skeleton from the roots of Isatis indigotica. Chin. Chem. Lett. 26(2015)931–936. DOI:10.1016/j.cclet.2015.05.052 |
| [18] | Y.F. Liu, M.H. Chen, Q.L. Guo, et al., Antiviral glycosidic bisindole alkaloids from the roots of Isatis indigotica. J. Asian Nat. Prod. Res. 17(2015)689–704. DOI:10.1080/10286020.2015.1055729 |
| [19] | Y.F. Liu, M.H. Chen, S. Lin, et al., Indole alkaloid glucosides from the roots of Isatis indigotica. J. Asian Nat. Prod. Res. 18(2016)1–12. DOI:10.1080/10286020.2015.1117452 |
| [20] | Y. Liu, X. Wang, M. Chen, et al., Three pairs of alkaloid enantiomers from the root of Isatis indigotica. Acta Pharm. Sin. B 6(2016)141–147. DOI:10.1016/j.apsb.2016.01.003 |
| [21] | M.H. Chen, S. Lin, Y.N. Wang, et al., Antiviral stereoisomers of 3, 5-bis(2-hydroxybut-3-en-1-yl)-1, 2, 4-thiadiazole from the roots Isatis indigotica. Chin. Chem. Lett. 27(2016)643–648. DOI:10.1016/j.cclet.2016.01.042 |
| [22] | D.W. Li, Q.L. Guo, X.H. Meng, et al., Two pairs of unusual scalemic enantiomers from Isatis indigotica leaves. Chin. Chem. Lett. 27(2016)1745–1750. DOI:10.1016/j.cclet.2016.08.006 |
| [23] | Y. Jiang, Y. Liu, Q. Guo, et al., Acetylenes and fatty acids from Codonopsis pilosula. Acta Pharm. Sin. B 5(2015)215–222. DOI:10.1016/j.apsb.2015.03.005 |
| [24] | Y.P. Jiang, Y.F. Liu, Q.L. Guo, et al., C14-Polyacetylene glucosides from Codonopsis pilosula. J. Asian Nat. Prod. Res. 17(2015)601–614. DOI:10.1080/10286020.2015.1041932 |
| [25] | Y.P. Jiang, Y.F. Liu, Q.L. Guo, J.G. Shi. C14-Polyacetylenol glycosides from the roots of Codonopsis pilosula. J. Asian Nat. Prod. Res. 17(2015)1166–1179. DOI:10.1080/10286020.2015.1112797 |
| [26] | Y.P. Jiang, Q.L. Guo, Y.F. Liu, J.G. Shi. Codonopiloneolignanin A, a polycyclic neolignan with a new carbon skeleton from the roots of Codonopsis pilosula. Chin. Chem. Lett. 27(2016)55–58. DOI:10.1016/j.cclet.2015.11.009 |
| [27] | Y. Jiang, Y. Liu, Q. Guo, et al., Sesquiterpene glycosides from the roots of Codonopsis pilosula. Acta Pharm. Sin. B 6(2016)46–54. DOI:10.1016/j.apsb.2015.09.007 |
| [28] | Z.B. Jiang, B.Y. Jiang, C.G. Zhu, et al., Aromatic acid derivatives from the lateral roots of Aconitum carmichaelii. J. Asian Nat. Prod. Res. 16(2014)891–900. DOI:10.1080/10286020.2014.939585 |
| [29] | Z.B. Jiang, X.H. Meng, B.Y. Jiang, et al., Two 2-(quinonylcarboxamino)benzoates from the lateral roots of Aconitum carmichaelii. Chin. Chem. Lett. 26(2015)653–656. DOI:10.1016/j.cclet.2015.04.011 |
| [30] | X.H. Meng, Z.B. Jiang, C.G. Zhu, et al., Napelline-type C20-diterpenoid alkaloid iminiums from an aqueous extract of fu zi:Solvent-/base-/acid-dependent transformation/equilibration between alcohol iminium and aza acetal forms. Chin. Chem. Lett. 27(2016)993–1003. DOI:10.1016/j.cclet.2016.05.013 |
| [31] | X.H. Meng, Z.B. Jiang, Q.L. Guo, et al., A minor arcutine-type C20-diterpenoid alkaloid iminium constituent of fu zi. Chin. Chem. Lett. 28(2017)588–592. DOI:10.1016/j.cclet.2016.11.010 |
