Biomass is the most attractive alternative feedstock of fossil resource to produce fuels and bulk chemicals since it is a widely available carbon source [1]. Furfural is an important biomass platform chemical that can be produced by acid-catalyzed dehydration from pentose [2-6]. In addition, it can also be prepared from hexose via isomerization step to form a ketose prior to the retro-aldol reactions [7-10]. Basically, furfural can be converted into many useful chemicals such as furan, furfuryl alcohol (FAL), tetrahydrofurfuryl alcohol (THFAL), 2-methylfuran, pentanediol, levulinic acid, etc. [11]. As a new route, furfural could be transformed into cyclopentanol (CPL) which is used for the production of fragrance chemicals and pharmaceuticals. Traditionally, CPL was obtained from an extra hydrogenation process of cyclopentanone (CPO) which was prepared by the pyrolysis of adipic acid or its derivatives and the oxidation of cyclopentene [12-14]. Therefore, the direct synthesis of CPL forms biomass-derived furfural by rearrangement is meaningful, which could reduce the social dependence on fossil resource. However, there were only a few related researches. The possible reason is that it is hard to fulfill the hydrogenation of CPO to CPL without the saturation of furan ring to side-product THFAL.
As another rearrangement product, CPO has been studied widely. In 2012, Hronec and Fulajtarová proposed the method to produce CPO from lignocellulose-derived furfural in the presence of metal catalysts and water [15]. The best yield of CPO was 76.5 mol% over 5% Pt/C catalyst and water was an essential part in the ring rearrangement. Then, they found the support had an influence on the product distribution [16]. A 44.7% CPO yield was obtained over the 1% Pt/Al2O3 while the main product was furfuryl alcohol over 1% Pt/MgO at 160 ℃, 8 MPa H2, 30 min. Other noble metal catalysts such as Ru/MIL-101 [17], Pd-Cu2O/C [18], Au/TiO2-A [19] and Ru/CNTs [20] were also reported and the CPO yield was more than 91%. Considering the high price and low reserves of noble metals, many non-noble metals had also been investigated, especially Cu and Ni. NiCu-50/SBA-15 [21], Cu-Ni-Al [22] and CuNi@C [23] were synthesized and exhibited good catalytic activity for the conversion of furfural into CPO. Our group developed CuZnAl catalyst for the conversion of furfural to CPO and achieved a 62% of CPO yield at 150 ℃ and 4 MPa H2 [24]. Among these catalysts, CPO is the main rearrangement product instead of CPL, which means an additional hydrogenation process is necessary.
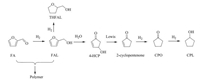
The reaction mechanism was proposed by Hronec et al. and shown in Scheme 1 [16, 25]. After the hydrogenation of furfuryl, furfuryl alcohol was obtained and converted by different pathways: rearrangement, polymerization and over-hydrogenation, which were competitive reactions. Through the rearrangement pathway, furfuryl alcohol was protonated by hydrogen ions and cationic intermediate was formed. Subsequently, it opened the ring by interacting with water, the furan ring was rearranged to 4-hydroxy-2-cycloentenone (4-HCP). It is an unstable intermediate and can be found in the spontaneous hydrolysis of furfuryl alcohol under N2 atmosphere without any catalyst [16]. Thus 4-HCP was hardly detected in the reaction system. Then 4-HCP was dehydrated to 2-cyclopentenone on Lewis acid sates. These two intermediates are high reactivity and the conversion was fast in the catalytic system [17]. 2-cyclopentenone underwent further hydrogenation to CPO and CPL. Polymerization and over-hydrogenation should be avoided. Therefore, the catalytic metals should be able to hydrogenate furfural and 2-cyclopentenone but not saturate the furan ring to THFAL. The weak Lewis acid support can contribute to dehydration [26].
|
Download:
|
| Scheme1. The reaction mechanism for the conversion of furfural into CPL. | |
It is a challenge to catalyze furfural into CPL directly. Therefore, it will be significant to develop new catalytic systems, especially catalysts with high activity to fulfill the conversion of CPL in one step without THFAL. Xiao's group reported the CPL was obtained as the main product over some non-noble metal catalysts. The yield of CPL was 83.6% at 140 ℃, 5 MPa H2 over 30% Ni/CNTs [27]. High loading of Ni was required to obtain CPL. CuMgAl [28] and CuZnAl [29] were highly active and the yield of CPL was 93.4% at 140 ℃, 4 MPa H2, 10 h and 84% at 150 ℃, 4 MPa H2, 10 h, respectively. Except for the high H2 pressure and long reaction time, these catalysts agglomerated during the recycling. Li et al. used Cu-Co catalysts to convert furfural into CPL under mild H2 pressure and short reaction time (2 MPa H2, 1 h). The CPL yield was 67 mol% at 170 ℃ [30]. Besides molecular H2, methanol can also be used as hydrogen donor [31].
