b Department of Chemistry, Faculty of Science, University of Maragheh, Maragheh, Iran;
c Dr. SSBhatnagar, University Institute of Chemical Engineering & Technology(SSB UICET), Panjab University, Chandigarh 160-014, India;
d Advanced Analysis Center, Korea Institute of Science and Technology, Seoul 136-791, South Korea
In recent years, there is a growing interest to the controlled synthesis of Cu2O nanocrystals with uniform morphology not only for the development of synthetic strategies, but also for the comprehensive study of the effect of the crystal plane on the surface chemistry and catalytic properties of Cu2O catalysts [1-6]. Furthermore, Cu2O nanocrystals are relatively easy to make, inexpensive, readily available, safe, and has low toxicity and good environmental acceptability, which favors the investigation of their properties and applications in catalysis [6-10].
Huang et al. reported the correlation between the morphology and catalytic activity of Cu2O nanocrystals in propylene oxidation, CO oxidation and the photocatalytic degradation of dyes [11-18]. These and many other examples clearly illustrate the importance of shape and morphology control to the efficient application of Cu2O nanocrystals [19-21].
The Cu(Ⅰ)-catalyzed azide-alkyne cycloaddition (CuAAC) as most famous click reaction are efficient method for the synthesis of 1, 4-disubstituted 1, 2, 3-triazoles that have tremendous applications in many areas such as polymers, drug discovery, and advanced material science, etc. [22-28]. In recent years, our group have reported some work on catalytic application of copperbased catalysts for azide-alkyne cycloaddition and achieved some meaningful results [29-31]. However, due to the complicated interaction between Cu species with ligands or supports, the Cu-based catalysts are not ideal model catalysts to study the relationships between structures and activities. Therefore, in this work, uniform Cu2O nanocrystals, including rhombic dodecahedrons, spheres, octahedrons and cubes were synthesized according to well-established procedures with some modification [13, 32]. We also report detailed structural characterization and the crystalplane effects on the catalytic properties in the 1, 3-dipolar cycloaddition of organic azides and alkynes. The catalytic performances of Cu2O nanocrystals normalized to the percentage of the exposed Cu atom were followed the order d-Cu2O > o-Cu2O~c-Cu2O > s-Cu2O.
2. Results and discussionThe Cu2O nanocubes, octahedra, spheres and truncated rhombic dodecahedra were synthesized in aqueous solutions following previously reported procedures [13, 32]. Exact amounts of CuCl2 solution, SDS surfactant, NaOH solution, and NH2OH·HCl as a reducing reagent were added in the sequence listed, and the mixture was aged for 1 h at 40 ℃ for growth the Cu2O nanocrystals.
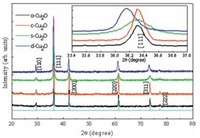
X-ray diffraction (XRD) was used to investigate the structures and phase purities of Cu2O nanocrystals. Fig. 1 shows typical diffraction patterns of the Cu2O nanopowders with no other crystalline impurities of CuO or metallic Cu, which fairly match with that of cubic bulk Cu2O (JC-PDS file No. 78-2076) [33].
