Water is a unique green solvent for chemical transformation in view of its low cost, safety, and abundance in nature. Recently, on the basis of environmental concern, the investigations on aqueous organic reaction have made great progress [1]. At the same time, transition-metal-catalyzed aqueous reactions have also got considerable attention [2]. However, since many organometallic compounds are sensitive to moisture, searching for novel efficient metal catalyst in aqueous reaction is still a challenge task. On the other hand, vinyleboranes play an important role in C—C bond formation reactions, which were widely applied in many chemical transformations [3]. The hydroboration of alkynes is the most straightforward method to access vinylboranes [4]. Although the transition metal catalyzed hydroborations of terminal alkynes have been well developed, the hydroboration of internal alkynes are less developed due to the low reactivity and difficulty of controlling stereo-and regioselectivity [5]. Bis(pinacolato)diboron (B2pin2) has been developed as a powerful and efficient borylation reagent, and was applied widely in the construction of C—B bond [6]. Although there have been a lot of reports on aqueous reaction involved by B2pin2, very few examples of the hydroborylation of internal alkynes with B2pin2 in water have been reported [7].
Herein, we reported the hydroborylation reaction of internal alkynes with B2pin2 in water catalyzed by spiro-SIPHOS/Cu(OTf)2 to give the products vinylboranes in high yields with exclusive regio-and stereoselectivities.
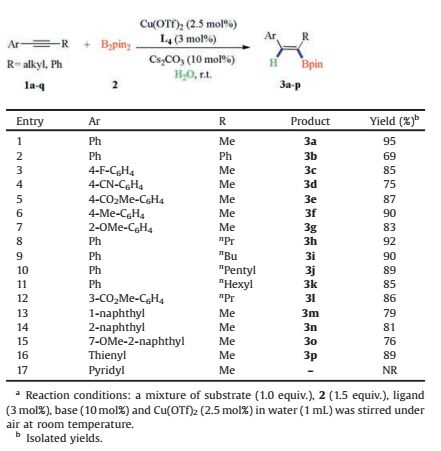
2. Results and discussionBesides several noble metal catalysts, copper-catalyst was the best choice for hydroboration of alkynes [8]. Yun and coworkers [9] had reported the Cu(Ⅰ)-catalyzed reaction of internal alkynes with B2pin2 in THF with methanol as additive. In their catalytic system, phosphorous ligands were necessary. Considering the instability of Cu(Ⅰ)-salts, we intended to employ Cu(Ⅱ)-salts to realize the hydroboration of internal alkynes. Initially, the reaction of 1-phenyl-propyne (1a) with B2pin2 (2) was carried out in water, in the presence of Cu(OTf)2 (2.5 mol%) and Cs2CO3 (10 mol%), without use of any ligands. Only trace amount of product Z-3a was obtained (Table 1, entry 1). However, when L1 (Fig. 1) was used as ligand, the vinylborane product Z-3a was obtained in 27% yield in the absence of Cs2CO3 (Table 1, entry 2). Luckily, in the presence of both ligand and base, the yield of Z-3a was increased remarkably to 63% (Table 1, entry 3). Z-4a was not detected in our reaction system, indicating that the aqueous hydroboration reaction underwent with exclusive β-regioselectivity [10]. The vinylborane product Z-3a was the only isomer, which was confirmed by NOESY analysis. Then various kinds of ligands were tested in our reaction system, and L2, L3 and L4 (Fig. 1) were all proved as effective ligands affording Z-3a in good yields (Table 1, entry 4-6). However, N, Pligand (L5) was ineffective in our catalytic system, and the yield of Z-3a was decreased to 23% (Table 1, entry 7). 1, 10-Phenanthroline (L6) which had been proven effective for the aqueous reaction between internal alkynes and Si-B compouds [11], was detrimental in the borylation of 1a with diboron compounds (Table 1, entry 8). It was probably due to the strong coordination between nitrogen of the ligand and boron in B2pin2, which may deactivates the ligand. At last, rac-SIPHOS (L4) was proven to be the most effective ligand in our catalytic system, and when the loading of L4 was reduced by half, it could give Z-3a in almost quantive yield (Table 1, entry 9 vs entry 6). To the best of our knowledge, up to date, although the spiro phosphoamidite-type ligands (such as SIPHOSE)/transition metal complex has achieved great success in asymmetric reactions [12], there has been no report on the application of SIPHOSE as an efficient ligand in transition-metal-catalyzed aqueous reaction of diboron compounds with internal alkynes. Then, several other bases were also tested, however they gave inferior yields comparing with Cs2CO3 (Table 1, entries 10-12). Thus, the optimized conditions for hydroborations of internal alkynes include Cu(OTf)2/L4 as catalysts with H2O as solvent under air at ambient temperature (entry 9, Table 1).
