Porous organic frameworks (POFs) constructed from light elements and linked by covalent bonds are a new generation ofporous materials, which have been of particular interest due totheir desirable properties of high stability, versatile functionality, large surface area [1-3]. To date, several types of POFs have been prepared including porous aromatic frameworks (PAFs) [4-6], polymers of intrinsic microporosity (PIMs) [7, 8], conjugated microporous polymers (CMPs) [9, 10], hyper-cross linked polymers(HCPs) [11], and covalent organic frameworks (COFs) [12-18], etc.Among these, COFs as one of the crystalline porous materials, which are mainly constructed from B, N, C, and O, and connected bycovalent bonds have attracted increasing attention in recent yearsnot only in the field of gas storage and separation [19, 20] but also invarious fields such as catalysis [21, 22], optoelectronics [23-25], sensing [26-28], organic molecules adsorption [29-32], andpotential drug delivery [33, 34]. Compared with the great concerned metal-organic frameworks (MOFs), COFs possess comparable surface area, inherent porosity, tunable pores, welldefined pore aperture, ordered channel structure, and versatile chemical composition. In addition, the main covalent linkages of B−O, C—N, and B—O—Si facilitate COFs with higher stability.Accordingly, many excellent works about COFs have been reported and their desirable properties enable such materials to bepromising in diverse fields [35, 36].
In this mini-review, we will represent a brief overview of the synthesis of COFs, whereas the growing types of linkers amendable to the synthesis of COFs are discussed. Our main focus of this review is to highlight the applications of COFs in various fields including gas storage and separation, catalysis, optoelectronics, sensing, small molecules adsorption, and drug delivery. Finally, the prospects and challenges of COFs have also been extensively discussed.
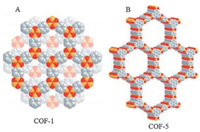
2. Synthesis of COFs by linking organic building blocks 2.1. B—O linkagesBoronic acids were first used to synthesize COFs via producing the linkages of boroxine anhydride in the form of B3O3 rings. COF-1(Fig. 1A) was synthesized by the self-condensation of 1, 4-phenylenediboronic acid (BDBA) to give an extended layers with hexagonal pores of 15 Å diameter and BET surface area of 711 m2/g [37]. Besides, COFs can also be synthesized by thereaction of boronic acids with catechols to produce boronateesters-based linkages as in case of COF-5 (Fig. 1B) and COF-105[37, 38]. Additionally, boronic acids can react with silanols toproduce borosilicate bonds and then to form structures of COFs such as COF-202 [39]. Undoubtedly, B—O linkages are remarkably general in the synthesis of COFs and COFs can be tailored by selecting diverse building blocks and reaction strategies, i.e., tunable topologies and multi-dimension of COFs can be obtained[40, 41]. For example, Lei's group fabricated a boronic ester-linked COF built from 4, 4'-phenylazobenzoyl diboronic acid and 2, 3, 6, 7, 10, 11-hexahydroxytriphenylene at room temperature and the liquid/solid interface [42]. Zhu's group reported an azobenzene-containing COF through the formation of borate ester [43].
|
Download:
|
| Fig. 1. Structural representations of (A) COF-1 and (B) COF-5. Carbon, boron, andoxygen are represented as gray, orange, and red spheres, respectively.Source: Reproduced from Ref. [14] with permission. Copyright 2015, AmericanChemical Society. | |
2.2. C—N linkages
In addition to the B—O bonds, COFs can also be preparedthrough the formation of C—N bonds. For example, COF-300 with asurface area of 1360 m2/g and a 5-fold interpenetrated dia topology was obtained by the imine condensation of aldehyde and amine linkers [44]. The formation of imine bonds is a versatile strategy forthe preparation of COFs and the polymerization reaction of Schiff bases is a similar method used to enhance the stability of iminebased COFs. For instance, Wan's group synthesized a bilayer COF byimine reaction between tetrathiafulvalene tetraaldehyde and pphenylenediamine [45]. Zhao's group prepared two kinds of 2D COFs which are constructed from truxene-based building blockand 1, 4-diaminobenzene or benzidine. More importantly, they studied the influence of substituents on interlayers stacking of 2D COFs, which may open a new path for the design of COF-basedfunctional materials [46]. To obtain higher stability, COFs havebeen synthesized through the formation of hydrazone linkages, another formation of C—N bonds with the condensation of aldehydes and hydrazide linkers. As in the case of COF-42 and COF-43 [47], these two examples of hydrazone COFs possessed enhanced chemical stabilities than imine-based COFs [48]. Inaddition to the abovementioned C—N bonds, other azine linkagesand triazine rings have also been applied for the synthesis of COFs[49, 50]. Remarkably, Wang's group indicated a facile strategy forthe synthesis of —C=N— linked COFs, i.e., imine-linked LZU-20, hydrazone-linked LZU-21, and azine-linked LZU-22 via thecondensation of dimethyl acetals and amines with good thermalstability [51]. Additionally, polyimide COFs with desirable stabilities have also been successfully prepared via the imidization reaction [52].
2.3. Other linkagesMany other linkages have also been explored for the synthesisof COFs in addition to the abovementioned general strategiesbecause of the diversity of reversible reactions, which is critical for the preparation of COFs with perfect order. Squaraine-linked COFs, which is composed of squaric acids and amines, allowed for the integration of zwitterion structures in their structures [53].Subsequently, another ionic COF was synthesized based onazodioxy linkages via the self-condensation of hydroxylamine with tetrabutylammonium fluoride as catalyst [54]. Afterwards, spiroborate-linked ionic COFs was prepared via the condensationof diols and trialkyl borate in the presence of basic catalysts [55]. Inaddition, double-stage strategies for the synthesis of COFs with multiple topologies also received wide attention, such as the combination of an imine and boronate ester or an imine andboroxine [17, 56].
