b National Engineering Research Center for Carbohydrate Synthesis, Jiangxi Normal University, Nanchang 330022, China;
c School of Life Science, Nanjing Normal University, Nanjing 210023, China;
d Anhui Academy of Science and Technology, Hefei 230031, China
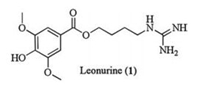
Leonurine (1, Fig. 1) [1] isolated from leonurus sibiricus L. which has been used as a folk medicine for the treatment of some gynecological diseases in China since ancient times [1-3], is found to possess some important biological functions, such as blood circulation, water swelling. It also holds great potential for the treatment of diseases including vascular thrombosis and hypertension [4-11].
|
Download:
|
| Fig. 1. Structure of Leonurine (1). | |
The availability of Leonurine is restricted both by its low concentration in the leonurus sibiricus L. and the difficulty associated with extraction from this plant. Therefore, a number of synthetic approaches have been developed to meet the demand of biological testing [12, 13-17]. Elegant in their own, most of these approaches employ expensive 4-aminobutan-1-ol as starting material and therefore are not suitable for scalable synthesis. Herein, we report a new synthesis of Leonurine (1) using 2, 3-dihydrofuran (7) as a latent equivalent of 4-aminobutan-1-ol. The operational simplicity coupled with the low price of the starting material allows a mass production of Leonurine. With large quantity of Leonurine in hand, we have conducted experiments to evaluate its toxicity effect on zebrafish.
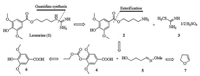
2. Results and discussionOur retrosynthetic analysis is presented in Scheme 1. We envisioned that Leonurine could be obtained by a direct guanidination of 2 with S-methylisothiourea hemisulfate (3) [18]. In order to increase the atom economy, we determined to try the reaction without protecting groups on both 2 and 3 based on the fact of the good crystallization ability of 1. Most of the previous syntheses of 2 involved the coupling of syringate derivative with phthalimide protected 4-aminobutan-1-ol and subsequent deprotection. The expensive price of 4-aminobutan-1-ol and huge amount waste generated from phthalimide render these strategy unfavorable. In light of that oxime can be reduced to give amine directly in high yield, we chose to explore Omethyloxime 9, readily available from 2, 3-dihydrofuran (7) as 1 equiv. of 4-aminobutan-1-ol for the synthesis of 2.
|
Download:
|
| Scheme1. Retrosynthesis of Leonurine (1). | |
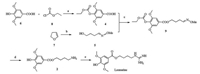
Bearing the synthetic plan in mind, we executed the synthesis of Leonurine (1) (Scheme 2). As depicted in Scheme 2, the compound 4 was prepared from commercially available materials, compound 6 and ethyl chloroformate (8) [14] in 72% yield. The other fragment oxime 5 was prepared from 2, 3-dihydrofuran (7) by the treatment of 2 mol/L hydrochloric acid and subsequent condensation with methoxylamine hydrochloride. Although the esterification of compounds 4 and 5 could proceed in many conditions, such as Fischer esterification, Steglich esterification, Yamaguchi esterification, a two-step procedure was adopted due to the nature of operational simplicity and mild conditions. Thus, the protected compound 4 was transformed into it corresponding acyl chloride with SOCl2 and then reacted with the 4-hydroxybutanal O-methyl oxime (5) in the presence of base to yield the desired ester 9. Deprotection of the compound 9 with ammonia solution in methanol and reduction of the oxime supposed to afford compound 2.
|
Download:
|
| Scheme2. Synthesis of Leonurine (1). Reagents and conditions: (a) 1 mol/L NaOH, 0-60 ℃, 6 h, 72%; (b) 1. 2 mol/L HCl, r.t., 6 h; 2. Methoxylamine hydrochloride, pyridine, ethanol, reflux, 2 h, 99%; (c) 1. SOCl2, reflux, 2 h; 2. triethylamine, DMAP, CH2Cl2, 2 h, 0 ℃, 91%; (d) 1. Ammonium hydroxide, methanol, r.t., 10 h; 2. Raney-Ni, H2, methanol, r.t., 10 h, 81%; (e) S-Methylisothiourea hemisulfate (3), DMF-H2O, 120 ℃, 12 h, 65%. | |
The conditions of the reduction of oxime (Scheme 2, step d) had also been screened (Table 1). While using of zinc powder only resulted in complicated products, low yield of compound 2 was achieved by hydrogenation with 10% Pd/C. At last, the experiments showed that Raney-Ni appeared to be the best catalyst and furnished the primary compound 2 in 81% yield under hydrogen atmosphere in methanol for 10 h (entry 4). Finally, Leonurine (1) was obtained on multi-gram scale by the reaction of the primary amine 2 and S-methylisothiourea hemisulfate (3) after recrystallization.