According to previous reported [32, 33], Co is a promising hydrogenation metal. In this study, we prepared several supported cobalt catalysts for the selective conversion of furfural to CPL in one step under mild conditions. The supports were the typical weak Lewis acid materials. Except for TiO2 and Al2O3, we also studied ZrO2 and changed its phase by doping La2O3 and characterized by X-ray diffraction (XRD) and temperature-programmed desorption of ammonia (NH3-TPD). The influences of various reaction conditions including reaction temperature, hydrogen pressure and reaction time on the catalytic activity were also investigated. The deactivation of the Co/ZrO2-La2O3 catalyst was also studied.
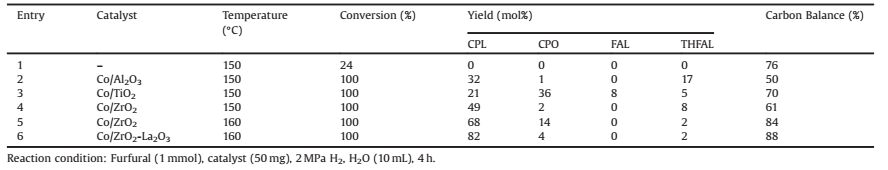
2. Results and discussion 2.1. Effect of different supports on the hydrogenation of furfuralBased on the previous reports [15], the rearrangement of furfuryl alcohol to form CPL or CPO occurred only in aqueousphase. Thus, water was employed as the solvent in the following reactions. Table 1 showed the results of furfural conversion in water by using different cobalt catalysts. The supports include Al2O3, TiO2, ZrO2 and ZrO2-La2O3. After the reaction, the main products were CPL, CPO and THFAL. CPL and CPO were the rearrangement products. THFAL came from over-hydrogenation of furfuryl alcohol. Furfural and furfuryl alcohol could be converted to oligomeric and polymeric compounds by heat [34, 35] or acid sites [36] when they were accumulated in the reaction system. The loss of carbon was related to polymerization. As showed in Table 1, furfural could be converted to polymer with a 24% carbon loss. The polymerization product was hardly detected, so we count the loss of carbon as its ratio. When Co/Al2O3 was used as catalyst, the byproduct THFAL was 17 mol%. Most of furfuryl alcohol was polymerized on the acid support, which was consistent with the previous reports [15, 37, 38]. It indicated that rearrangement reaction was hindered by the polymerization and over-hydrogenation. As for Co/TiO2, because the cobalt particles were encapsulated in the TiO2 in the effect of SMSI [39], the polymer formed by heat could block the some caves resulting in the partial loss of hydrogenation activity. Hence, the yield of CPO was higher than CPL. When Co was supported on ZrO2, the polymerization reduced and the main product of CPL increased to 49 mol%. Changing the temperature to 160 ℃, the yield of THFAL reduced to 2 mol%, which showed high temperature contributed to the rearrangement by increasing the concentration of hydrogen ions which was related to temperature. The catalytic activity increased and product distribution tended to be CPL. Therefore, the furfuryl alcohol underwent the rearrangement reaction instead of over-hydrogenation and polymerization. After doping La2O3, the catalytic activity raised and CPO was further hydrogenation into CPL. The 82 mol% yield of CPL was obtained at 160 ℃, 2 MPa, 4 h over Co/ZrO2-La2O3.