3. Applications of COFsCOFs' desirable properties of inherent porosity, well-definedpore aperture, ordered channel structure, large surface area, lowdensity, high stability, multi-dimension, and designable functionality make these materials promising for use in the fields of gasstorage and separation, catalysis, optoelectronics, sensing, smallmolecules adsorption, and drug delivery [57]. In the following part, we will briefly outline the applications of COFs, providing anoutlook for the development of COFs.
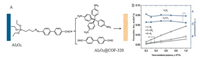
3.1. Gas storage and separationAs promising materials for gas storage and separation, permanent porous COFs have attracted increasing attention sincethe initial synthesis of COFs and many excellent works have proventhat COFs possess superior capacity for the storage and separationof important gases such as carbon dioxide, hydrogen, and methane[58-61]. Three-dimensional COFs with higher surface area andpore volumes exhibited larger storage capacities compared withtwo-dimensional layered structures [62]. For instance, COF-102, athree-dimensional non-interpenetrating structure with a Langmuir surface area of 4650 m2/g, showed a higher methane storage capacity of 25 wt% (203 cm3/cm) at 298 K and 80 bar compared with the 15 wt% capacity of the 9-fold interpenetrating COF-320 under the identical conditions. Additionally, COFs exhibited appreciable hydrogen storage capacity, for example, COF-102 and COF-103 performed well in the hydrogen storage with uptake values of 72.4 and 70.5 mg/g, respectively. Recently, a novel 3D COFs membrane was fabricated on a porous a-Al2O3 ceramic support via the formation of the covalent linkage by 3-aminopropyltriethoxysilane and 4, 4'-biphenyldicarboxaldehyde linkersbetween the 3D COF-320 and the porous a-Al2O3 support (Fig. 2A).Experimental results indicated that the gases permeation fluxacross the as-fabricated novel 3D COF-320 membranes wereincreased as H2 > CH4 > N2 and the obtained 3D COF-320 membrane was H2 selective with high permeance of 5.67 ×107 mol/(m2 s Pa)(Fig. 2B), suggesting that such COFs' membrane has great potentialin the field of membrane-separation [63]. Subsequently, Gascon's group prepared a mixed-matrix membrane composed of Matrimidand azine-linked COFs, the obtained COFs-based mixed-matrixmembrane possessed high adsorption selectivity for the separation of CO2 from an equimolar mixture of CO2 and CH4 [64]. Zhao's group developed a heteropore COF via the condensation of 4, 4'-bis(bis(4-formylphenyl)amino)-[1, 1':4', 1''-terphenyl]-2', 5'-dicarbaldehyde and 1, 4-diaminobenzene with desirable iodine capturecapacity (Fig. 3) [65].
|
Download:
|
| Fig. 2. Schematic presentation of (A) COF-320 membrane grown on porous a-Al2O3 substrate and (B) gas permeation performance of the supported 3D COF-320 membrane.Source: Reproduced from Ref. [63] with permission. Copyright 2015, Royal Society of Chemistry. | |

|
Download:
|
| Fig. 3. (A) Schematic illustration of iodine capture in the obtained COF. (B) Gravimetric uptake of iodine as a function of time at 75 ℃ (Inset: photographs of the COF before andafter being exposed to iodine vapor). Source: Reproduced from Ref. [65] with permission. Copyright 2017, Royal Society of Chemistry. | |
3.2. Catalysis
In terms of catalytic applications, COFs have been successfully applied to reduce the gap between heterogeneous materials and homogeneous catalysis. For example, base-functionalized COFs of BF-COF-1 and BF-COF-2 with intrinsic catalytic activity have beenused as catalysts in Knoevenagel condensation reactions [66]. In 2015, COFs composed of cobalt porphyrin catalysts and 1, 4-benzenedicarboxaldehyde or biphenyl-4, 4'-dicarboxaldehyde were synthesized through imine bonds. Such catalytic materialsperformed well for aqueous electrochemical reduction of CO2 to COwith high Faradaic efficiency and satisfactory turnover numbers[67]. COFs with additional catalytic species have also aroused wideattention. Wang's group constructed a Pd(Ⅱ)-containing COF, Pd/COF-LZU1, via a simple treatment of COF-LZU1 with Pd(OAc)2 asthe catalysis of Suzuki-Miyaura coupling reaction with desirablestability and easy recyclability as well as high efficiency [68].Lotsch's group reported a hydrazone-based COF constructed from1, 3, 5-tris-(4-formyl-phenyl)triazine (TFPT) and 2, 5-diethoxy-terephthalohydrazide (DETH), which was capable of visible-lightdriven hydrogen generation with Pt as a proton reduction catalyst[48]. Similarly, two kinds of covalent porphyrinic frameworks withmeso-tetra(4-hydrazidocarbonylphenyl)porphyrin and terephthalaldehyde or squaric acid as building blocks were fabricated andmanganese(Ⅲ) ions was modified in the COFs, presenting catalyticproperties for the selective oxidation of olefins [53]. Interestingly, Wang's group constructed a chiral COF assembled from 4, 4'-(1Hbenzo[d]-imidazole-4, 7-diyl)dianiline and various of chiral moieties, demonstrating that these obtained COFs are active as heterogeneous organocatalysts [69]. Subsequently, Cui's groupdeveloped a homochiral 2D COFs via embedding chiral functionalities into the COFs as an efficient heterogeneous catalysts [21].