| [32] | Y. Wang, S. Lin, M. Chen, et al., Chemical constituents from aqueous extract of Gastrodia elata. China J. Chin. Mater. Med. 37(2012)1775–1781. |
| [33] | Y. Zhang, M. Li, R.X. Kang, et al., NHBA isolated from Gastrodia elata exerts sedative and hypnotic effects in sodium pentobarbital-treated mice. Pharmacol. Biochem. Behav. 102(2012)450–457. DOI:10.1016/j.pbb.2012.06.002 |
| [34] | Q. Guo, Y. Wang, S. Lin, et al., 4-Hydroxybenzyl-substituted amino acid derivatives from Gastrodia elata. Acta Pharm. Sin. B 5(2015)350–357. DOI:10.1016/j.apsb.2015.02.002 |
| [35] | Q.L. Guo, Y.N. Wang, C.G. Zhu, et al., 4-Hydroxybenzyl-substituted glutathione derivatives from Gastrodia elata. J. Asian Nat. Prod. Res. 17(2015)439–454. DOI:10.1080/10286020.2015.1040000 |
| [36] | J. He, Z. Luo, L. Huang, et al., Ambient mass spectrometry imaging metabolomics method provides novel insights into the action mechanism of drug candidates. Anal. Chem. 87(2015)5372–5379. DOI:10.1021/acs.analchem.5b00680 |
| [37] | Q.L. Guo, S. Lin, Y.N. Wang, et al., Gastrolatathioneine, an unusual ergothioneine derivative from an aqueous extract of tian ma:A natural product co-produced by plant and symbiotic fungus. Chin. Chem. Lett. 27(2016)1577–1581. DOI:10.1016/j.cclet.2016.06.040 |
| [38] | Z. Liu, W. Wang, N. Fen, et al., Parishin C's prevention Aβ1-42-induced inhibition of long-term potentiation is related to NMDA receptors. Acta Pharm. Sin. B 6(2016)189–197. DOI:10.1016/j.apsb.2016.03.009 |
| [39] | G.P. Moss. Nomenclature of lignans and neolignans (IUPAC recommendations 2000). Pure Appl. Chem. 72(2000)1493–1523. |
| [40] | R.K. Thappa, M.S. Bhatia, S.G. Aggarwal, K.L. Dhar, C.K. Atal. Ocimin, a novel neolignan from Ocimum americanum. Phytochemistry 18(1979)1242. DOI:10.1016/0031-9422(79)80152-1 |
| [41] | A.J. Kadam, R.B. Mane, A new synthesis of ocimin, Indian J. Chem. 39B (2000) 628. |
| [42] | L.B. Davin, M. Jourdes, A.M. Patten, et al., Dissection of lignin macromolecular configuration and assembly:Comparison to related biochemical processes in allyl/propenyl phenol and lignan biosynthesis. Nat. Prod. Rep. 25(2008)1015–1090. DOI:10.1039/b510386j |
| [43] | J.B. Broderick, B.R. Duffus, K.S. Duschene, E.M. Shepard. Radical Sadenosylmethionine enzymes. Chem. Rev. 114(2014)4229–4317. DOI:10.1021/cr4004709 |
| [44] | L. Wang, H.B. Xiao, X.M. Liang. Chemical constituents from Gastrodia elata (Ⅲ). Chin. Tradit. Herb. Drugs 40(2009)1186–1189. |
| [45] | J.S. Yang, Y.L. Su, Y.L. Wang, et al., Chemical constituents of Armillaria mellea mycelium Ⅶ:Isolation and characterization of chemical constituents of the acetone extract. Acta Pharm. Sin. 26(1991)117–122. |
| [46] | M. Misiek, J. Williams, K. Schmich, et al., Structure and cytotoxicity of arnamial and related fungal sesquiterpene aryl esters. J. Nat. Prod. 72(2009)1888–1891. DOI:10.1021/np900314p |
| [47] | H. Kobori, A. Sekiya, T. Suzuki, et al., Bioactive sesquiterpene aryl esters from the culture broth of Armillaria sp. J. Nat. Prod. 78(2015)163–167. DOI:10.1021/np500322t |
 2017, Vol. 28
2017, Vol. 28 