|
|
Table 1 Hydrogenation of furfural with various cobalt catalysts. |
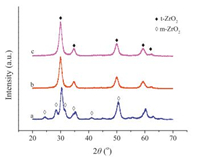
The reason of high catalytic activity with the addition of La2O3 was studied by XRD and NH3-TPD. All Zr-based supports were characterized by XRD patterns to study the crystal morphology. As showed in Fig. 1, the diffraction peaks of ZrO2 could be indexed to two different phases: the diffraction peaks at 24.3°, 28.1°, 31.3°, 34.6°, 41.2° and 50.1° were monoclinic zirconia (m-ZrO2) while 30.3°, 35.2°, 50.6°, 60.0° and 62.23° were tetragonal zirconia (tZrO2). The mixture phases were existed in ZrO2. However, after adding some La, the diffraction peaks of m-ZrO2 were disappeared. Only the diffraction peaks of t-ZrO2 were observed without La species appeared. The shift of diffraction peaks in ZrO2-La2O3 to lower angle suggested that La had been doped into ZrO2 lattice and increased the interplanar spacing. Thus, t-ZrO2 can be obtained from the mixture phase of t-ZrO2 and m-ZrO2 by doping La2O3, which may be the main reason for the good performance. After impregnation and calcination, Co/ZrO2-La2O3 was synthesized and the t-ZrO2 phase did not change compared with ZrO2-La2O3. There was not the diffraction peak contributed by metallic Co, which demonstrated that Co species were highly dispersed on the surface of supports. Then, Co/ZrO2-La2O3 was investigated at different conditions.
|
Download:
|
| Fig. 1. XRD patterns of (a) ZrO2, (b) ZrO2-La2O3 and (c) Co/ZrO2-La2O3. | |
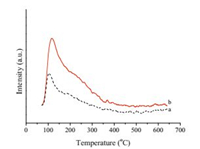
The acidic properties of the synthesized supports were investigated by NH3-TPD. The results were shown in Fig. 2. The acid sites can be classified as weak acid sites ( < 200 ℃), medium acid sites (200-400 ℃) and strong acid sites (>400 ℃) depending on NH3 desorption temperatures [40]. The sample showed a desorption peak at about 120 ℃, which meant the existence of weak acid sites. It was obvious that the peaks increased after doping of La2O3. This could be explained that the amount of acid sites in t-ZrO2 was more than that in m-ZrO2, which was consistent with previous reports [41]. This phenomenon indicated the dispersion of La species in ZrO2 lattice was important for the increase of the acidity which may contributed to the conversion of furfural to CPL.
|
Download:
|
| Fig. 2. NH3-TPD profiles of (a) ZrO2, (b) ZrO2-La2O3. | |
2.2. Effect of reaction conditions on the conversion of furfural to CPL
The yields of furfural at different temperature catalyzed by Co/ ZrO2-La2O3 were illustrated in Fig. 3a. The volcanic type curve of CPL could be recognized. The results showed that temperature had an influence on the product distribution during the hydrogenation process. In the investigated temperature range, the yield of CPL reached the maximum at 160 ℃. Some CPO and furfural alcohol were remained at 150 ℃. Lower temperature limited both the hydrogenation activity of the catalyst and the dissociation constant of water [42]. When the temperature was at 170 ℃ or higher, the polymerization was dominant. Based on the previous studies, furfuryl alcohol could polymerize [43]. It occurred at α-position and β-position of furan ring in the reaction system [36]. The polymer could attach on the surface of the catalyst and impair the activity. Therefore, 160 ℃ was optimal temperature for the rearrangement and hydrogenation. This moderate temperature not only produced enough hydrogen ions but also minimized the polymerization of furfural and furfuryl alcohol by heat.

|
Download:
|
| Fig. 3. Catalytic performance of Co/ZrO2-La2O3 at different temperature (a), hydrogen pressure (b), reaction time (c). | |
The hydrogen pressure was one of the important factors affecting the final products. The initial hydrogen pressure was studied for the hydrogenation of furfural by Co/ZrO2-La2O3. Fig. 3b showed the results of furfural hydrogenation carried out on different hydrogen pressure. When the initial pressure was 1 MPa, the main product was CPO. When the initial pressure raised to 2 MPa, CPO was hydrogenated to CPL. The yield of CPL improved obviously when the initial pressure raised from 1 MPa to 2 MPa, which was similar to the Ni/CNTs [27]. Thus, the low pressure was suitable for producing CPO while the high pressure can increase the selectivity to CPL. A further increase in hydrogen pressure showed no improvement in selectivity, but a slight slip for CPL. A part of furfuryl alcohol was over-hydrogenated and 6 mol% THFAL was obtained when the pressure was up to 4 MPa. Therefore, the higher pressure could lead to a high selectivity to THFAL, which was the product of over-hydrogenation of furfuryl alcohol during the competing reaction with rearrangement. Therefore, 2 MPa was the optimal pressure for the high selectivity for CPL.