3.3. Optoelectronics 3.3.1. Semiconduction and photoconductionCOFs built from aromatic building blocks with periodic arrayspossess favorable semiconductive and photoconductive behaviors, thus, photoelectronic applications of COFs are of particular interestand many good research results have been reported. [70-72]. Twokinds of porphyrin-based COFs, COF-66 and COF-366, have beenshown to possess high charge carrier mobility with chargemobility values of 3.0 and 8.1 cm2/V/s, respectively [73]. Similarly, phthalocyanine-based COFs and CS-COF also demonstrated chargecarrier mobility [74, 75]. Subsequently, Fullerene was mixed withprototypical light-absorbing phthalocyanine phenylenebis (boronic acid) COF and Kinetic Monte Carlo simulations was used to studythe dominant pore-filling mechanisms. Results indicated COF withlarger pores were favorable for electron conduction [76]. Afterwards, Banerjee's group mechanochemically synthesized bipyridine based COFs for the first time and the obtained materials outperformed its conventional solvothermal counterpart as amembrane electrode assembly with a stable open circuit voltage of 0.93 V at 50 ℃ and a proton conductivity of 1.4 ×10-2 S/cm [77]. In 2016, Zhu's group reported the first example of cationic COFs forthe development of charged COFs (EB-COF:X, X = F, Cl, Br, I) throughthe process of ion exchange. Moreover, the proton conductivity ofionic COFs could be enhanced by incorporating PW12O403- intothese COFs [78].
3.3.2. Energy storageCOFs with semiconductivity and facile-modified pore environment facilitate the applications of such materials for energy storageand conversion [79]. Dichtel and coworkers reported the first COF bycondensing the 2, 6-diaminoanthraquinone with 1, 3, 5-triformylphloroglucinol that exhibited the reversible electrochemical processes [80]. Moreover, the pre-synthesized COF modified electrodes possessed enhanced capacitance and showed unconspicuous changes after 5000 charge-discharge cycles. Recently, they prepareda redox-active 2D COF film via the electropolymerization of 3, 4-ethylenedioxythiophene within the pores of COF endow such formulations with enhanced electrical conductivity and improved electrochemical responses as well as stable capacitances (Fig. 4)[81]. Besides, COFs can also perform as host materials for sulfur impregnation to show a positive effect on Li-S batteries as in thecase of Wang's work. They constructed a sulfur electrode by introducing sulfur into the pores of CTF-1, showing stable cycling performance and agood rate capability [82].They also designed a 2D porphyrin-based COF for sulfur storage in Li-S batteries by impregnating sulfur into the COF to improve the performance of the sulfur cathode with 633 mA h/g capacity after 200 cycles of charge/discharge [83].

|
Download:
|
| Fig. 4. Depiction of preparation of conducting polymer-modified COF film and the cross-section of pore following the oxidation and reduction of the DAAQ moieties.Source: Reproduced from Ref. [81] with permission. Copyright 2016, American Chemical Society. | |
3.4. Sensing
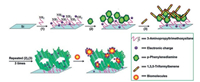
The diverse compositions and synergistic functions of COFs endow these materials with unique sensing characteristics and successful attempts on sensing applications of COFs have beenreported. For instance, Jiang and coworkers fabricated an azinelinked COFs, which was constructed from hydrazine and 1, 3, 6, 8-tetrakis(4-formylphenyl)pyrene and the as-prepared COFs werehighly luminescent [50]. Experimental results demonstrated thatthe obtained COFs possessed high sensitivity and selectivity in the detection of 2, 4, 6-trinitrophenol explosive and it's worth mentioning that this was the first report on the employment of COFs for chemosensing systems. Moreover, Fang et al. reported an iminelinked COF on amino functionalized silicon substrate, which wasapplied as biosensor for bovine serum albumin adsorption andprobe DNA immobilization owing to the strong electrostatic interactions (Fig. 5) [28]. Such COF films constructed via step-wisereaction between 1, 3, 5-benzenetricarboxaldehyde and 1, 4-diaminobenzene were used as the sensitive layer of biosensors to extendthe applications of such materials. In addition, Wang's groupfabricated a thioether-based fluorescent COF (COF-LZU8) for sensitive removal of Hg2+ (Fig. 6) [27] and Liu's group reported asimilar work of luminescent COF-JLU3 through the co-condensation of 1, 3, 5-tris(3'-tert-butyl-4'-hydroxy-5'-formylphenyl)benzene and hydrazine hydrate for selective detection of Cu2+ [84].Wang's group synthesized a novel 3D pyrene-based COF composedof tetra(p-aminophenyl) methane and 1, 3, 6, 8-tetrakis (4-formylphenyl)pyrene and then applied for the explosive detection withhigh selectivity [85].
|
Download:
|
| Fig. 5. Schematic presentation of imine-linked COF on surface for bio-molecular adsorption. Source: Reproduced from Ref. [28] with permission. Copyright 2014 SIOC, CAS, Shanghai & WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim. | |

|
Download:
|
| Fig. 6. Schematic introduction for the application of synthesized COF-LZU8 for both detection and removal of Hg2+. Source: Reproduced from Ref. [27] with permission.Copyright 2016, American Chemical Society. | |
3.5. Small molecules adsorption
COFs have aroused sustainable attention in the field of adsorption of small molecules, especially for the separation and enrichment due to their fascinating properties. In 2015, a novel benzimidazole-functionalized 2D COF containing a number of carboxylic groups was developed and applied as a matrix of solidphase extractant for the separation and enrichment of uranium [86]. Such COFs-based solid phase extractant indicated high selectivity to uranium by comparing with 11 kinds of competingions and all these results demonstrated that the COFs-basedmaterials could be potential functional solid-phase matrixes.Similarly, a new "stereoscopic" 2D super-microporous phosphazene-based COF by linking hexachorocyclotriphosphazene and p-phenylenediamine was prepared and used for selective sorptiontowards uranium [87]. In addition, Zhang's group designed ahydrazone COF-based micro-solid phase extraction (Fig. 7) forenrichment and analysis of trace Sudan dyes in chilli powder andsausage samples with low detection limit of 0.03-0.15 g/L, indicating that COFs could be promising sample preparationmedia for sampling, enrichment and separation purpose [88].Furthermore, we fabricated two kinds of COFs-based solid phase microextraction adsorbent for the enrichment of pesticideresidues with desirable enhancement factors and low detectionlimits, confirmed that COFs with fascinating properties arepromising adsorbents for pretreatment technology (Fig. 8)[89, 90]. Similarly, Yan and coworkers reported a spherical COF for high-resolution chromatographic separation of various important industrial analytes [91]. The abovementioned results indicated that the COF materials play an important role in the adsorptionof small molecules.
