b Department of Biomedical Science, College of Natural Science, Catholic University of Daegu, Gyeungsan-Si 38430, South Korea
Inflammation is an early protective reaction of a host, initiated after infection and/or injury. This complex but highly coordinated process results in the increased production of various soluble mediators including chemokines, cytokines, free radicals (such as nitric oxide (NO)), and eicosanoids (prostaglandins) by resident cells (that is, tissue macrophages, lymphocytes, fibroblasts, endothelial cells and mast cells) in the injured or infected tissue [1]. Recently, inflammatory related diseases treatments are mainly associated with interrupting the synthesis or action of these mediators. NO, a multifunctional gaseous free radical, is one of the key signaling molecules that is synthesized from the amino acid Larginine by nitric oxide synthase (endothelial-NOS, neuronal-NOS and inducible-NOS). It regulates various physiological and pathophysiological processes and its role in the pathogenesis of inflammation tightly depends upon its concentration [2]. Prolonged and overproduction of NO could potentially result in tissue damage and activation of proinflammatory mediators associated with acute and chronic inflammations such as rheumatoid arthritis, asthma, diabetes, stroke, cancer and neurodegenerative disorders [3]. Hence, suppression of No overproduction represents a beneficial therapeutic strategy.
Nonsteroidal anti-inflammatory drugs (NSAIDs) which act on cyclooxygenase (COX-1 and -2), classical steroidal anti-inflammatory drugs (SAIDs), antihistamines and selective COX-2 inhibitors (COXIBs) are generally used to treat a wide spectrum of inflammatory diseases. Although these small-molecule inhibitors have provided some relief to patients suffering from pain and inflammation, they are not without their shortcomings. Therefore, there is a need for the identification and development of safe, effective and novel anti-inflammatory agents.
Isoflavones, as members of the flavonoid family, are important bioactive secondary metabolites of plants, chiefly, found in legumes (Fabaceae) such as soya, chick pea and fenugreek. Generally, these compounds exist as their glycosides. Their beneficial health effects are known for a long time. Nowadays, soy products/soy isoflavones (such as genistein, daidzein and glycitein) are very common in human diet for their various presumed health benefits [4]. The broad spectrum biological activities of isoflavones include anti-inflammatory [5], antioxidant [6], anticancer [7], antiviral [8], antifungal [9], antibacterial [10], anticataracts [11] and antifertility [12]. Apart from this, some isoflavones are investigated as kinase inhibitors [13]. They are also effective in human obesity and have a positive influence on plasma cholesterol [14]. Low rates of prostate and breast cancers incidents among Asians when compared to people in the Western world could be related to the difference in consumption of isoflavones in Asian diets (15-47 mg/day) compared with Western diets (0.15-1.7 mg/day) [15]. In recent decades, research on isoflavone and related compounds has been received considerable attention around the globe due to their ample occurrence in nature, accessible structural modifications, effortless synthesis and multifarious biological activities.
As part of our continuing research efforts focused on the synthesis and biological evaluation of bioactive natural products and their analogues as potential NO inhibitors [16], herein, we describe the first synthesis of naturally occurring C-methylisoflavones isosideroxylin (1), 6, 8-dimethylgenistein (2) and their derivatives (3-8) (Fig. 1) and preliminary investigation of their NO inhibitory potency in lipopolysaccharide (LPS) macrophage cell line RAW264.7 as an indicator of anti-inflammatory effect. Compound 1 was isolated from Leiophyllum buxifolium and displayed a selective antiproliferative effect against MDAMB-231 cells with an IC50 value of 7.0 μmol/L and also showed weak inhibition against the MCF-7 cells [17]. Compound 2 was isolated from Henriettella fascicularis and showed significant competitive binding to estrogen receptor-β with an IC50 value of 0.88 μmol/L and moderate antiestrogenic activity with cultured Ishikawa cells [18].