The kinetics curves could reflect the reaction pathway clearly. The Fig. 3c exhibited the product distribution for the conversion of furfural at different reaction time. The zero time was the moment that the reaction temperature reached 160 ℃. Firstly, furfural was fast hydrogenated into furfuryl alcohol and then CPO began to accumulation by rearrangement. With the extension of time, CPL was obtained by the hydrogenation of CPO. The carbon balance was decreased significantly at the beginning since furfuryl alcohol polymerized partly to the compounds having conjugated diene structures with 5-30 furanic moieties [44]. It increased a little owing to the decomposition reaction according to the former report [45]. When the polymer formed, it would deposit on the surface of the catalyst, which could block the active sites for hydrogenation and decrease the catalytic activity [46].
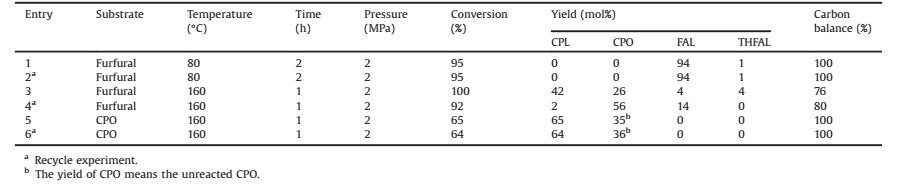
2.3. The study of deactivationA series of experiments were designed over Co/ZrO2-La2O3 to study the deactivation in Table 2. When the reaction was preform at 80 ℃ for 2 h, the conversion was 95% without carbon loss. There was not polymer. The products were furfuryl alcohol and THFAL. The rearrangement did not occur duo to lower concentration of hydrogen ions. After the recycle, the catalyst activity did not change. Then, when the reaction was carried out at 160 ℃, reaction time was set at 1 h to show the difference obviously. The conversion was 100% and the main product was CPL with a 42 mol% yield. There was 24% carbon loss which was caused by polymerization. However, after the reuse of catalysts, the main product was CPO without further hydrogenation and the conversion reduced to 92%. When CPO was the substrate, the conversion was 65% without polymerization under the same conditions. The catalytic activity was almost unchanged after the recycle, indicating the structure of the catalyst was intact. Therefore, the polymer could be the reason for the decrease the catalytic activity and the inhibition of the polymer formation should improve over the supported Co catalysts
|
|
Table 2 Hydrogenation of furfural and CPO at different conditions over Co/ZrO2-La2O3. |
3. Conclusion
In summary, cobalt catalysts loading on different supports were synthesized and applied in the hydrogenation of furfural into CPL in one step. Among the different supports, t-ZrO2 obtained by doping La2O3 was high-efficient for the conversion of furfural into CPL after loading cobalt metal. Under the optimized conditions (160 ℃, 2 MPa H2 pressure and 4 h), the yield of CPL could reach up to 82 mol%. The change of phase in ZrO2 was proved by XRD and the increase of acidity was demonstrate by NH3-TPD that could improve the catalytic activity. Further studies to control the polymer formation during the reaction are currently undergoing.
4. Experiment 4.1. Chemicals materialsFurfural was purified by vacuum distillation and stored at -15 ℃. Furfuryl alcohol was not purified. Furfural (AR, >99%) Furfuryl alcohol (AR, >99%), tetrahydrofurfuryl alcohol (AR, >97%), cyclopentanone (AR, >97%), cyclopentanol (AR, >97%), Co (NO3)2·6H2O (AR, >99%), La(NO3)3·6H2O (AR, >44%), ZrOCl2·8H2O (AR, >99%), (NH4)2Ce(NO3)6 (AR, >99%) and CTAB (AR, >99%) were from Sinopharm Chemical Reagent Co., Ltd. Al2O3 was purchased from Aladdin Chemical Reagent Co., Ltd. TiO2 was purchased from Sigma-Aldrich Chemical Reagent Co., Ltd.