|
Download:
|
| Fig. 7. Schematic introduction of hydrazone COF-based micro-solid phase extraction system. Source: Reproduced from Ref. [88] with permission. Copyright 2015 Elsevier B.V.All rights reserved. | |
|
Download:
|
| Fig. 8. Schematic introduction for the synthesis of the hydrazone COF-based SPME fiber. Source: Reproduced from Ref. [89] with permission. Copyright 2016, Elsevier B.V. | |
3.6. Drug delivery
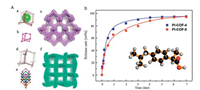
Compared with MOFs, the application of COFs in the field ofdrug delivery is still in its infancy, but it is undeniable that the useof COFs for drug delivery have gained increasing attention andgreat efforts have been made to construct COFs-based drugdelivery systems. For example, Yan et al. constructed two 3D polyimide COFs, which is composed of pyromellitic dianhydride and 1, 3, 5, 7-tetraaminoadamantane for PI-COF-4 and tetra(4-aminophenyl)methane for PI-COF-5, respectively [92]. Importantly, this was the first example of applying COFs to drug delivery andboth PI-COFs featured high ibuprofen loading and well-controlledrelease profiles (Fig. 9). All these results paved a new way for the subsequent development of COFs for pharmaceutical applications.In 2016, a surface-confined, photoresponsive single-layer COF wasfabricated, in which an azobenzene group was introduced into thebackbone of diboronic acid. The surface of the COF could bedestructed under UV irradiation via isomerization and then thedecomposed surface COF could be recovered after annealing due tothe reversibility of the condensation of boronic acid. Such reversible photoinduced decomposition-recovering of COF demonstrated controlled loading and release of copper phthalocyanine(Fig. 10), opening a new avenue to the development of photosensitive COFs which can be applicable in the field of drugdelivery [93]. Furthermore, Zhao et al. synthesized two nanoscale COFs by a condensation reaction of aldehyde and aminecompounds and three different drug molecules, 5-FU, captopril, and ibuprofen were selected to further study the potential of COFs as drug carriers [34]. Experimental results indicated that the asprepared COFs presented high drug loading capacity andappreciable release performance with low cytotoxicity, promotingfurther studies of COFs-based systems for disease therapy.Additionally, a nanoscale covalent triazine polymer was synthesized via the Friedel−Crafts reaction as a potential nanocarrier forcancer therapy and imaging [94]. Doxorubicin, an anticancer drug, was loaded onto the material and could be controlled release at pH4.8 and 7.4 with appropriate toxicity. Cell imaging experiments demonstrated that such material was potential for bioimaging anddisease theranostics.
|
Download:
|
| Fig. 9. (A) Structural representations of 3D PI-COFs. (a-c) Extended structure of PI-COF-4; (d-f) Extended structure of PI-COF-5. (B) Release profiles of IBU-loaded 3D PI-COFs.Inset: the structure of IBU. C, black; H, gray; O, red. Source: Reproduced from Ref. [92] with permission. Copyright 2015, American Chemical Society. | |

|
Download:
|
| Fig. 10. Corresponding STM images of the controlled capture and release of CuPc using the photoresponsive surface COF and it0s recovered after heating at 100 ℃.Source: Reproduced from Ref. [93] with permission. Copyright 2016 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim. | |
4. Conclusion
Varieties of COFs have been synthesized and attracted muchattention since the pioneering work by Yaghi et al. in 2005, as outlined in this mini-review, many remarkable researches of COFs with advantages of predictable structures, versatile functionalities, and potential applications have been reported. In summary, weintroduced the main linkages of COFs and briefly highlighted the applications of COFs in various fields including gas storage and separation, catalysis, optoelectronics, sensing, small molecules adsorption, and drug delivery. While remarkable progress has beenmade in terms of the synthesis and applications of COFs, there arestill many challenges need to be addressed and greater spacer for improvement. Firstly, the future challenges pertain to extending building blocks of COFs to provide excellent properties with facile synthetic strategies. Secondly, more efforts should be made toexpand the applications of COFs such as in the field of energy storage and stimulus responsiveness. Finally, the applications ofCOFs in disease theranostics are still in its early stage and moreresearch developments are expected. We envision a bright futurefor the development of COFs materials in sweeping fields.
AcknowledgmentsWe thank the National Natural Science Foundation of China(Nos. 51673084, 51473061), and the JLU Cultivation Fund for theNational Science Fund for Distinguished Young Scholars, forfinancial support.