|
|
Table 1 Cu(Ⅱ)-catalyzed reaction of 1a with 2 in water.a |
|
Download:
|
| Fig. 1. The structure of ligands. | |
With the optimized conditions in hand, we began to explore the substrate scope of internal alkynes (Table 2). It was found that the electronic effect had little influence on the yield of the vinylborane products. The substrates bearing either electron-donating or electron-withdrawing groups, could react smoothly with B2pin2 under the optimized conditions. However the solid internal alkynes, gave relatively low yields due to the poor solubility (3b). The reaction system had good functional group compatibility, substrates with nitriles and esters went through hydroborylations smoothly, and no hydrolysis of nitriles or esters was detected (3d-3i). Interestingly, subatrates with long alkyl chains which were hydrophobic, could also react well, affording the Z-selective vinylboranes in good to excellent yields (3h-3l). Polycyclic aromatic substrates were also tested in our reaction systems, and they could give the desired products in high yields (3m-3o). Internal alkynes with hetero-aromatic cycles could also afford desired product. For example, 2-(prop-1-yn-1-yl)thiophene (1p) provided vinylborane product Z-3p in excellent yield. All products were obtained in exclusive β-regio and Z-stereoselectivities, which was determined by NOESY spectrum. However when 2-(1-propynyl)pyridine (1q) was used, no desired product was obtained. Although the reason was unclear, we deemed that strong interaction between N-and B-atoms may preclude the B-B bond activation, thus impeding the formation of CuⅡ-B species (Fig. 2), which was considered as the key intermediate (Scheme 2).
|
|
Table 2 Substrates' scope of the hydroborations of unactivated internal alkynes.a |

|
Download:
|
| Fig. 2. The strong coordination between 1q and 2. | |
|
Download:
|
| Scheme 2. Plausible mechanism. | |
In the regio-and stereoselective hydroborations of internal alkynes, the origin of hydrogen was believed coming from the solvent (H2O), which was further proven by the deuterium-labeled experiments (Scheme 1). For example, the hydroboration reaction of 1a was conducted in D2O, which afforded deuterated-labled vinylborane 5 in high yield (95%) with exclusively β-regio and Z-stereospecifity.

|
Download:
|
| Scheme1. Deuterium-labeled experiments. | |
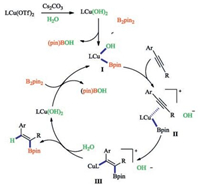
Diboron compounds (B2pin2) belong to interelement compounds, and it had been proven that B-B bond could be activated through transmetalation processe. Based on precedent reports, we proposed a tentative mechanism which is shown in Scheme 2. LCu (OTf)2 can be converted to more activated LCu(OH)2 assisted by Cs2CO3 in water. Then LCu(OH)2 undergoes σ-bound metathesis with B2pin2, generating the key Cu-B intermediate Ⅰ. Then the intermediate Ⅰ coordinated with the C-C triple bond, forming intermediate Ⅱ. Subsequently, the C-C triple bond inserts into the Cu-B bond, affording intermediate Ⅲ. Finally the vinyl copper species undergoes hydrolysis to afford vinylboranes and LCu(OH)2 was regenerated.
3. ConclusionIn conclusion, we have developed an efficient catalytic system for the synthesis of multisubstituted vinylboranes via borylcupration of unactivated internal alkynes. The reactions were conducted in water which was environmentally friendly. The aqueous hydroborylation exhibited high efficiency and exclusive regioand stereoselectivity. This method, using low cost Cu(OTf)2 as catalyst with rac-SIPHOS (L4) as ligand, and water as solvent and proton source, providing a convenient and practical route to multisubstituted vinylboranes, which are very important synthons in organic synthesis.