|
|
Table 1 Screening of the reduction conditions. |
With large quantity of Leonurine (1) in hand, we next evaluate its toxicity on a zebrafish model. Fig. 2 showed that, at concentration of 5 μg/mL, the hatching rate would achieve 100% in 48 h, and decrease a little as the concentration increasing. At the concentration of 300 μg/mL, the rate was the lowest. From the figure we can see that the hatching would finish in 24 to 48 h at all the concentrations and the increasing rate of the hatching rate decreased after 48 h. The hatching of the control group also finished in 48 h indicated that Leonurine (1) would promote the hatching at low concentration.

|
Download:
|
| Fig. 2. The effect on the hatching rate of embryos. | |
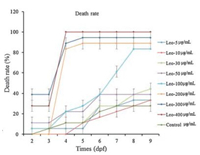
Fig. 3 showed that Leonurine (1) would lead to acute death when the concentration more than or equal to 200 μg/mL, resulting in death in 4 days. At the concentration of 100 μg/mL, the mortality rate increased. When the concentration of Leonurine (1) was lower than 100 μg/mL, the mortality rate increased with the increasing of the concentration, but no more than 50% and unchanged any more after 8 days. The LC50 of Leonurine (1) on zebrafish was 267.8795 μg/mL, calculated by linear regression method of SCSS.
|
Download:
|
| Fig. 3. The effect on the mortality of zebrafish. | |
3. Conclusion
In summary, We have developed a new route for the synthesis of Leonurine (1) from commercially available compound 6 and 2, 3-dihydrofuran (7). The longest liner step is six from 2, 3-dihydrofuran (7). This route avoids the expensive 4-aminobutan-1-ol and employed a more atom-economic protecting group for the amino group. The operational simplicity coupled with the low price of the starting material allows a mass production of Leonurine (1). The toxicity study shows that Leonurine (1) would promote the hatching of zebrafish embryos at low concentration and result in acute death or chronic lethal toxicity at high concentration.
4. ExperimentalAll reagents were used as received from commercial sources without further purification or prepared as described in the literature. Reactions were stirred using Teflon-coated magnetic stirring bars. Analytical TLC was performed with 0.20 mm silica gel 60F plates with 254 nm fluorescent indicator. TLC plates were visualized by ultraviolet light. Chromatographic purification of products was carried out by flash column chromatography on silica gel (200-300 mesh). NMR spectra were measured in CDCl3 (with TMS as internal standard), DMSO-d6 or CF3COOD on a Bruker AV400 (1H at 400 MHz, 13C at 101 MHz). Chemical shifts (δ) are reported in ppm, and coupling constants (J) are in Hz. Highresolution mass spectra (HRMS) were recorded on an LTQ/FT linear ion trap mass spectrometer.
4.1. Chemical syntheses 4.1.1. Preparation of compound 4To a stirred solution of 1 mol/L NaOH solution was added compound 6 (66 g, 0.33 mol at 0 ℃). After all the solids were dissolved, ethyl chloroformate (36.17 g, 0.33 mol) was added to the mixture slowly, keeping the temperature below 10 ℃. After the addition was complete, the mixture was stirred at this temperature for 6 h. Hydrochloric acid (12 mol/L) was added to acidify the mixture, and lots of white solids came out. The solids were filtered out and recrystallized in acetone to afford compound 4 (64 g, 72% yield) as a white crystals. 1H NMR (400 MHz, DMSO-d6): δ 13.22 (s, 1H), 7.30 (s, 2H), 4.24 (q, 2H, J = 7.1 Hz), 3.84 (s, 6H), 1.28 (t, 3H, J = 7.1 Hz). [lit.[19] 1H NMR 7.38 (2H, Ar-H), 4.29 (2H, —CH2—), 3.39 (3H, CH3O—), 1.33 (3H, CH3CH2—)].