|
Download:
|
| Figure 1. Structures of isosideroxylin (1), 6, 8-dimethylgenistein (2) and their analogues (3-8). | |
2. Results and discussion 2.1. Chemistry
Our retrosynthetic analysis of isoflavones 1-8 is outlined in Scheme 1. We envisioned a selective or complete O-demethylation of 3, 9-13 which in turn obtained by a Suzuki coupling between 3-iodochromone 14 and corresponding boronic acids results the target compounds. Compound 14 can be easily accessed from a fully functionalized acetophenone, 15 by Gammill's protocol [19] which involves the condensation of 15 with DMF-DMA followed by iodine-mediated ring closure of the resulting enaminoketone. Compound 15 can be conveniently prepared from phloroglucinol (16) by 4 step synthetic sequence comprising Vilsmeier-Haack reaction, reduction, methylation and Friedel-Crafts acylation.

|
Download:
|
| Scheme 1. Retrosynthetic analysis of 1-8. | |
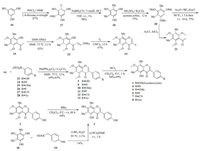
Thus, our synthetic approach commenced with the Vilsmeier- Haack reaction of phloroglucinol (16) (Scheme 2). Treatment of 16 with 2 equiv. Vilsmeier reagent generated in situ from DMF and POCl3 afforded dialdehyde 17 in 87% yield. Facile reduction of two carbonyl groups gave compound 18 using a Clemmensen reduction type condition. Reaction of 17 with 5 equiv. sodium cyanoborohydride/HCl (3 mol/L) system in THF and methyl orange as an indicator offered 18 in almost quantitative yield (98%). Previously, two methods reported to prepare 18, however, these processes were plagued by low yields, harsh conditions and usage of toxic reagent [20]. When we tried the one-pot reaction between 18 and 2-(4-hydroxyphenyl)acetic acid (19) followed by treatment with PCl5/DMF as a model reaction, compound 2 was obtained in low yield (14%) [21]. Next, Friedel-Crafts acylation over 18 using AcCl or Ac2O in presence of boron trifluoride diethyl etherate (BF3·Et2O) or AlCl3 led to only the O-acetylation product as major compound. To circumvent this detrimental effect of the hydroxy groups, 18 was converted as 20 using dimethyl sulfate. Now, compound 20 was subjected to Friedel-Crafts acylation using AcCl and AlCl3. Reaction in diethyl ether and THF solvents gave acetylated product 21 in 51% and 76%, respectively, whereas with Ac2O/BF3·Et2O in solvent-free condition the desired 2'-hydroxyacetophenone 15 was obtained in 75% yield. Subsequently, compound 15 was applied to the Gammill's method to generate iodochromone 14 via enaminoketone 22 formation. Condensation of 15 with N, N-dimethylformamide dimethyl acetal (DMF-DMA) gave 22 in 92% yield. Iodinemediated tandem cyclization and iodination of the enaminoketone 22 in CHCl3 produced the 3-iodochromone 14 in 67% yield.
|
Download:
|
| Scheme 2. Synthesis of isosideroxylin (1), 6, 8-dimethylgenistein (2) and their analogues (3-8). | |
With the requisite precursor 14 in hand, next we executed the Suzuki coupling to achieve 1-8. Treatment of 14 with 1.5 equiv. of boronic acids 23-28, 1.5 equiv. of Cs2CO3 and 0.1 equiv. of Pd (PPh3)2Cl2 in sealed tube at 75 ℃ led to the formation of isoflavones 3 and 9-13 in 69%-85% yields. Finally, we were delighted to observe that the selective ortho-demethylation of 3 and 9-13 using 1.0 mol/L boron trichloride solution in CH2Cl2 at 0 ℃ for 1 h gave isosideroxylin (1) and analogues 4-8 in 94%-99% yields.
Another natural isoflavone, 6, 8-dimethylgenistein (2) was obtained from 3 by complete O-demethylation using 1.0 mol/L boron tribromide solution in CH2Cl2. All the products 1-8 were structurally confirmed by their spectral data (1H NMR & 13C NMR and MS).