4.2. Catalyst preparationZrO2 was synthesized by co-precipitation/hydrothermal crystallization with a modification. Typically, a certain amount of hexadecyltrimethylammonium bromide (CTAB) was dissolved in deionized water at 60 ℃ with agitation. Zirconium oxychloride solution was added to give a clear homogeneous solution. The mixed solution contains Zr, CTAB and H2O with molar ratio of 1:0.5:100, respectively. After 0.5 h, 1 mol/L sodium hydroxide solution was prepared and added dropwise under vigorous stirring to a constant pH of 9. Then, the mixture was aged at 90 ℃ for 10 h and followed by filtration and washing with deionized water and ethanol. Finally, the precipitate was dried at 105 ℃ overnight and then calcined in air at 550 ℃ for 4 h.
ZrO2-La2O3 was synthesized by adding zirconium oxychloride solution and lanthanum solution to the clear homogeneous solution. The mixed solution contains Zr, La, CTAB and H2O with a molar ratio of 1:0.2:0.5:100, respectively. After that, the process was same as that for preparing ZrO2.
The Co based catalysts were prepared by impregnation method. Al2O3 and TiO2 were calcined in air at 750 ℃ for 4 h to remove the impurities. The support (0.5 g) was dispersed in acetone (45 mg) with stirring at 45 ℃. Cobalt nitrate (0.246 mg) was dissolved in 5 ml acetone and then added drop by drop to the above solution.After stirring for 24 h, acetone was removed by rotary evaporation. The catalyst was dried at 105 ℃ overnight and calcined at 300 ℃ for 2 h to remove the nitrates with a heating rate of 1 ℃/min. Finally, the catalyst was calcined at 600 ℃ for 2 h at a rate of 1 ℃/min again. The calcined catalysts were reduced in a H2 atmosphere at 600 ℃ for 2 h with a heating rate of 1 ℃/min before reaction. All the loadings were 8.8 wt% for the Co based catalysts according to ICP-AES.
Catalytic hydrogenation of furfural was carried out in a 25 ml stainless steel autoclave equipped with a magnetic stirrer. Typically, a mixture of furfural (1 mmol), catalyst (50 mg) and water (10 mL) were put into the reactor and purged with H2 for several times. The reactor was pressured with H2 to 2 MPa. Then the autoclave was heated to the desired temperature for 4 h. After reaction, the reactor was cooled to ambient temperature. The liquid products were extracted by ethyl acetate and then analyzed by a gas chromatograph (GC, Kexiao 1690) with a HP-INNOMAX capillary column (30 m × 0.25 mm × 0.25 μm) and GC-MS (Agilent 7890A). The GC detecting conditions were as follows: nitrogen was the carrier gas; injection port temperature was 280 ℃; detector (FID) temperature was 280 ℃. Column temperature heated from 40 ℃ to 250 ℃ with a heating rate of 10 ℃/min. The n-hexanol was used as internal standard to quantify the products.
4.3. Characterization methodsX-ray diffraction (XRD) was conducted on an X-ray diffractometer (TTR-Ⅲ, Rigaku Corp, Japan) using Cu Kα radiation (λ = 1.54056 Å). The data were recorded over 2θ ranges of 20-70°.
Temperature-programmed desorption was carried out in a home-built reactor system coupled to a gas chromatograph. All the gas flow was set to 40 mL/min. Temperature-programmed desorption of ammonia (NH3-TPD) was employed to determine the total acidity of the catalysts. Prior to absorption of ammonia, 80 mg catalyst sample was heated at 500 ℃ for 1 h under Ar flow and then cooled to 80 ℃ followed by saturating with pure NH3 for 1 h. Then after flushing with Ar for 1 h, the NH3-TPD was performed from 80 to 650 ℃ with a heating rate of 10 ℃/min. Desorbed ammonia was monitored by an on-line gas chromatograph equipped with a thermal conductivity detector (TCD).