4.1.2. Preparation of compound 5To a stirred solution of 2 mol/L HCl (100 mL) was slowly added 7 (10 g, 0.14 mol) at room temperature. After the addition was complete, the mixture was stirred for 6 h. Then, the mixture was concentrated in vacuo. The crude product obtained in the last step was dissolved in ethanol (100 mL), pyridine (12.9 mL, 0.16 mol) and methoxylamine hydrochloride (12.53 g, 0.15 mol) was added as the mixture stirring. After the addition was finished, the mixture was heated to reflux and stirred at this temperature for 2 h. The mixture was concentrated in vacuo. The residue was then dissolved in CH2Cl2 (100 mL) and washed with water. The organic layer was dried with MgSO4 and concentrated in vacuo to afford the product 5 (16.5 g, 99% yield) as a light yellow oil. 1H NMR (400 MHz, CDCl3): δ 7.34 (t, 0.54H, J = 6.0 Hz), 6.62 (t, 0.38H, J = 5.8 Hz), 3.80 (s, 1.26H), 3.74 (s, 1.71H), 3.63-3.52 (m, 2H), 2.53 (s, 1H), 2.34 (td, 1H, J = 7.4, 5.8 Hz), 2.23 (td, 1H, J = 7.3, 6.2 Hz), 1.73-1.60 (m, 2H). 13C NMR (101 MHz, CDCl3): δ 151.23, 150.56, 61.67, 61.57, 61.55, 61.20, 29.28, 28.91, 26.09, 22.07. HRMS (ESI): m/z calcd. for C5H11NO2 117.07898 [M+H]+; found: 117.07872.
4.1.3. Preparation of compound 9To a stirred solution of SOCl2 (2.7 mL, 37.0 mmol) was slowly added the protected compound 4 (2 g, 7.4 mmol), the the mixture was heated to reflux. After 1 h, the excess SOCl2 was concentrated in vacuo to afford the carboethoxy syringate acid chloride. To a stirred solution of compound 8 (1.0 g, 8.9 mmol) in CH2Cl2 (30 mL) was added TEA (1.5 mL, 11.1 mmol) and DMAP (90 mg, 0.74 mmol), and then, the freshly prepared carboethoxy syringate acid chloride was slowly added into the solution at 0 ℃. After the completion of addition, the mixture was stirred for 6 h at room temperature. Water was added to quench the reaction, and then the resulting mixture was extracted with CH2Cl2 (2 ×10 mL). The combined organic layer was dried with MgSO4 and concentrated in vacuo. The residue was purified by column chromatography (petroleum ether/EtOAc = 15:1) to afford compound 9 (2.48 g, 91% yield) as a white semi-solid. 1H NMR (400 MHz, CDCl3): δ 7.44 (q, 0.63H, J = 5.5 Hz), 7.32 (dd, 2H, J = 2.5, 0.4 Hz), 6.72 (t, 0.41H, J = 5.5 Hz), 4.41-4.29 (m, 4H), 3.90 (s, 6H), 3.85 (d, 1.38H, J = 0.6 Hz), 3.80 (d, 1.78H, J = 0.7 Hz), 2.48 (dd, 1H, J = 13.0, 7.4 Hz), 2.36 (dd, 1H, J = 13.6, 7.0 Hz), 1.99 (tt, 2H, J = 13.8, 6.9 Hz), 1.39 (td, 3H, J = 7.1, 0.4 Hz). 13C NMR (101 MHz, CDCl3): δ 165.74, 152.43, 152.16, 150.23, 149.39, 132.81, 128.25, 106.31, 65.21, 64.73, 64.54, 61.64, 61.26, 56.36, 26.46, 25.78, 25.51, 22.53, 14.11. HRMS (ESI): m/z calcd. for C17H23NO8 369.1424 [M+H]+; found: 369.1424.