|
Download:
|
| Figure 1. XRD patterns of the Cu2O nanocrystals synthesized with various morphologies. | |
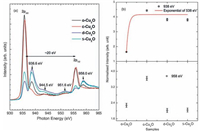
In order to probe the 3d character of Cu in the Cu2O samples, the Cu L-edge XANES spectra were collected from all Cu2O nanocrystals with cubic, octahedral, spheres and truncated rhombic dodecahedral structures. According to Fig. 2, it is clear that all samples shows two distinct peaks at ~935.6 eV and ~955.6 eV which are assigned to the transitions of Cu2p3/2 (L3) and 2p1/2 (L2) electrons into the empty d-states, respectively. The difference among L3 and L2, which is determined by the spin-orbit coupling, has value of ~20 eV. The post-L3-edge structures at ~938.6, 944.5 and 951.6 eV are the characteristics of Cu2O and relevant with previous reports [34]. Similarly, post L2-edge structure at 958.0 eV in Cu2O is reported in previous published reports [34]. It is seen that shape and intensity of various spectral features of o-Cu2O and c-Cu2O resembles to that of reported Cu2O [34]. Thus, it appears that only c-Cu2O represents Cu+1 states. Drastic changes for these parameters for s-Cu2O and d-Cu2O reflect the possibility of different oxidation state of Cu. It is reported that Cu2 p3/2 peak appear at 1 eV and 2.5 eV lower photon energy for Cu metal and CuO than that of Cu2O except the significant change of energy [34]. In this case, no energy shift is observed for this edge, hence, copper ions has +1 oxidation state in s-Cu2O and d-Cu2O samples too. It is shown that intensity of Cu2p3/2 peak is associated with density of states of d-character at the bottom of conduction band, distorted by strong core-hole potential [34]. Thus modifications of dcharacter seem to be associated with low intense Cu2p3/2 peaks of s-Cu2O and d-Cu2O. The structures at 938.6 and 958.0 eV also exhibit intensity variations. To study the intensity variation of these structures, XANES spectra is de-convoluted in Fig. S1 in Supporting information as shown in Fig. 2b. The intensity of spectral feature at 938.6 eV is almost thrice for c-Cu2O, s-Cu2O and d-Cu2O to that of o-Cu2O. The intensity for c-Cu2O is maximum for structure appearing at 958.0 eV and almost same for rest of the samples.
|
Download:
|
| Figure 2. (a) The Copper L-edge XANES spectra of the Cu2O nanocrystals synthesized with various morphologies; (b) reflects the intensity variations of structures appearing at 938.6 and 958eV. | |
Figs. 3 and 4 show the SEM and TEM images of typically synthesized Cu2O nanocubes with sizes of 500-600 nm, octahedral with sizes of 250-350 nm, rhombic dodecahedra with sizes of 0.7-1.1 mm and spheres with sizes of 0.8-1.3 mm respectively. Cubes and octahedral are more uniformly-sized, whereas rhombic dodecahedra and spheres have larger size distributions.

|
Download:
|
| Figure 3. SEM images of different morphologies of c-Cu2O (a), o-Cu2O (b), s-Cu2O (c), d-Cu2O (d). | |

|
Download:
|
| Figure 4. TEM images of different morphologies of c-Cu2O (a), o-Cu2O (b), s-Cu2O (c), d-Cu2O (d). | |
To compare the catalytic performance of all Cu2O nanocrystals, cycloaddition reaction of benzyl chloride, phenyl acetylene and NaN3 to synthesize 1-benzyl-4-phenyl-1, 2, 3-traizole were investigated in present of water as the standard "green" solvent without any additive under air at 70 ℃. The specific cycloaddition reaction rate of benzyl chloride, phenyl acetylene and NaN3 normalized to the percentage of the exposed Cu atoms for various Cu2O nanocrystals followed the order d-Cu2O > o-Cu2O~c-Cu2O > s-Cu2O (Table 1). It took 120 min of reaction for the Cu2O spheres to attain a product yield of 91%, but when Cu2O nanocubes and octahedra were used, the product yield was improved to an excellent yield of 97% at 120 min. Remarkably, the use of Cu2O rhombic dodecahedra rhombic dodecahedra as a catalyst significantly reduced the time of reaction to just 90 min with a yield of 97%. The above results clearly confirm that the morphology of Cu2O nanocrystals controls their catalytic activity.
|
|
Table 1 Comparison of catalytic potential of different Cu2O nanocrystals for the synthesis of 1-benzyl-4-phenyl-1, 2, 3-traizole. |
The morphology effect of Cu2O nanocrystals on the product yield was also studied (Fig. 5). Results clearly demonstrated that on the basis of per unit mass of catalyst, the crystal-plane-controlled catalytic performance of Cu2O nanocrystals in the 1, 3-dipolar cycloaddition follows the order octahedral > cubes > rhombic dodecahedra~ spheres. The above results clearly confirm that the morphology of Cu2O nanocrystals controls their catalytic activity.