2.2. PharmacologyThe synthesized isoflavonoids 1-8 were examined for their capacity to inhibit the production of NO in murine RAW 264.7 macrophage cells stimulated with LPS. As we know that NO is a highly reactive molecule with very short half-life ( < 1 s in blood) in the presence of O2 and other scavenging molecules and hence difficult to quantify [22]. Nitrite (NO2-) and nitrate (NO3-), the stable oxidation end products of NO, are measured spectrophoto-metrically by employing the acidic Griess reagent as an index of systemic NO production. Cell viability was determined by the MTT assay and NG-monomethyl-L-arginine acetate (L-NMMA) [23] was employed as positive control. Initially, cells were pretreated with compounds 1-8 at 0.1, 1, 10, 25, 50 and 100 μmol/L concentrations for 12 h followed by activation with LPS (500 ng/mL) for an additional 18 h. However, significant NO inhibition activity changes were observed at 1 μmol/L-10 μmol/L. At 25, 50 and 100 μmol/L concentrations, compounds 1-8 exhibited almost same level activity. Hence, we discussed the activity at 1 μmol/L and 10 μmol/L concentrations only. As shown in Table 1, all the isoflavonoids (1-8) had a concentration-dependent inhibition on NO production. At the lowest concentration (1 μmol/L), compound 2 (80.53 ± 3.07) followed by compounds 3 (86.79 ±1.58) and 4 (88.78 ± 2.49) released the lower amount of NO that means the better NO inhibition potency compared to the positive control, LNMMA (88.88 ± 9.32). At the highest concentration (10 μmol/L), compound 3 (51.58 ± 2.27) followed by compounds 1 (66.34 ± 3.46) and 6 (67.69 ± 5.33) released the comparable amount of NO with L-NMMA (40.95 ± 5.98).
|
|
Table 1 NO production inhibitory effects of isosideroxylin (1), 6, 8-dimethylgenistein (2) and their analogues (3-8). |
Next, the cytotoxicity of isoflavones against RAW 264.7 cells viability was analyzed to ensure that cell death was not responsible for the decreased NO production. Incubation with 1 μmol/L and 10 μmol/L concentrations of isoflavones for 24 h did not cause any significant viability changes compared to the control group which indicates that the compounds exerted no cytotoxic effect and do not affect normal cell growth (Table 1). The IC50 values of 1-8 were evaluated by using GraphPad Prism 4.0 software and the values were 13.2, 10.17, 20.09, 20.89, 33.88, 21.93, 13.21 and 14.67μmol/L, respectively compared to L-NMMA (7.82 μmol/L). Overproduction of NO is due to the iNOS high expression, hence, we studied the ability of compounds 1-8 to modulate the LPS-induced iNOS expression. As shown in Fig. 2, expression was markedly attenuated in RAW 264.7 macrophages by pretreatment with 3 which was also correlated with the NO measurement (Table 1). Therefore, the reduced expression of iNOS due to this compound exposure was responsible for the significant inhibition of NO production. Based on these pharmacological results, it can be concluded that compound 3 which strongly inhibited LPSstimulated NO production without significant cytotoxicity could be deserved as a promising NO production inhibitor agent for further investigation.

|
Download:
|
| Figure 2. Effects of compounds 1-8 on iNOS expression (Western blot). | |
3. Conclusion
This work highlights the first concise and efficient synthesis of naturally occurring bioactive C-methylisoflavones isosideroxylin (1), 6, 8-dimethylgenistein (2) and their derivatives (3-8) in 7-8 steps with an overall yields of 16%-24% from commercially available precursors. In this strategy Vilsmeier-Haack reaction, Friedel-Crafts acylation, Gammill's protocol and Suzuki coupling reactions were applied as the key steps to realize the synthesis. Subsequently, compounds 1-8 were assayed for their potential to inhibit the NO production in LPS-induced RAW 264.7 macrophage cells. All the compounds reduced NO production in a concentration-dependent manner and did not show obvious cytotoxic effect at the highest concentration (10 μmol/L) leading to the effective inhibition with IC50 values ranged from 10.17 to 33.88μmol/L. Of note, compounds 3 (IC50 = 10.17 μmol/L) followed by 1 (IC50 = 13.2 μmol/L), 7 (IC50 = 13.21 μmol/L) and 8 (IC50 = 14.67μmol/L) showed comparable inhibitory activity with L-NMMA (IC50 = 7.82 μmol/L), used as positive control. This modular approach could also be used for the synthesis of other C-methylisoflavone analogues.