| [1] | R. Dawson, A.I. Cooper, D.J. Adams. Nanoporous organic polymer networks. Prog. Polym. Sci. 37(2012)530–563. DOI:10.1016/j.progpolymsci.2011.09.002 |
| [2] | X. Zou, H. Ren, G. Zhu. Topology-directed design of porous organic frameworks and their advanced applications. Chem. Commun. 49(2013)3925–3936. DOI:10.1039/c3cc00039g |
| [3] | V. Guillerm, L.J. Weselinski, M. Alkordi, et al., Porous organic polymers with anchored aldehydes:a new platform for post-synthetic amine functionalization en route for enhanced CO2 adsorption properties. Chem. Commun. 50(2014)1937–1940. DOI:10.1039/c3cc48228f |
| [4] | T. Ben, H. Ren, S. Ma, et al., Targeted synthesis of a porous aromatic framework with high stability and exceptionally high surface area. Angew. Chem. Int. Ed. 48(2009)9457–9460. DOI:10.1002/anie.200904637 |
| [5] | T. Ben, S. Qiu. Porous aromatic frameworks:Synthesis. structure and functions, CrystEngComm 15(2013)17–26. |
| [6] | M. Wu, G. Chen, P. Liu, et al., Preparation of porous aromatic framework/ionic liquid hybrid composite coated solid-phase microextraction fibers and their application in the determination of organochlorine pesticides combined with GC-ECD detection. Analyst 141(2016)243–250. DOI:10.1039/C5AN01372K |
| [7] | J. Zhang, J. Jin, R. Cooney, et al., Fluoride-mediated polycondensation for the synthesis of polymers of intrinsic microporosity. Polymer 76(2015)168–172. DOI:10.1016/j.polymer.2015.08.066 |
| [8] | D. Becker, N. Konnertz, M. Böhning, et al., Light-switchable polymers of intrinsic microporosity. Chem. Mater. 28(2016)8523–8529. DOI:10.1021/acs.chemmater.6b02619 |
| [9] | C. Gu, N. Huang, J. Gao, et al., Controlled synthesis of conjugated microporous polymer films:versatile platforms for highly sensitive and label-free chemoand biosensing. Angew. Chem. Int. Ed. 53(2014)4850–4855. DOI:10.1002/anie.201402141 |
| [10] | T. Ratvijitvech, R. Dawson, A. Laybourn, et al., Post-synthetic modification of conjugated microporous polymers. Polymer 55(2014)321–325. DOI:10.1016/j.polymer.2013.06.004 |
| [11] | H. Gao, L. Ding, W. Li, et al., Hyper-cross-linked organic microporous polymers based on alternating copolymerization of bismaleimide. ACS Macro. Lett. 5(2016)377–381. DOI:10.1021/acsmacrolett.6b00015 |
| [12] | N. Huang, P. Wang, D. Jiang. Covalent organic frameworks:a materials platform for structural and functional designs. Nat. Rev. Mater. 1(2016)16068. DOI:10.1038/natrevmats.2016.68 |
| [13] | L.A. Baldwin, J.W. Crowe, D.A. Pyles, et al., Metalation of a mesoporous threedimensional covalent organic framework. J. Am. Chem. Soc. 138(2016)15134–15137. DOI:10.1021/jacs.6b10316 |
| [14] | P.J. Waller, F. Gandara, O.M. Yaghi. Chemistry of covalent organic frameworks. Acc. Chem. Res. 48(2015)3053–3063. DOI:10.1021/acs.accounts.5b00369 |
| [15] | B.J. Smith, N. Hwang, A.D. Chavez, et al., Growth rates and water stability of 2D boronate ester covalent organic frameworks. Chem. Commun. 51(2015)7532–7535. DOI:10.1039/C5CC00379B |
| [16] | N. Huang, X. Ding, J. Kim, et al., A photoresponsive smart covalent organic framework. Angew. Chem. Int. Ed. 54(2015)8704–8707. DOI:10.1002/anie.201503902 |
| [17] | X. Chen, M. Addicoat, E. Jin, et al., Designed synthesis of double-stage twodimensional covalent organic frameworks. Sci. Rep. 5(2015)14650. DOI:10.1038/srep14650 |
| [18] | G. Das, D. Balaji Shinde, S. Kandambeth, et al., Mechanosynthesis of imine beta-ketoenamine, and hydrogen-bonded imine-linked covalent organic frameworks using liquid-assisted grinding. Chem. Commun. 50(2014)12615–12618. |
| [19] | Y. Pramudya, J.L. Mendoza-Cortes. Design principles for high H2 storage using chelation of abundant transition metals in covalent organic frameworks for 0-700 bar at 298 K. J. Am. Chem. Soc. 138(2016)15204–15213. DOI:10.1021/jacs.6b08803 |
| [20] | H. Ma, H. Ren, S. Meng, et al., A 3D microporous covalent organic framework with exceedingly high C3H8/CH4 and C2 hydrocarbon/CH4 selectivity. Chem. Commun. 49(2013)9773–9775. DOI:10.1039/c3cc45217d |
| [21] | X. Wang, X. Han, J. Zhang, et al., Homochiral 2D porous covalent organic frameworks for heterogeneous asymmetric catalysis. J. Am. Chem. Soc. 138(2016)12332–12335. DOI:10.1021/jacs.6b07714 |
| [22] | H. Li, Q. Pan, Y. Ma, et al., Three-dimensional covalentorganic frameworks with dual linkages for bifunctional cascade catalysis. J. Am. Chem. Soc. 138(2016)14783–14788. DOI:10.1021/jacs.6b09563 |
| [23] | H. Xu, S. Tao, D. Jiang. Proton conduction in crystalline and porous covalent organic frameworks. Nat. Mater. 15(2016)722–726. DOI:10.1038/nmat4611 |
| [24] | J.W. Crowe, L.A. Baldwin, P.L. McGrier. Luminescent covalent organic frameworks containing a homogeneous and heterogeneous distribution of dehydrobenzoannulene vertex units. J. Am. Chem. Soc. 138(2016)10120–10123. DOI:10.1021/jacs.6b06546 |