4.1.4. Preparation of compound 2To a stirred solution of compound 9 (2 g, 5.4 mmol) in MeOH (25 mL) was added ammonia (2 mL) at room temperature, lots of solid began to appear. After 4 h, TLC indicated the disappearance of the starting material. The solid was collected by filtration, transformed into hydrochloride salt and then dissolved in methanol (25 mL) again. The resulting mixture was added catalytic amount Raney-Ni (0.2 g) and stirred under H2 atomsphere (1 atm) at room temperature for 10 h. The solid was filtered off and MeOH was removed in vacuo. The residue was dissolved in water, and NaHCO3 (0.5 g, 6.0 mmol) was added. Lots of solid began to appear as the mixture was stirring. The solid was collected and dried to afford compound 2 (1.18 g, 81% yield) as a white solid. mp: 214-215 ℃; 1H NMR (400 MHz, DMSO-CF3COOD): δ 7.21 (s, 2H), 4.25 (t, 2H, J = 6.2 Hz), 3.81 (s, 6H), 2.86 (dd, 2H, J = 12.8, 5.4 Hz), 1.76 (dt, 2H, J = 12.7, 6.2 Hz), 1.66 (dt, 2H, J = 9.3, 7.0 Hz). HRMS (ESI): m/z calcd. for C13H19NO5 269.12632 [M+H]+; found: 269.12638.
4.1.5. Preparation of Leonurine (1)To a stirred solution of compound 2 (5.6 g, 20 mmol) in DMF (15 mL) was added S-methylisothiourea hemisulfate (3) (15% in water, 23.1 g, 25 mmol), the mixture was heated to reflux and stirred at 120 ℃ for 6 h. HPLC indicated the disappearance of starting material, the solvent was removed in vacuo and the residue was dissolved in water. NaHCO3 (2.31 g, 27.5 mmol) was added and lots of solid began to appear as the mixture stirring. The solid was collected by filtration and dried to give Leonurine (1) (4.0 g, 65% yield) as a white solid. 1H NMR (400 MHz, CF3COOD): δ 7.38 (s, 2H), 4.42 (t, 2H, J = 6.2 Hz), 3.91 (s, 6H), 3.30 (t, 2H, J = 6.7 Hz), 1.89 (dt, 2H, J = 13.3, 6.5 Hz), 1.83-1.71 (m, 2H). lit. [18] 1H NMR (400 MHz, CF3COOD): δ 7.36 (s, 2H), 4.3 ~ 4.55 (br, 2H), 3.94 (s, 6H), 3.15 ~ 3.45 (br, 2H), 1.75 ~ 2.05 (br, 4H).
4.2. Toxicity study on zebrafishAdult zebrafish were obtained from Model Animal Research Center of Nanjing University and kept in 5 L acrylic tank with the following conditions: 28.5 ℃, with a 14/10 h light/dark cycle. Zebrafish were fed two times a day, 6 days/week, with Tetramin flake food supplemented with live brine shrimps (Artemia salina). Embryos were obtained from natural spawning that was induced in the morning by turning on the light. Collection of embryos was completed within 30 min.
After the completion of zebrafish fertilization, the fertilized eggs with the survival rate above 95% were selected for the experiment. The selected embryos were randomly divided into 9 groups, and each group put 6 embryos into 24-well plate with 1.5 mL solution of different concentration of Leonurine (0, 5, 10, 30, 50, 100, 200, 300 and 400 μg/mL). At 28 ± 0.5 ℃, all groups were growing in a 12/12 h light/dark cycle. The mortality and hatching rate was determined by daily observation of heartbeats, autonomous movement, and stress response. Dead ones should be taken away as soon as possible.
AcknowledgmentsThe research was supported by Anhui Ph.D. Programs of Postdoctoral Workstation, Talents Program of Hefei and funds from the sixth group of Anhui '115' Industrial Innovation Team (Team of Key Technology Innovation on Botanical Pesticides).