4. ExperimentalAll chemicals were obtained from commercial suppliers and were used without further purification unless stated otherwise. All solvents used for reactions were freshly distilled from proper dehydrating agents under nitrogen atmosphere. All solvents used for chromatography were purchased and directly used without further purification. 1H NMR spectra were recorded on a Varian Mercury-300 MHz FT-NMR and 75 MHz for 13C, with the chemical shift (δ) reported in parts per million (ppm) downfield relative to TMS and the coupling constants (J) quoted in Hz. Peak splitting patterns were abbreviated as s (singlet), d (doublet), t (triplet), q (quartet), dd (doublet of doublet) and m (multiplet) and CDCl3/CD3OD/acetone-d6/DMSO-d6 were used as a solvent and an internal standard. Low resolution mass spectra (electron ionization, EI) were recorded using Agilent-5977E spectrometer. High resolution mass spectra (electron ionization, EI) were recorded on a JMS-700 (JEOL, Japan) spectrometer. Melting points were measured on a MEL-TEMP Ⅱ apparatus and were uncorrected. Thin-layer chromatography (TLC) was performed on DC-Plastikfolien 60, F254 (Merck, layer thickness 0.2 mm) plastic-backed silica gel plates and visualized by UV light (254 nm) or staining with panisaldehyde and phosphomolybdic acid (PMA) stain. Chromatographic purification was carried out using Kieselgel 60 (60- 120 mesh, Merck).
4.1. General procedure for Suzuki couplingTo a 15 mL sealed tube equipped with a magnetic stir bar were added the iodo compound 14 (0.07 g, 0.2 mmol, 1.0 equiv.), boronic acid (23-28) (1.5 equiv.), Cs2CO3 (0.10 g, 0.3 mmol, 1.5 equiv.), and N, N-dimethylformamide (2 mL) and the mixture was degassed for 2 min. Pd(PPh3)2Cl2 (0.01 g, 0.02 mmol, 0.1 equiv.) was added to the mixture and degassed for another 2 min. The reaction was then stirred at 75 ℃ for 12 h. After cooling to room temperature, water (10 mL) was added and extracted with ether (3 ×15 mL). The combined organic layer was washed with H2O (3 ×10 mL), brine (2 ×10 mL), dried over anhyd. Na2SO4 and concentrated in vacuo. The crude was purified by column chromatography (EtOAc/ hexane = 1/5-1/3) to afford the pure isoflavone product (3, 9-13).
4.2. General procedure for ortho-demethylation of isoflavone using boron trichlorideTo a stirred solution of isoflavone (3, 9-13) (0.12 mmol, 1.0 equiv.) in anhydrous CH2Cl2 (3 mL) at 0 ℃ was added boron trichloride (0.6 mL, 1.0 mol/L in CH2Cl2, 5.0 equiv.) dropwise under nitrogen atmosphere and stirred for 1 h. After completion of the reaction, excess reagent was quenched with MeOH (1 mL) and extracted with CH2Cl2 (2 ×15 mL). Combined organic layer washed with H2O (2 ×10 mL), brine (2 ×10 mL), dried over anhyd. Na2SO4 and concentrated in vacuo. The crude was purified by column chromatography (EtOAc/hexane = 1/5-1/3) to afford the pure product (1, 4-8).
Physical and spectroscopic characterization data of the compounds and experimental procedures described in this article were given in Supporting information.