|
Download:
|
| Figure 5. The morphology effect of Cu2O nanocrystals for the synthesis of 1-benzyl-4-phenyl-1, 2, 3-traizole. | |
To illustrate the scope of this method, we extended the cycloaddition reaction of a wide variety of terminal alkynes and organic halides in presence of d-Cu2O, o-Cu2O and c-Cu2O. In most cases similar results were obtained (Table 2). First, various benzyl chlorides/bromides and phenyl acetylenes with the substitution of electron withdrawing and electron donating groups on the phenyl ring were investigated and did not have any appreciable influence on the outcome of the reaction (entries 1-17). Furthermore, steric hindrance of the ortho substituents on the benzyl chloride had little effect on the reaction yields (entries 2 and 3). As can be seen from Table 1, in case of deactivated alkynes such as propargyl alcohol and 2-methyl-3-butyn-2-ol, when benzyl bromide was used under the similar reaction conditions, the corresponding products were obtained in higher yields than the case of benzyl chloride (entries 7, 8 and 17, 18). On the other hand, t-butyl bromide as a deactivated halide reacted nicely with various alkynes to obtain the corresponding 1, 4-disubstituted triazoles in excellent yields (entries 13-15).
|
|
Table 2 Cycloaddition of benzyl halides with terminal alkynes in the presence of d-Cu2O, o-Cu2O and c-Cu2O. |
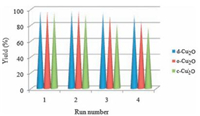
The recyclability of catalyst is an important and critical topic in the field of heterogeneous catalysis. Therefore the recyclability of the d-Cu2O, c-Cu2O and o-Cu2O as catalysts was also surveyed (Fig. 6). After completing one run of reaction, the reaction mixture was centrifuged and another cycle of the reaction was carried out using the same particles. The results revealed that catalysts could be reused at least four times without appreciable dramatic yield loss.
|
Download:
|
| Figure 6. Recycling studies of d-Cu2O, c-Cu2O and o-Cu2O catalysts in azide–alkyne cycloaddition. | |
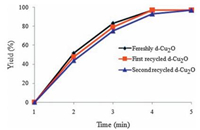
Expectedly, in presence of the recovered d-Cu2O, the reaction rate slightly decreased as compared to fresh d-Cu2O (Fig. 7).
|
Download:
|
| Figure 7. Reaction kinetic plots by using the fresh and recovered d-Cu2O catalyst, respectively. | |
The SEM images of the recycled catalysts after the fourth cycle show some morphological and surface changes and therefore these changes and also small losses of catalyst mass (10%-15% of the total mass of catalyst) during washing procedure may be caused in the low diminution in the activity from the first to the fourth cycle (Fig. 8).

|
Download:
|
| Figure 8. SEM images of a) d-Cu2O; b) o-Cu2O and c) c-Cu2O after azide–alkyne cycloaddition. | |
To further confirm the structures and phase purities of recycled Cu2O nanocrystals, XRD patterns of the recycled nanocrystals were recorded (Fig. 9). The XRD patterns of the recycled d-Cu2O and c-Cu2O showed only peaks of the Cu2O nanopowders with no impurities, but recycled o-Cu2O indicated mixed phases of Cu2O and CuO, probably arising from slight oxidation of CuI during cycloaddition reaction.

|
Download:
|
| Figure 9. XRD patterns of the recycled Cu2O nanocrystals. | |
To investigate heterogeneous nature of the d-Cu2O, c-Cu2O and o-Cu2O nanocrystals, the cycloaddition of benzyl chloride, NaN3 and phenylacetylene after hot filtration was done and it was observed that no product is formed after separation of nanocrystals and therefore they act heterogeneously in the reaction. Also, ICP analysis was employed to be sure that no leaching of copper took place during cycloaddition reaction.