AcknowledgmentsThe authors appreciate financial support from the NSFC (No. 21572213), the National Basic Research Program of China (No. 2013CB228103), Program for Changjiang Scholars and Innovative Research Team in University of the Ministry of Education of China and the Fundamental Research Funds for the Central Universities (No. wk 2060190040).
| [1] | R.J. van Putten, J.C. van der Waal, E.D. De Jong, et al., Hydroxymethylfurfural, a versatile platform chemical made from renewable resources. Chem. Rev. 113(2013)1499–1597. DOI:10.1021/cr300182k |
| [2] | A. Gandini, T.M. Lacerda, A.J.F. Carvalho, E. Trovatti. Progress of polymers from renewable resources:furans, vegetable oils, and polysaccharides. Chem. Rev. 116(2015)1637–1669. |
| [3] | R. Karinen, K. Vilonen, M. Niemelä. Biorefining:heterogeneously catalyzed reactions of carbohydrates for the production of furfural and hydroxymethylfurfural. ChemSusChem 4(2011)1002–1016. DOI:10.1002/cssc.201000375 |
| [4] | L. Hu, G. Zhao, W.W. Hao, et al., Catalytic conversion of biomass-derived carbohydrates into fuels and chemicals via furanic aldehydes. RSC Adv. 2(2012)11184–11206. DOI:10.1039/c2ra21811a |
| [5] | M.J. Climent, A. Corma, S. Iborra. Converting carbohydrates to bulk chemicals and fine chemicals over heterogeneous catalysts. Green Chem. 13(2011)520–540. DOI:10.1039/c0gc00639d |
| [6] | Y. Nakagawa, M. Tamura, K. Tomishige. Catalytic reduction of biomass-derived furanic compounds with hydrogen. ACS Catal. 3(2013)2655–2668. DOI:10.1021/cs400616p |
| [7] | E.I. Gürbüz, J.M.R. Gallo, D.M. Alonso, et al., Gallo M.R.. Conversion of hemicellulose into furfural using solid acid catalysts in γ-valerolactone, Angew. Chem. Int. Ed. 52(2013)1270–1274. |
| [8] | J.L. Cui, J.J. Tan, T.S. Deng, et al., Conversion of carbohydrates to furfural via selective cleavage of the carbon-carbon bond:the cooperative effects of zeolite and solvent. Green Chem. 18(2016)1619–1624. DOI:10.1039/C5GC01948F |
| [9] | T.M. Aida, Y. Sato, M. Watanabe, et al., Dehydration of d-glucose in high temperature water at pressures up to 80 MPa. J. Supercrit. Fluids 40(2007)381–388. DOI:10.1016/j.supflu.2006.07.027 |
| [10] | F.M. Jin, H. Enomoto. Rapid and highly selective conversion of biomass into value-added products in hydrothermal conditions:chemistry of acid/basecatalysed and oxidation reactions. Energy Environ. Sci. 4(2011)382–397. DOI:10.1039/C004268D |
| [11] | K. Yan, G. Wu, T. Lafleur, C. Jarvis. Production. properties and catalytic hydrogenation of furfural to fuel additives and value-added chemicals, Renew. Sustain. Energy Rev. 38(2014)663–676. |
| [12] | M. Renz. Ketonization of carboxylic acids by decarboxylation:mechanism and scope. Eur. J. Org. Chem. 2005(2005)979–988. DOI:10.1002/(ISSN)1099-0690 |
| [13] | K.A. Dubkov, G.I. Panov, E.V. Starokon, V.N. Parmon. Non-catalytic liquid phase oxidation of alkenes with nitrous oxide. 2. Oxidation of cyclopentene to cyclopentanone. React. Kinet. Catal. Lett. 77(2002)197–205. DOI:10.1023/A:1020372726494 |
| [14] | J. Marquié, A. Laporterie, J. Dubac, N. Roques. Graphite-supported ketodecarboxylation of carboxylic diacids. Synlett 2001(2001)0493–0496. DOI:10.1055/s-2001-12319 |
| [15] | M. Hronec, K. Fulajtarová. Selective transformation of furfural to cyclopentanone. Catal. Commun. 24(2012)100–104. DOI:10.1016/j.catcom.2012.03.020 |