AcknowledgmentsThis research was financially supported by industrial co-work program with Sun Chem(Sun Kyung Chemical Co., South Korea).
Appendix A. Supplementary dataSupplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.cclet.2016.12.041.
| [1] | Fullerton J.N., Gilroy D.W.. Resolution of inflammation:a new therapeutic frontier. Nat. Rev. Drug Discov. 15 (2016) 551–567. DOI:10.1038/nrd.2016.39 |
| [2] | Bogdan C.. Nitric oxide and the immune response. Nat. Immunol. 2 (2001) 907–916. DOI:10.1038/ni1001-907 |
| [3] |
(a) A. J. Duncan, S. J. R. Heales, Nitric oxide and neurological disorders, Mol. Aspects Med. 26(2005) 67-96; (b) K. Bian, F. Murad, Nitric oxide (NO)-biogeneration, regulation, and relevance to human diseases, Front Biosci. 8(2003) d264-d278. |
| [4] | Gilbert E.R., Liu D.. Anti-diabetic functions of soy isoflavone genistein:mechanisms underlying its effects on pancreatic b-cell function. Food Funct. 4 (2013) 200–212. DOI:10.1039/C2FO30199G |
| [5] |
(a) J. Yu, X. Bi, B. Yu, D. Chen, Isoflavones: anti-inflammatory benefit and possible caveats, Nutrients 8(2016) 361; (b) B. H. Kim, E. Y. Chung, B. -K. Min, et al. , Anti-inflammatory action of legume isoflavonoid sophoricoside through inhibition on cyclooxygenase-2 activity, Planta Med. 69(2003) 474-476. |
| [6] |
(a) C. E. Rüfer, S. E. Kulling, Antioxidant activity of isoflavones and their major metabolites using different in vitro assays, J. Agric. Food Chem. 54(2006) 2926-2931; (b) C. H. Lee, L. Yang, J. Z. Xu, et al. , Relative antioxidant activity of soybean isoflavones and their glycosides, Food Chem. 90(2005) 735-741. |
| [7] |
(a) S. Andres, K. Abraham, K. E. Appel, A. Lampen, Risks and benefits of dietary isoflavones for cancer, Crit. Rev. Toxicol. 41(2011) 463-506; (b) Y. Kwon, Effect of soy isoflavones on the growth of human breasttumors: findings from preclinical studies, Food Sci. Nutr. 2(2014) 613-622. |
| [8] | Andres A., Donovan S.M., Kuhlenschmidt M.S.. Soy isoflavones and virus infections. J. Nutr. Biochem. 20 (2009) 563–569. DOI:10.1016/j.jnutbio.2009.04.004 |
| [9] | Krämer R.P., Hindorf H., Jha H.C., et al., Antifungal activity of soybean and chickpea isoflavones and their reduced derivatives. Phytochemistry 23 (1984) 2203–2205. DOI:10.1016/S0031-9422(00)80520-8 |
| [10] |
(a) C. Morel, F. R. Stermitz, G. Tegos, K. Lewis, Isoflavones as potentiators of antibacterial activity, J. Agric. Food Chem. 51(2003) 5677-5679; (b) M. Sato, H. Tanaka, N. Tani, et al. , Different antibacterial actions of isoflavones isolated from Erythrina poeppigiana against methicillin-resistant Staphylococcus aureus, Lett. Appl. Microbiol. 43(2006) 243-248. |
| [11] | Varma S.D., Mikuni I., Kinoshita J.H.. Flavonoids as inhibitors of lens aldose reductase. Science 188 (1975) 1215–1216. DOI:10.1126/science.1145193 |
| [12] | Moersch G.W., Morrow D.F., Neuklis W.A.. The antifertility activity of isoflavones related to genistein. J. Med. Chem. 10 (1967) 154–158. DOI:10.1021/jm00314a005 |
| [13] | Ogawara H., Akiyama T., Watanabe S.I., et al., Inhibition of tyrosine protein kinase activity by synthetic isoflavones and flavones. J. Antibiot. 42 (1989) 340–343. DOI:10.7164/antibiotics.42.340 |