| [1] | T. Goto, N. Kato, Y. Hirata, Y. Hayashi. The structure of leonurine. Tetrahedron Lett. 3(1962)545–548. DOI:10.1016/S0040-4039(00)76926-7 |
| [2] | C.X. Chen, C.Y. Kwan. Endothelium-independent vasorelaxation by leonurine. a plant alkaloid purified from Chinese motherwort,. Life Sci. 68(2001)953–960. DOI:10.1016/S0024-3205(00)00987-5 |
| [3] | K. Kuchta, J. Ortwein, D. Briel, H. Rauwald. Leonurine in leonurus and leonotis drugs?. Detection and quantitative determination by a newly developed HPLC method. Planta Med. 76(2010)1330–1331. |
| [4] | H.W. Yeung, Y.C. Kong, W.P. Lay, K.F. Cheng. The structure and biological effect of leonurine-uterotonic principle from Chinese drug. I-Mu-Tsao, Planta Med. 31(1977)51–56. DOI:10.1055/s-0028-1097489 |
| [5] | Z. Wang, P.L. Zhang, Y. Ju. Effect of leonurine on the activity of creatine kinase. J. Asian Nat. Prod. Res. 6(2004)281–287. DOI:10.1080/10286020310001595962 |
| [6] | J. Qi, Z.Y. Hong, H. Xin, Y.Z. Zhu. Neuroprotective effects of leonurine on ischemia/reperfusion-induced mitochondrial dysfunctions in rat cerebral cortex. Biol. Pharm. Bull. 33(2010)1958–1964. DOI:10.1248/bpb.33.1958 |
| [7] | X. Li, F.L. Yuan, Y.Q. Zhao, et al., Effects of leonurine hydrochloride on medically induced incomplete abortion in early pregnancy rats. Eur. J. Obstetr. Gynecol. Reprod. Biol. 159(2011)375–380. DOI:10.1016/j.ejogrb.2011.09.006 |
| [8] | G.D. Norata, A.L. Catapano. Leonurine:a new comer in the natural compounds affecting atherosclerosis. Atherosclerosis 224(2012)37–38. DOI:10.1016/j.atherosclerosis.2012.02.036 |
| [9] | H. Xin, M. Gu, W.W. Wang, et al., Effects of leonurine on L-type calcium channel in rat ventricular myocytes. Biol. Pharm. Bull. 35(2012)1249–1256. DOI:10.1248/bpb.b12-00011 |
| [10] | X. Li, F.L. Yuan, Y.Q. Zhao, et al., Effect of leonurine hydrochloride on endothelin and the endothelin receptor-mediated signal pathway in medically-induced incomplete abortion in rats. Eur. J. Obstetr. Gynecol. Reprod. Biol. 169(2013)299–303. DOI:10.1016/j.ejogrb.2013.02.022 |
| [11] | X.H. Liu, L.L. Pan, H.Y. Deng, et al., Leonurine (SCM-198) attenuates myocardial fibrotic response via inhibition of NADPH oxidase 4. Free Radic. Biol. Med. 54(2013)93–104. DOI:10.1016/j.freeradbiomed.2012.10.555 |
| [12] | S.S. Luo, X.F. Gu, Y.Z. Zhu. Improved synthesis of leonurine as cardioprotective agent. J. Chin. Pharm. Sci. 21(2012)292–295. |
| [13] | Y. Kishi, S. Sugiura, S. Inoue, Y. Hayashi, T. Goto. Synthesis of leonurine. Tetrahedron Lett (1968)637–640. |
| [14] | S. Sugiura, S. Inoue, Y. Hayashi, Y. Kishi, T. Goto. Structure and synthesis of leonurine. Tetrahedron 25(1969)5155–5161. DOI:10.1016/0040-4020(69)80036-0 |
| [15] | K.F. Cheng, C.S. Yip, H.W. Yeung, Y.C. Kong. Leonurine. an improved synthesis. Experientia 35(1979)571–572. DOI:10.1007/BF01960323 |
| [16] | X.H. Liu, L.L. Pan, Q.H. Gong, Y.Z. Zhu. Leonurine (SCM-198) improves cardiac recovery in rat during chronic infarction. Eur. J. Pharmacol. 649(2010)236–241. DOI:10.1016/j.ejphar.2010.08.056 |
| [17] | G.C. Zhong, J.Z. Peng, J.M. Li, et al., Design. Synthesis and sodium hydrogen exchanger isoform-1(NHE-1) inhibitory activity of leonurine analogues. Chin. J. Org. Chem. 31(2011)1445–1451. |
| [18] | G.X. Shao, R.Y. Mo, L.L. Zhu, W.Y. He. Synthesis of leonurine and syringic acid amino-esters. Acta Pharm. Sin. 19(1984)419–424. |
| [19] | X.X. Li, Z.A. Xu. Synthesis of leonurine. China Patent CN1415602A. |
 2017, Vol. 28
2017, Vol. 28 