3. ConclusionFor the first time, Cu2O nanocubes, octahedra, rhombic dodecahedra and icosahedra have been used as catalysts in the 1, 3-dipolar cycloaddition reaction of benzyl chloride, phenyl acetylene and NaN3 for the generation of 1, 4-disubstituted triazoles. The catalytic performance of Cu2O nanocrystals on the basis of the exposed Cu atoms followed the order d-Cu2O > o-Cu2O~c-Cu2O > s-Cu2O. A wide variety of triazoles with excellent yields have been synthesized, demonstrating that o-Cu2O and c-Cu2O are broadly useful and highly efficient catalysts for the azidealkyne cycloaddition reaction. Also these nanocrystals are recyclable catalysts at least four times.
4. Experimental 4.1. ChemicalsAnhydrous copper(Ⅱ) chloride, sodium hydroxide, SDS, and hydroxylamine hydrochloride were purchased from Aldrich and Merck. All chemicals were used as received without further purification.
4.2. Cu2O nanocrystal synthesisFor the synthesis of Cu2O nanocrystals with cubic, octahedral, spheres and truncated rhombic dodecahedral structures, 5 mL of 0.1 mol/L CuCl2 solution and 0.87 g of SDS powder were respectively added to four beakers containing 89.2, 83.2, 78.2, and 69.2 mL of deionized water with vigorous stirring. After complete dissolution of SDS powder, 1.8 mL of 1.0 mol/L NaOH solution was added. Finally, 4.0, 10.0, 15.0 and 24.0 mL of 0.1 mol/L NH2OH.HCl were quickly injected into beakers respectively. The solutions were kept in the water bath for 1 h at 40 ℃ to growth of nanocrystals. The product was collected by centrifugation, washed by excessive water and ethanol for several times to remove unreacted chemicals and SDS surfactant, and finally dried in vacuum at 50 ℃.
4.3. General procedure for azide-alkyne cycloadditionTo a solution of Cu2O (1 mg) in H2O (2 mL) were added alkyne (0.5 mmol), the organic halide (0.55 mmol) and NaN3 (0.55 mmol). The reaction mixture was warmed to 70 ℃ continuing stirring with monitoring by TLC until total conversion of the starting materials. After addition of water (3 mL), the resulting mixture was extracted with EtOAc (2×10 mL). The collected organic phases were dried with anhydrous CaCL2 and the solvent was removed in vaccum to give the corresponding triazoles, which did not require any further purification.
AcknowledgmentsM. Bagherzadeh and M. Amini thank the Iranian National Science Foundation (INSF), Sharif University of Technology and University of Maragheh for financial supports of this work.
Appendix A. Supplementary dataSupplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.cclet.2017.01.022.
| [1] | Kuo C.H., Huang M.H.. Facile synthesis of Cu2O nanocrystals with systematic shape evolution from cubic to octahedral structures. J. Phys. Chem.C 112 (2008) 18355–18360. DOI:10.1021/jp8060027 |
| [2] | Geng Z., Zhang Y., Yuan X., et al., Incorporation of Cu2O nanocrystals into TiO2 photonic crystal for enhanced UV? visible light driven photocatalysis. J. Alloys Compd. 644 (2015) 734–741. DOI:10.1016/j.jallcom.2015.05.075 |
| [3] | Liang X.D., Gao L., Yang S.W., Sun J.. Facile synthesis and shape evolution of single-crystal cuprous oxide. Adv. Mater. 21 (2009) 2068–2071. DOI:10.1002/adma.v21:20 |