| [14] | Ørgaard A., Jensen L.. The effects of soy isoflavones on obesity. Exp. Biol. Med. 233 (2008) 1066–1080. DOI:10.3181/0712-MR-347 |
| [15] |
(a) T. Marugame, K. Katanoda, International comparisons of cumulative risk of breast and prostate cancer, from cancer incidence in five continents Vol. Ⅷ, Jpn. J. Clin. Oncol. 36(2006) 399-400; (b) A. H. Wu, R. G. Ziegler, A. ]M. Y. Nomura, et al. , Soy intake and risk of breast cancer in Asians and Asian Americans, Am. J. Clin. Nutr. 68(1998) 1437S-1443S; (c) S. Medjakovic, M. Mueller, A. Jungbauer, Potential health-modulating effects of isoflavones and metabolites via activation of PPAR and AhR, Nutrients 2(2010) 241-279. |
| [16] |
(a) K. Damodar, J. K. Kim, J. G. Jun, Synthesis and pharmacological properties of naturally occurring prenylated and pyranochalcones as potent antiinflammatory agents, Chin. Chem. Lett. 27(2016) 698-702; (b) Y. H. Seo, K. Damodar, J. K. Kim, J. G. Jun, Synthesis and biological evaluation of 2-aroylbenzofurans, rugchalcones A B and their derivatives as potent antiinflammatory agents, Bioorg. Med. Chem. Lett. 26(2016) 1521-1524. |
| [17] | Tian D., Porter J.R.. An isoflavone from Leiophyllum buxifolium and its antiproliferative effect. J. Nat. Prod. 78 (2015) 1748–1751. DOI:10.1021/acs.jnatprod.5b00100 |
| [18] | Calderón A.I., Terreaux C., Schenk K., et al., Isolation and structure elucidation of an isoflavone and a sesterterpenoic acid from Henriettella fascicularis. J. Nat. Prod. 65 (2002) 1749–1753. DOI:10.1021/np0201164 |
| [19] | Gammill R.B.. A new and efficient synthesis of 3-halogenated 4H-1-benzopyran-4-ones. Synthesis (1979) 901–903. |
| [20] |
(a) C. Dittmer, G. Raabe, L. Hintermann, Asymmetric cyclization of 2'-hydroxychalcones to flavanones: catalysis by chiral Brønsted acids and bases, Eur. J. Org. Chem. (2007) 5886-5898; (b) L. Shi, X. E. Feng, J. R. Cui, et al. , Synthesis and biological activity of flavanone derivatives, Bioorg. Med. Chem. Lett. 20(2010) 5466-5468. |
| [21] | Balasubramanian S., Nair M.G.. An efficient 'one pot' synthesis of isoflavones. Synth. Commun. 30 (2000) 469–484. DOI:10.1080/00397910008087343 |
| [22] | D. Giustarini, R. Rossi, A. Milzani, I. Dalle-Donne, Nitrite and nitrate measurement by Griess reagent in human plasma: Evaluation of interferences and standardization, in: E. Cadenas, L. Packer (Eds. ), Methods in Enzymology, Elsevier Inc. , Richmond, 2008, pp. 361-380. |
| [23] |
(a) L. Salerno, V. Sorrenti, C. Di Giacomo, G. Romeo, M. A. Siracusa, Progress in the development of selective nitric oxide synthase (NOS) inhibitors, Curr. Pharm. Des. 8(2002) 177-200; (b) J. L. Song, Y. Yuan, H. B. Tan, et al. , Euryachins A and B, a new type of diterpenoids from Eurya chinensis with potent NO production inhibitory activity, RSC Adv. 6(2016) 85958-85961; (c) C. A. Kontogiorgis, D. Hadjipavlou-Litina, Current trends in QSAR on NO donors and inhibitors of nitric oxide synthase (NOS), Med. Res. Rev. 22(2002) 385-418. |
 2017, Vol. 28
2017, Vol. 28