| [16] | M. Hronec, K. Fulajtarová, T. Liptaj. Effect of catalyst and solvent on the furan ring rearrangement to cyclopentanone. Appl. Catal. A:Gen. 437(2012)104–111. |
| [17] | R.Q. Fang, H.L. Liu, R. Luque, Y.W. Li. Efficient and selective hydrogenation of biomass-derived furfural to cyclopentanone using Ru catalysts. Green Chem. 17(2015)4183–4188. DOI:10.1039/C5GC01462J |
| [18] | M. Hronec, K. Fulajtarová, I. Vávra, et al., Carbon supported Pd-Cu catalysts for highly selective rearrangement of furfural to cyclopentanone. Appl. Catal. B:Environ. 181(2016)210–219. DOI:10.1016/j.apcatb.2015.07.046 |
| [19] | G.S. Zhang, M.M. Zhu, Q. Zhang, et al., Towards quantitative and scalable transformation of furfural to cyclopentanone with supported gold catalysts. Green Chem. 18(2016)2155–2164. DOI:10.1039/C5GC02528A |
| [20] | Y.H. Liu, Z.H. Chen, X.F. Wang, et al., Highly selective and efficient rearrangement of biomass-derived furfural to cyclopentanone over interface-active Ru/carbon nanotubes catalyst in water. ACS Sustain. Chem. Eng. 5(2017)744–751. DOI:10.1021/acssuschemeng.6b02080 |
| [21] | Y.L. Yang, Z.T. Du, Y.H. Huang, et al., Conversion of furfural into cyclopentanone over Ni-Cu bimetallic catalysts. Green Chem. 15(2013)1932–1940. DOI:10.1039/c3gc37133f |
| [22] | H.Y. Zhu, M.H. Zhou, Z. Zeng, G.M. Xiao, R. Xiao. Selective hydrogenation of furfural to cyclopentanone over Cu-Ni-Al hydrotalcite-based catalysts. Korean J. Chem. Eng. 31(2014)593–597. DOI:10.1007/s11814-013-0253-y |
| [23] | Y. Wang, S.Y. Sang, W. Zhu, L.J. Gao, G.M. Xiao. CuNi@C catalysts with high activity derived from metal-organic frameworks precursor for conversion of furfural to cyclopentanone. Chem. Eng. J. 299(2016)104–111. DOI:10.1016/j.cej.2016.04.068 |
| [24] | J.H. Guo, G.Y. Xu, Z. Han, et al., Selective conversion of furfural to cyclopentanone with CuZnAl catalysts. ACS Sustain. Chem. Eng. 2(2014)2259–2266. DOI:10.1021/sc5003566 |
| [25] | M. Hronec, K. Fulajtarová, T. Soták. Highly selective rearrangement of furfuryl alcohol to cyclopentanone. Appl. Catal. B:Environ. 154(2014)294–300. |
| [26] | J. Ohyama, R. Kanao, Y. Ohira, A. Satsuma. The effect of heterogeneous acid-base catalysis on conversion of 5-hydroxymethylfurfural into a cyclopentanone derivative. Green Chem. 18(2016)676–680. DOI:10.1039/C5GC01723H |
| [27] | M.H. Zhou, H.Y. Zhu, L. Niu, G.M. Xiao, R. Xiao. Catalytic hydroprocessing of furfural to cyclopentanol over Ni/CNTs catalysts:model reaction for upgrading of bio-oil. Catal. Lett. 144(2014)235–241. DOI:10.1007/s10562-013-1149-5 |
| [28] | M.H. Zhou, Z. Zeng, H.Y. Zhu, G.M. Xiao, R. Xiao. Aqueous-phase catalytic hydrogenation of furfural to cyclopentanol over Cu-Mg-Al hydrotalcites derived catalysts:model reaction for upgrading of bio-oil. J. Energy Chem. 23(2014)91–96. DOI:10.1016/S2095-4956(14)60109-1 |
| [29] | Y. Wang, M.H. Zhou, T.Z. Wang, G.M. Xiao. Conversion of furfural to cyclopentanol on Cu/Zn/Al catalysts derived from hydrotalcite-like materials. Catal Lett. 145(2015)1557–1565. DOI:10.1007/s10562-015-1539-y |
| [30] | X.L. Li, J. Deng, J. Shi, et al., Selective conversion of furfural to cyclopentanone or cyclopentanol using different preparation methods of Cu-Co catalysts. Green Chem. 17(2015)1038–1046. DOI:10.1039/C4GC01601G |