| [25] | D.H. Yang, Z.Q. Yao, D. Wu, et al., Structure-modulated crystalline covalent organic frameworks as high-rate cathodes for Li-ion batteries. J. Mater. Chem. A 4(2016)18621–18627. DOI:10.1039/C6TA07606H |
| [26] | L. Guo, X. Zeng, D. Cao. Porous covalent organic polymers as luminescent probes for highly selective sensing of Fe3+ and chloroform:functional group effects. Sens. Actuators B:Chem. 226(2016)273–278. DOI:10.1016/j.snb.2015.11.108 |
| [27] | S.Y. Ding, M. Dong, Y.W. Wang, et al., Thioether-based fluorescent covalent organic framework for selective detection and facile removal of mercury(Ⅱ). J. Am. Chem. Soc. 138(2016)3031–3037. DOI:10.1021/jacs.5b10754 |
| [28] | P. Wang, M. Kang, S. Sun, et al., Imine-linked covalent organic framework on surface for biosensor. Chin. J. Chem. 32(2014)838–843. DOI:10.1002/cjoc.201400260 |
| [29] | J. Gao, D. Jiang. Covalent organic frameworks with spatially confined guest molecules in nanochannels and their impacts on crystalline structures. Chem. Commun. 52(2016)1498–1500. DOI:10.1039/C5CC09225F |
| [30] | M.S. Lohse, T. Stassin, G. Naudin, et al., Sequential pore wall modification in a covalent organic framework for application in lactic acid adsorption. Chem. Mater. 28(2016)626–631. DOI:10.1021/acs.chemmater.5b04388 |
| [31] | X. Niu, S. Ding, W. Wang, et al., Separation of small organic molecules using covalent organic frameworks-LZU1 as stationary phase by open-tubular capillary electrochromatography. J. Chromatogr. A 1436(2016)109–117. DOI:10.1016/j.chroma.2016.01.066 |
| [32] | J. Plas, O. Ivasenko, N. Martsinovich, et al., Nanopatterningof a covalentorganic framework host-guest system. Chem. Commun. 52(2016)68–71. DOI:10.1039/C5CC07557B |
| [33] | V.S. Vyas, M. Vishwakarma, I. Moudrakovski, et al., Exploiting noncovalent interactions in an imine-based covalent organic framework for quercetin delivery. Adv. Mater. 28(2016)8749–8754. DOI:10.1002/adma.201603006 |
| [34] | L. Bai, S.Z. Phua, W.Q. Lim, et al., Nanoscale covalent organic frameworks as smart carriers for drug delivery. Chem. Commun. 52(2016)4128–4131. DOI:10.1039/C6CC00853D |
| [35] | X. Feng, X. Ding, D. Jiang. Covalent organic frameworks. Chem. Soc. Rev. 41(2012)6010–6022. DOI:10.1039/c2cs35157a |
| [36] | S.Y. Ding, W. Wang. Covalent organic frameworks (COFs):from design to applications. Chem. Soc. Rev. 42(2013)548–568. DOI:10.1039/C2CS35072F |
| [37] | A.P. Côte, A.I. Benin, N.W. Ockwig, et al., Porous crystalline. covalent organic frameworks. Science 310(2005)1166–1170. DOI:10.1126/science.1120411 |
| [38] | H.M. El-Kaderi, J.R. Hunt, J.L. Mendoza-Cortes, et al., Designed synthesis of 3D covalent organic frameworks. Science 316(2007)268–272. DOI:10.1126/science.1139915 |
| [39] | J.R. Hunt, C.J. Doonan, J.D. LeVangie, et al., Reticular synthesis of covalent organic borosilicate frameworks. J. Am. Chem. Soc. 130(2008)11872–11873. DOI:10.1021/ja805064f |
| [40] | A.P. Côte, H.M. El-Kaderi, H. Furukawa, et al., Reticular synthesis of microporous and mesoporous 2D covalent organic frameworks. J. Am. Chem. Soc. 129(2007)12914–12915. DOI:10.1021/ja0751781 |
| [41] | E.L. Spitler, B.T. Koo, J.L. Novotney, et al., A 2D covalent organic framework with 4.7nm pores and insight into its interlayer stacking. J. Am. Chem. Soc. 133(2011)19416–18421. DOI:10.1021/ja206242v |
| [42] | C. Liu, Y. Yu, W. Zhang, et al., Room-temperature synthesis of covalent organic frameworks with a boronic ester linkage at the liquid/solid interface. Chemistry 22(2016)18412–18418. DOI:10.1002/chem.v22.51 |
| [43] | J. Zhang, L. Wang, N. Li, et al., A novel azobenzene covalent organic framework. CrystEngComm 16(2014)6547–6551. DOI:10.1039/C4CE00369A |
| [44] | F.J. Uribe-Romo, J.R. Hunt, H. Furukawa, et al., A crystalline imine-linked 3D porous covalent organic framework. J. Am. Chem. Soc. 131(2009)4570–4571. DOI:10.1021/ja8096256 |
| [45] | W.L. Dong, S.Y. Li, J.Y. Yue, et al., Fabrication of bilayer tetrathiafulvalene integrated surface covalent organic frameworks. Phys. Chem. Chem. Phys. 18(2016)17356–17359. DOI:10.1039/C6CP01804A |
| [46] | X. Zhao, Y. Fan, Q. Wen, et al., A case study on the influence of substituents on interlayer stacking of 2D covalent organic frameworks. Chemistry (2017). DOI:10.1002/chem.201700915 |
| [47] | F.J. Uribe-Romo, C.J. Doonan, H. Furukawa, et al., Crystalline covalent organic frameworks with hydrazone linkages. J. Am. Chem. Soc. 133(2011)11478–11481. DOI:10.1021/ja204728y |
| [48] | L. Stegbauer, K. Schwinghammer, B.V. Lotsch. A hydrazone-based covalent organic framework for photocatalytic hydrogen production. Chem. Sci. 5(2014)2789–2793. DOI:10.1039/C4SC00016A |