| [4] | Yao K.X., Yin X.M., Wang T.H., Zeng H.C.. Synthesis self-assembly, disassembly, and reassembly of two types of Cu2O Nanocrystals unifaceted with {001} or {110} planes. J. Am. Chem. Soc. 132 (2010) 6131–6144. DOI:10.1021/ja100151f |
| [5] | Lan X., Zhang J.Y., Gao H., Wang T.M.. Morphology-controlled hydrothermal synthesis and growth mechanism of microcrystal Cu2O. Cryst. Eng. Commun. 13 (2011) 633–636. DOI:10.1039/C0CE00232A |
| [6] | Golden T.D., Shumsky M.G., Zhou Y.C., et al., Electrochemical deposition of copper(Ⅰ) oxide films. Chem. Mater. 8 (1996) 2499–2504. DOI:10.1021/cm9602095 |
| [7] | Liu R., Kulp E.A., Oba F., et al., Epitaxial electrodeposition of high-aspect-ratio Cu2O(110) nanostructures on InP(111). Chem. Mater. 17 (2005) 725–729. DOI:10.1021/cm048296l |
| [8] | Zhao H.Y., Wang Y.F., Zeng J.H.. Hydrothermal synthesis of uniform cuprous oxide microcrystals with controlled morphology. Cryst. Growth Des. 8 (2008) 3731–3734. DOI:10.1021/cg8003678 |
| [9] | Li J., Shi Y., Cai Q., et al., Patterning of nanostructured cuprous oxide by surfactant-assisted electrochemical deposition. Cryst. Growth Des. 8 (2008) 2652–2659. DOI:10.1021/cg070266i |
| [10] | Bao H.Z., Zhang W.H., Shang D.L., et al., Shape-dependent reducibility of cuprous oxide nanocrystals. J. Phys. Chem.C 114 (2010) 6676–6680. DOI:10.1021/jp101617z |
| [11] | Bao H., Zhang W., Hua Q., et al., Crystal-plane-controlled surface restructuring and catalytic performance of oxide nanocrystals. Angew. Chem. Int. Ed. 50 (2011) 12294–12298. DOI:10.1002/anie.v50.51 |
| [12] | Hua Q., Cao T., Bao H.Z., Jiang Z.Q., Huang W.X.. Crystal-plane-controlled surface chemistry and catalytic performance of surfactant-free Cu2O nanocrystals. Chemsuschem 6 (2013) 1966–1972. DOI:10.1002/cssc.v6.10 |
| [13] | Huang W.C., Lyu L.M., Yang Y.C., Huang M.H.. Synthesis of Cu2O nanocrystals from cubic to rhombic dodecahedral structures and their comparative photocatalytic activity. J. Am. Chem. Soc. 134 (2012) 1261–1267. DOI:10.1021/ja209662v |
| [14] | Zhang Y., Deng B., Zhang T.R., Gao D.M., Xu A.W.. Shape effects of Cu2O polyhedral microcrystals on photocatalytic activity. J. Phys. Chem.C 114 (2010) 5073–5079. DOI:10.1021/jp9110037 |
| [15] | Ho J.Y., Huang M.H.. Synthesis of submicrometer-sized Cu2O crystals with morphological evolution from cubic to hexapod structures and their comparative photocatalytic activity. J. Phys. Chem.C 113 (2009) 14159–14164. DOI:10.1021/jp903928p |
| [16] | Xu Y., Wang H., Yu Y.F., et al., Cu2O nanocrystals:surfactant-free roomtemperature morphology-modulated synthesis and shape-dependent heterogeneous organic catalytic activities. J. Phys. Chem.C 115 (2011) 15288–15296. DOI:10.1021/jp204982q |
| [17] | Li L.L., Nan C.Y., Peng Q., Li Y.D.. Selective synthesis of Cu2O nanocrystals as shape-dependent catalysts for oxidative arylation of phenylacetylene. Chem. Eur. J. 18 (2012) 10491–10496. DOI:10.1002/chem.v18.34 |
| [18] | Chanda K., Rej S., Huang M.H.. Facet-dependent catalytic activity of Cu2O nanocrystals in the one-pot synthesis of 1, 2, 3-triazoles by multicomponent click reactions. Chem. Eur. J. 19 (2013) 16036–16043. DOI:10.1002/chem.201302065 |