4. ExperimentalLigand L4 (0.003 mmol, 1.0 mg), diboron compound (B2pin2) and Cs2CO3 were weighed and added to a reaction tube. Then 1 mL solution of Cu(OTf)2 (0.9 mg/mL), and internal alkyne 1a (0.1 mmol, 12.6 mL) were added via syringe. After the reaction mixture was stirred for 12 h at room temperature, the reaction solution was extracted with ether for 3 times. The organic layer was combined and dried over Na2SO4, and the organic solvent was evaporated. The residue was subjected to flash column chromatography on silica gel with petroleum ether as eluent to afford the desired product 3a.
| [1] |
(a) J. Cornella, J.T. Edwards, T. Qin, et al., Practical Ni-catalyzed aryl-alkyl crosscoupling of secondary redox-active esters, J. Am. Chem. Soc. 138(2016) 2174-2177; (b) F. Toriyama, J. Cornella, L. Wimmer, et al., Redox-active esters in Fecatalyzed C-C coupling, J. Am. Chem. Soc. 138(2016) 11132-11135; (c) J. Wang, T. Qin, T.G. Chen, et al., Nickel-catalyzed cross-coupling of redoxactive esters with boronic acids, Angew. Chem. Int. Ed. 55(2016) 9676-9679; (d) C.J. Li, Organic reactions in aqueous media with a focus on carbon-carbon bond formations:a decade update, Chem. Rev. 105(2005) 3095-3166. |
| [2] | B. Cornils, W.A. Herrmann. Aqueous-phase Organometallic Catalysis, WileyVCH. Co. Weinheim (2004). |
| [3] |
(a) N. Miyaura, A. Suzuki, Palladium-catalyzed cross-coupling reactions of organoboron compounds, Chem. Rev. 95(1995) 2457-2483; (b) T. Hayashi, K. Yamasaki, Rhodium-catalyzed asymmetric 1, 4-addition and its related asymmetric reactions, Chem. Rev. 103(2003) 2829-2844; (c) T.R. Wu, J.M. Chong, asymmetric conjugate alkenylation of enones catalyzed by chiral diols, J. Am. Chem. Soc. 129(2007) 4908-4909. |
| [4] |
(a) X. He, J.F. Hartwig, True metal-catalyzed hydroboration with titanium, J. Am. Chem. Soc. 118(1996) 1696-1702; (b) N. Iwadate, M. Suginome, Synthesis of B-protected β-styrylboronic acids via Iridium-catalyzed hydroboration of alkynes with 1, 8-naphthalenediaminatoborane leading to iterative synthesis of oligo(phenylenevinylene)s, Org. Lett. 11(2009) 1899-1902; (c) Ⅰ. Beletskaya, A. Pelter, Hydroborations catalysed by transition metal complexes, Tetrahedron 53(1997) 4957-5026; (d) R. Barbeyron, E. Benedetti, J. Cossy, et al., Recent developments in alkyne borylations, Tetrahedron 70(2014) 8431-8452; (e) H. Yoshida, S. Kawashima, Y. Takemoto, et al., Copper-catalyzed borylation reactions of alkynes and arynes, Angew. Chem. Int. Ed. 51(2012) 235-238; (f) Y.W. Zhao, Q. Feng, Q.L. Song, Copper-catalyzed decarboxylative hydroboration of phenylpropiolic acids under ligand-free or both ligand-and basefree conditions, Chin. Chem. Lett. 27(2016) 571-574. |
| [5] | Y.D. Bidal, F. Lazreg, C.S.J. Cazin. Copper-catalyzed regioselective formation of tri-and tetrasubstituted vinylboronates in air. ACS Catal 4(2014)1564–1569. DOI:10.1021/cs500130y |
| [6] |