| [49] | P. Kuhn, M. Antonietti, A. Thomas. Porous. covalent triazine-based frameworks prepared by ionothermal synthesis. Angew. Chem. Int. Ed. 47(2008)3450–3453. DOI:10.1002/(ISSN)1521-3773 |
| [50] | S. Dalapati, S. Jin, J. Gao, et al., An azine-linked covalent organic framework. J. Am. Chem. Soc. 135(2013)17310–17313. DOI:10.1021/ja4103293 |
| [51] | Z.J. Li, S.Y. Ding, H.D. Xue, et al., Synthesis of -C=N linked covalent organic frameworks via the direct condensation of acetals and amines. Chem. Commun. 52(2016)7217–7220. DOI:10.1039/C6CC00947F |
| [52] | Q. Fang, Z. Zhuang, S. Gu, et al., Designed synthesis of large-pore crystalline polyimide covalent organic frameworks. Nat. Commun. 5(2014)4503. |
| [53] | W. Zhang, P. Jiang, Y. Wang, et al., Bottom-up approach to engineer two covalent porphyrinic frameworks as effective catalysts for selective oxidation. Catal. Sci. Technol. 5(2015)101–104. DOI:10.1039/C4CY00969J |
| [54] | B. Nath, W.H. Li, J.H. Huang, et al., A new azodioxy-linked porphyrin-based semiconductive covalent organic framework with I2 doping-enhanced photoconductivity. CrystEngComm 18(2016)4259–4263. DOI:10.1039/C6CE00168H |
| [55] | Y. Du, H.S. Yang, J.M. Whiteley, et al., Ionic covalent organic frameworks with spiroborate linkage. Angew. Chem. Int. Ed. 55(2016)1737–1741. DOI:10.1002/anie.201509014 |
| [56] | Y. Zeng, R. Zou, Z. Luo, et al., Covalent organic frameworks formed with two types of covalent bonds based on orthogonal reactions. J. Am. Chem. Soc. 137(2015)1020–1023. DOI:10.1021/ja510926w |
| [57] | H. Wang, H. Ding, X. Meng, et al., Two-dimensional porphyrin-and phthalocyanine-based covalent organic frameworks. Chin. Chem. Lett. 27(2016)1376–1382. DOI:10.1016/j.cclet.2016.05.020 |
| [58] | L. Xia, Q. Liu. Lithium doping on covalent organic framework-320 for enhancing hydrogen storage at ambient temperature. J. Solid State Chem. 244(2016)1–5. |
| [59] | H. Wei, S. Chai, N. Hu, et al., The microwave-assisted solvothermal synthesis of a crystalline two-dimensional covalent organic framework with high CO2 capacity. Chem. Commun. 51(2015)12178–12181. DOI:10.1039/C5CC04680G |
| [60] | Z. Yang, D. Cao. Effect of Li doping on diffusion and separation of hydrogen and methane in covalent organic frameworks. J. Phys. Chem. C 116(2012)12591–12598. DOI:10.1021/jp302175d |
| [61] | J.H. Guo, H. Zhang, Z.P. Liu, et al., Multiscale study of hydrogen adsorption diffusion. and desorption on Li-doped phthalocyanine covalent organic frameworks, J. Phys. Chem. C 116(2012)15908–15917. |
| [62] | H.Y. Furukawa, O.M. Yaghi. Storage of hydrogen. methane, and carbon dioxide in highly porous covalent organic frameworks for clean energy applications, J. Am. Chem. Soc. 131(2009)8875–8883. |
| [63] | H. Lu, C. Wang, J. Chen, et al., A novel 3D covalent organic framework membrane grown on a porous alpha-Al2O3 substrate under solvothermal conditions. Chem. Commun. 51(2015)15562–15565. DOI:10.1039/C5CC06742A |
| [64] | M. Shan, B. Seoane, E. Rozhko, et al., Azine-linked covalent organic framework (COF)-based mixed-matrix membranes for CO2/CH4 separation. Chemistry 22(2016)14467–14470. DOI:10.1002/chem.201602999 |
| [65] | Z.J. Yin, S.Q. Xu, T.G. Zhan, et al., Ultrahigh volatile iodine uptake by hollow microspheres formed from a heteropore covalent organic framework. Chem. Commun (2017). DOI:10.1039/c7cc01045a |
| [66] | Q. Fang, S. Gu, J. Zheng, et al., 3D microporous base-functionalized covalent organic frameworks for size-selective catalysis. Angew. Chem. Int. Ed. 53(2014)2878–2882. DOI:10.1002/anie.v53.11 |
| [67] | S. Lin, C.S. Diercks, Y.B. Zhang, et al., Covalent organic frameworks comprising cobalt porphyrins for catalytic CO2 reduction in water. Science 349(2015)1208–1213. DOI:10.1126/science.aac8343 |
| [68] | S.Y. Ding, J. Gao, Q. Wang, et al., Construction of covalentorganic framework for catalysis:Pd/COF-LZU1 in Suzuki-Miyaura coupling reaction. J. Am. Chem. Soc. 133(2011)19816–19822. DOI:10.1021/ja206846p |
| [69] | H.S. Xu, S.Y. Ding, W.K. An, et al., Constructing crystalline covalent organic frameworks from chiral building blocks. J. Am. Chem. Soc. 138(2016)11489–11492. DOI:10.1021/jacs.6b07516 |
| [70] | L. Yang, D.C. Wei. Semiconducting covalent organic frameworks:a type of twodimensional conducting polymers. Chin. Chem. Lett. 27(2016)1395–1404. DOI:10.1016/j.cclet.2016.07.010 |
| [71] | L. Ma, S. Wang, X. Feng, et al., Recent advances of covalent organic frameworks in electronic and optical applications. Chin. Chem. Lett. 27(2016)1383–1394. DOI:10.1016/j.cclet.2016.06.046 |
| [72] | H. Ding, Y. Li, H. Hu, et al., A tetrathiafulvalene-based electroactive covalent organic framework. Chem. Eur. J. 20(2014)14614–14618. DOI:10.1002/chem.v20.45 |