| [19] | Zou W.X., Liu L.C., Zhang L., et al., Crystal-plane effects on surface and catalytic properties of Cu2O nanocrystals for NO reduction by CO. Appl. Catal. A Gen. 505 (2015) 334–343. DOI:10.1016/j.apcata.2015.08.021 |
| [20] | Chanda K., Rej S., Huang M.H.. Investigation of facet effects on the catalytic activity of Cu2O nanocrystals for efficient regioselective synthesis of 3, 5-disubstituted isoxazoles. Nanoscale 5 (2013) 12494–12501. DOI:10.1039/c3nr03790h |
| [21] | Zhang H., Ren X., Cui Z.L.. Shape-controlled synthesis of Cu2O nanocrystals assisted by PVP and application as catalyst for synthesis of carbon nanofibers. J. Cryst. Growth 304 (2007) 206–210. DOI:10.1016/j.jcrysgro.2007.01.043 |
| [22] | Padwa A.. 3-Dipolar Cycloaddition Chemistry, Wiley, New York: 1984 . |
| [23] | Ul Islam R., Taher A., Choudhary M., Witcomb M.J., Mallick K.. A polymer supported Cu(Ⅰ) catalyst for the 'click reaction' in aqueous media. Dalton Trans. 44 (2015) 1341–1349. DOI:10.1039/C4DT02962C |
| [24] | Donnelly K.F., Petronilho A., Albrecht M.. Application of 1, 2, 3-triazolylidenes as versatile NHC-type ligands:synthesis, properties, and application in catalysis and beyond. Chem. Commun. 49 (2013) 1145–1159. DOI:10.1039/C2CC37881G |
| [25] | Thirumurugan P., Matosiuk D., Jozwiak K.. Click chemistry for drug development and diverse chemical-biology applications. Chem. Rev. 113 (2013) 4905–4979. DOI:10.1021/cr200409f |
| [26] | Gonda Z., Novák Z.. Highly active copper-catalysts for azide-alkyne cycloaddition. Dalton Trans. 39 (2010) 726–729. DOI:10.1039/B920790M |
| [27] | Becer C.R., Hoogenboom R., Schubert U.S.. Click chemistry beyond metalcatalyzed cycloaddition. Angew. Chem. Int. Ed. 48 (2009) 4900–4908. DOI:10.1002/anie.v48:27 |
| [28] | Moses J.E., Moorhouse A.D.. The growing applications of click chemistry. Chem. Soc. Rev. 36 (2007) 1249–1262. DOI:10.1039/B613014N |
| [29] | Amini M., Bayrami A., Marashi M.N., et al., Synthesis structure, and catalytic properties of copper, palladium and cobalt complexes containing an N, O-type bidentate thiazoline ligand. Inorg. Chim. Acta 443 (2016) 22–27. DOI:10.1016/j.ica.2015.12.015 |
| [30] | Faraji M., Amini M., Anbari A.P.. Preparation and characterization of TiO2-nanotube/Ti plates loaded Cu2O nanoparticles as a novel heterogeneous catalyst for the azide-alkyne cycloaddition. Catal. Commun. 76 (2016) 72–75. DOI:10.1016/j.catcom.2016.01.002 |
| [31] | Akbari A., Arsalani N., Amini M., Jabbari E.. Cube-octameric silsesquioxanemediated cargo copper Schiff baseforefficient clickreaction inaqueous media. J. Mol. Catal. A Chem. 414 (2016) 47–54. DOI:10.1016/j.molcata.2015.12.022 |
| [32] | Kuo C.H., Huang M.H.. Morphologically controlled synthesis of Cu2O nanocrystals and their properties. Nano Today 5 (2010) 106–116. DOI:10.1016/j.nantod.2010.02.001 |
| [33] | Wang Z.L., Liu Y.X., Martin D.J., et al., CuOx-TiO2 junction:what is the active component for photocatalytic H2 production?. Phys. Chem. Chem. Phys. 15 (2013) 14956–14960. DOI:10.1039/c3cp52496e |
| [34] | Sharma A., Varshney M., Park J., et al., XANES, EXAFS and photocatalytic investigations on copper oxide nanoparticles and nanocomposites. RSC Adv. 5 (2015) 21762–21771. DOI:10.1039/C4RA16217J |
 2017, Vol. 28
2017, Vol. 28