| [31] | Y. Xua, S.B. Qiu, J.X. Long, et al., In situ hydrogenation of furfural with additives over a RANEY®Ni catalyst. RSC Adv. 5(2015)91190–91195. DOI:10.1039/C5RA12844G |
| [32] | G. Zhang, K.V. Vasudevan, B.L. Scott, S.K. Hanson. Understanding the mechanisms of cobalt-catalyzed hydrogenation and dehydrogenation reactions. J. Am. Chem. Soc. 135(2013)8668–8681. DOI:10.1021/ja402679a |
| [33] | X.H. Liu, L.J. Xu, G.Y. Xu, et al., Selective hydrodeoxygenation of lignin-derived phenols to cyclohexanols or cyclohexanes over magnetic CoNx@NC catalysts under mild conditions. ACS Catal. 6(2016)7611–7620. DOI:10.1021/acscatal.6b01785 |
| [34] | R.T. Conley, I. Metil. An investigation of the structure of furfuryl alcohol polycondensates with infrared spectroscopy. J. Appl. Polym. Sci. 7(1963)37–52. DOI:10.1002/app.1963.070070104 |
| [35] | E.M. Wewerka. An investigation of the polymerization of furfuryl alcohol with gel permeation chromatography. J. Appl. Polym. Sci. 12(1968)1671–1681. DOI:10.1002/app.1968.070120716 |
| [36] | E.M. Wewerka. Study of the γ-alumina polymerization of furfuryl alcohol. J. Polym. Sci. Part A:Polym. Chem. 9(1971)2703–2715. DOI:10.1002/pol.1971.150090923 |
| [37] | T. Kim, R.S. Assary, R.E. Pauls, et al., Thermodynamics and reaction pathways of furfuryl alcohol oligomer formation. Catal. Commun. 46(2014)66–70. DOI:10.1016/j.catcom.2013.11.030 |
| [38] | N. Guigo, A. Mija, L. Vincent, N. Sbirrazzuoli. Chemorheological analysis and model-free kinetics of acid catalysed furfuryl alcohol polymerization. Phys. Chem. Chem. Phys. 9(2007)5359–5366. DOI:10.1039/b707950h |
| [39] | J. Lee, S.P. Burt, C.A. Carrero, et al., Stabilizing cobalt catalysts for aqueousphase reactions by strongmetal-support interaction. J. Catal. 330(2015)19–27. DOI:10.1016/j.jcat.2015.07.003 |
| [40] | P. Kumar, V.C. Srivastava, I.M. Mishra. Dimethyl carbonate synthesis from propylene carbonate with methanol using Cu-Zn-Al catalyst. Energy Fuels 29(2015)2664–2675. DOI:10.1021/ef502856z |
| [41] | Souza P.M. de, R.C. Rabelo-Neto, L.E. Borges, et al., Effect of zirconia morphology on hydrodeoxygenation of phenol over Pd/ZrO2. ACS Catal. 5(2015)7385–7398. DOI:10.1021/acscatal.5b01501 |
| [42] | A.V. Bandura, S.N. Lvov. The ionization constant of water over wide ranges of temperature and density. J. Phys. Chem. Ref. Data 35(2006)15–30. DOI:10.1063/1.1928231 |
| [43] | S.Q. Xia, Y. Li, Q.Y. Shang, C.W. Zhang, P.S. Ma. Liquid-phase catalytic hydrogenation of furfural in variable solvent media. Trans. Tianjin Univ. 22(2016)202–210. DOI:10.1007/s12209-016-2804-x |
| [44] | M. Choura, N.M. Belgacem, A. Gandini. Acid-catalyzed polycondensation of furfuryl alcohol:mechanisms of chromophore formation and cross-linking. Macromolecules 29(1996)3839–3850. DOI:10.1021/ma951522f |
| [45] | M. Hronec, K. Fulajtárova, M. Mičušik. Influence of furanic polymers on selectivity of furfural rearrangement to cyclopentanone. Appl. Catal. A:Gen. 468(2013)426–431. DOI:10.1016/j.apcata.2013.08.052 |
| [46] | X.H. Zhang, T.J. Wang, L.L. Ma, C.Z. Wu. Aqueous-phase catalytic process for production of pentane from furfural over nickel-based catalysts. Fuel 89(2010)2697–2702. DOI:10.1016/j.fuel.2010.05.043 |
 2017, Vol. 28
2017, Vol. 28