| [73] | S. Wan, F. Gándara, A. Asano, et al., Covalent organic frameworks with high charge carrier mobility. Chem. Mater. 23(2011)4094–4097. DOI:10.1021/cm201140r |
| [74] | S. Wan, J. Guo, J. Kim, et al., A belt-shaped blue luminescent. and semiconducting covalent organic framework, Angew. Chem. Int. Ed. 47(2008)8826–8830. |
| [75] | X. Ding, J. Guo, X. Feng, et al., Synthesis of metallophthalocyanine covalent organic frameworks that exhibit high carrier mobility and photoconductivity. Angew. Chem. Int. Ed. 50(2011)1289–1293. DOI:10.1002/anie.v50.6 |
| [76] | B.T. Koo, P.G. Berard, P. Clancy. A kinetic monte carlo study of fullerene adsorptionwithin a Pc-PBBA covalent organic framework and implications for electron transport. J. Chem. Theory Comput. 11(2015)1172–1180. DOI:10.1021/ct501044u |
| [77] | D.B. Shinde, H.B. Aiyappa, M. Bhadra, et al., A mechanochemically synthesized covalent organic framework as a proton-conducting solid electrolyte. J. Mater. Chem. A 4(2016)2682–2690. DOI:10.1039/C5TA10521H |
| [78] | H. Ma, B. Liu, B. Li, et al., Cationic covalent organic frameworks:a simple platform of anionic exchange for porosity tuning and proton conduction. J. Am. Chem. Soc. 138(2016)5897–5903. DOI:10.1021/jacs.5b13490 |
| [79] | C.R. DeBlase, K.H. Burgos, K.E. Silberstein, et al., Rapid and efficient redox processes within 2D covalent organic framework thin films. ACS Nano 9(2015)3173–3183. |
| [80] | C.R. DeBlase, K.E. Silberstein, T.T. Truong, et al., β-Ketoenamine-linked covalent organic frameworks capable of pseudocapacitive energy storage. J. Am. Chem. Soc. 135(2013)16821–16824. DOI:10.1021/ja409421d |
| [81] | C.R. Mulzer, L. Shen, R.P. Bisbey, et al., Superior charge storage and power density of a conducting polymer-modified covalent organic framework. ACS Cent. Sci. 2(2016)667–673. DOI:10.1021/acscentsci.6b00220 |
| [82] | H. Liao, H. Ding, B. Li, et al., Covalent-organic frameworks:potential host materials for sulfur impregnation in lithium-sulfur batteries. J. Mater. Chem. A 2(2014)8854–8858. DOI:10.1039/C4TA00523F |
| [83] | H. Liao, H. Wang, H. Ding, et al., A 2D porous porphyrin-based covalent organic framework for sulfur storage in lithium-sulfur batteries. J. Mater. Chem. A 4(2016)7416–7421. DOI:10.1039/C6TA00483K |
| [84] | Z. Li, Y. Zhang, H. Xia, et al., A robust and luminescent covalent organic framework as a highly sensitive and selective sensor for the detection of Cu2+ ions. Chem. Commun. 52(2016)6613–6616. DOI:10.1039/C6CC01476C |
| [85] | G. Lin, H. Ding, D. Yuan, et al., A pyrene-based. fluorescent three-dimensional covalent organic framework, J. Am. Chem. Soc. 138(2016)3302–3305. |
| [86] | J. Li, X. Yang, C. Bai, et al., A novel benzimidazole-functionalized 2D COF material:synthesis and application as a selective solid-phase extractant for separation of uranium. J. Colloid Interface Sci. 437(2015)211–218. DOI:10.1016/j.jcis.2014.09.046 |
| [87] | S. Zhang, X. Zhao, B. Li, et al., Stereoscopic 2D super-microporous phosphazene-based covalent organic framework:design. synthesis and selective sorption towards uranium at high acidic condition, J. Hazard. Mater. 314(2016)95–104. |
| [88] | C. Zhang, G. Li, Z. Zhang. A hydrazone covalent organic polymer based microsolid phase extraction for online analysis of trace Sudan dyes in food samples. J. Chromatogr. A 1419(2015)1–9. DOI:10.1016/j.chroma.2015.09.059 |
| [89] | M. Wu, G. Chen, P. Liu, et al., Polydopamine-based immobilization of a hydrazone covalent organic framework for headspace solid-phase microextraction of pyrethroids in vegetables and fruits. J. Chromatogr. A 1456(2016)34–41. DOI:10.1016/j.chroma.2016.05.100 |
| [90] | M. Wu, G. Chen, J. Ma, et al., Fabrication of cross-linked hydrazone covalent organic frameworks by click chemistry and application to solid phase microextraction. Talanta 161(2016)350–358. DOI:10.1016/j.talanta.2016.08.041 |
| [91] | C.X. Yang, C. Liu, Y.M. Cao, et al., Facile room-temperature solution-phase synthesis of a spherical covalent organic framework for high-resolution chromatographic separation. Chem. Commun. 51(2015)12254–12257. DOI:10.1039/C5CC03413B |
| [92] | Q. Fang, J. Wang, S. Gu, et al., 3D porous crystalline polyimide covalent organic frameworks for drug delivery. J. Am. Chem. Soc. 137(2015)8352–8355. DOI:10.1021/jacs.5b04147 |
| [93] | C. Liu, W. Zhang, Q. Zeng, et al., A photoresponsive surface covalent organic framework:surface-confined synthesis. isomerization, and controlled guest capture and release, Chemistry 22(2016)6768–6773. |
| [94] | A. Rengaraj, P. Puthiaraj, Y. Haldorai, et al., Porous covalent triazine polymer as a potential nanocargo for cancer therapy and imaging. ACS Appl. Mater. Interface 8(2016)8947–8955. DOI:10.1021/acsami.6b00284 |
 2017, Vol. 28
2017, Vol. 28 