(a) T. Kitanosono, P. Xu, S. Isshiki, L. Zhu, S. Kobayashi, Cu(ii)-Catalyzed asymmetric boron conjugate addition to α, β-unsaturated imines in water, Chem. Commun. 50(2014) 9336-9339; (b) S. Radomkit, A.H. Hoveyda, Enantioselective synthesis of boron-substituted quaternary carbon stereogenic centers through NHC-catalyzed conjugate additions of (Pinacolato)boron units to enones, Angew. Chem. Int. Ed. 53(2014) 3387-3391; (c) A.L. Moure, R.G. Arrayás, D.J. Cárdenas, I. Alonso, J.C. Carretero, Regiocontrolled CuI-catalyzed borylation of propargylic-functionalized internal alkynes, J. Am. Chem. Soc. 134(2012) 7219-7222; (d) T. Kitanosono, P. Xu, S. Kobayashi, Heterogeneous and homogeneous chiral Cu(ii) catalysis in water:enantioselective boron conjugate additions to dienones and dienoesters, Chem. Commun. 49(2013) 8184-8186; (e) Y. Sasaki, Y. Horita, C. Zhong, M. Sawamura, H. Ito, Copper(Ⅰ)-catalyzed regioselective monoborylation of 1, 3-enynes with an internal triple bond:selective synthesis of 1, 3-dienylboronates and 3-alkynylboronates, Angew. Chem. Int. Ed. 50(2011) 2778-2782; (f) S. Mannathan, M. Jeganmohan, C.H. Cheng, Nickel-catalyzed borylative coupling of alkynes, enones, and bis(pinacolato)diboron as a route to substituted alkenyl boronates, Angew. Chem. Int. Ed. 48(2009) 2192-2195. |
| [7] | C.C. Tai, M.S. Yu, Y.L. Chen, et al., Synthesis of a guanidine NHC complex and its application in borylation reactions. Chem. Commun 50(2014)4344–4346. DOI:10.1039/C4CC00550C |
| [8] |
(a) H. Jang, A.R. Zhugralin, Y. Lee, A.H. Hoveyda, Highly selective methods for synthesis of internal (α-) vinylboronates through efficient NHC-Cu-catalyzed hydroboration of terminal alkynes. utility in chemical synthesis and mechanistic basis for selectivity, J. Am. Chem. Soc. 133(2011) 7859-7871; (b) H.R. Kim, J. Yun, Highly regio-and stereoselective synthesis of alkenylboronic esters by copper-catalyzed boron additions to disubstituted alkynes, Chem. Commun. 47(2011) 2943-2945. |
| [9] | J.E. Lee, J. Kwon, J. Yun. Copper-catalyzedaddition of diboronreagents to[small alpha], [small beta]-acetylenic esters:efficient synthesis of β-boryl-α, β-ethylenic esters. Chem. Commun 44(2008)733–734. |
| [10] | K. Semba, T. Fujihara, J. Terao, Y. Tsuji. Copper-catalyzed highly regio-and stereoselective directed hydroboration of unsymmetrical internal alkynes:controlling regioselectivity by choice of catalytic species. Chem. Eur. J. 18(2012)4179–4184. DOI:10.1002/chem.v18.14 |
| [11] | Q.Q. Xuan, C.L. Ren, L. Liu, D. Wang, C.J. Li. Copper(ii)-catalyzed highly regioand stereo-selective hydrosilylation of unactivated internal alkynes with silylborate in wate. Org. Biomol. Chem 13(2015)5871–5874. DOI:10.1039/C5OB00694E |
| [12] |
(a) M.L. Li, S. Yang, X.C. Su, et al., Mechanism studies of Ir-catalyzed asymmetric hydrogenation of unsaturated carboxylic acids, J. Am. Chem. Soc. 139(2017) 541-547; (b) L. Yu, et al., Enantioselective iridium-catalyzed hydrogenation of α, β-disubstituted nitroalkenes, Chem. Commun. 52(2016) 4812-4815; (c) C. Guo, D.W. Sun, S. Yang, et al., Iridium-catalyzed asymmetric hydrogenation of 2-Pyridyl cyclic imines:a highly enantioselective approach to nicotine derivatives, J. Am. Chem. Soc. 137(2015) 90-93; (d) D.H. Bao, H.L. Wu, C.L. Liu, J.H. Xie, Q.L. Zhou, Developmentof chiral spiro PN-S ligands for iridium-catalyzed asymmetric hydrogenation of β-alkylb-ketoesters, Angew. Chem. Int. Ed. 54(2015) 8791-8794; (e) J.H. Xie, D.H. Bao, Q.L. Zhou, Recent advances in the development of chiral metal catalysts for the asymmetric hydrogenation of ketones, Synthesis 47(2015) 460-471. |
 2017, Vol. 28
2017, Vol. 28