BaTiO3 has been widely used for its ferroelectricity at room temperature, high dielectric constant and low-loss. During the last decades, much effort has been carried out on controlling BaTiO3 in nanoscale. Nanopowders can be prepared through ball-milling, molten salts, sol-gel and hydrothermal processes. Among them, the last method has been well studied. For example, nanoparticles [1], nanowires [2, 3] and nanotubes [4] are prepared using TiO2 and Ba(OH)2·8H2O through this method. Ferroelectric and dielectric properties of nanosized BaTiO3 have also been studied. It was found that Curie temperature was significant increased [5]. The ferroelectric phases were observed in the shells due to the complicated surface structure. Moreover, the dielectric constant decreased with decreasing particle size [6].
Single-handed helical nanofibers and nanotubes have been prepared through both self-templating and external templating approaches using chiral low-molecular-weight gelators (LMWGs) [7, 8]. The TiO2 nanotubes and tubular nanoribbons prepared through the external templating approach exhibit interested optical activity and asymmetric catalysis property [9–12]. The optical activity is proposed to originate from the chiral defect on the inner surfaces of the tubular structures. A post-preparation approach has been developed by us for the preparation of LiTaO3 tubular nanoribbons with optical activity using Ta2O5 tubular nanoribbons as starting materials [13]. Herein, single-handed helical BaTiO3 nanotubes were prepared using TiO2 nanotubes as starting materials. The BaTiO3 nanotubes exhibited optical activity and dielectric properties.
2. Results and discussionThe single-handed helical TiO2 nanotubes were prepared through a supramolecular templating approach. The formation of them was shown as follows. First, the LMWGs self-assembled into single-handed twisted nanoribbons in the reaction mixture. Second, TiO2 nanoparticles adsorbed and polycondensed on the surfaces of the organic twisted nanoribbons. Finally, after removing the templates with ethanol, the TiO2 nanotubes were obtained. Single-handed helical BaTiO3 nanotubes were prepared through the hydrothermal process using the TiO2 nanotubes and Ba(OH)2·8H2O. Their FE-SEM and TEM images are shown in Fig. 1. The morphologies were almost kept after the impregnation of Ba2+ ions. The outer diameter and wall thickness are about 200–500 nm and 50 nm, respectively. These nanotubes are constructed by fine nanoparticles.

|
Download:
|
| Figure 1. (a) FE-SEM and (c) TEM images of P-BaTiO3. (b) FE-SEM and (d) TEM images of M-BaTiO3. | |
The WAXRD pattern of P-BaTiO3 is shown in Fig. 2a. The WAXRD pattern shows the pervoskite BaTiO3 with cubic/tetragonal symmetries. A broad peak is identified at 2θ=22.7°, indicating an amorphous structure. Therefore, P-BaTiO3 should be constructed by partially crystalline nanoparticles. Raman spectroscopy is used to identify the tetragonal or cubic phase structure of BaTiO3 (Fig. 2b) [14]. The band at about 715 cm-1 is related to the highest frequency longitudinal optical mode A1(LO). The bands at 185, 251, and 514 cm-1 are assigned to the fundamental TO mode of the A1 symmetry. The sharp band at 307 cm-1 indicates the tetragonal phase. Therefore, P-BaTiO3 should exhibits ferroelectric properties [5].

|
Download:
|
| Figure 2. (a) WAXRD pattern and (b) Raman spectrum of P-BaTiO3. | |
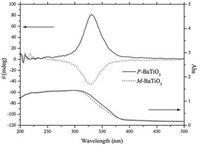
The DRCD and DRUV-vis spectra of M-BaTiO3 and P-BaTiO3 are shown in Fig. 3. Broad UV absorption bands at 200–383 nm were identified. The BaTiO3 nanotubes exhibited DRCD signals at approximately 330 nm, indicating that the chirality originated from the intrinsic electronic structure. It was reported that asprepared titania tubular nanoribbons exhibited DRCD signals at 320 nm [12]. The DRCD signals shifted to long wavelength with the molecular structural transition and crystallization. Because the tubular structures were formed through an external templating approach, chiral defects on the inner surfaces were proposed to be the origin of the optical activity of the BaTiO3 nanotubes.
|
Download:
|
| Figure 3. DRCD and DRUV-vis spectra of M-BaTiO3 and P-BaTiO3. | |
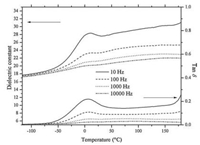
The dielectric constant and tanδ for P-BaTiO3 are plotted vs. temperature in Fig. 4. The data were collected under nitrogen as temperature increased from low to high. At 10 and 100 Hz, one dielectric constant peak at 9.6 ℃ and one tanδ peak at 5.0 ℃ are observed at -120 ℃ to 180 ℃. The values of the dielectric constants are significantly lower than the reported bulk BaTiO3 ceramics value [15]. The tanδ is lower than 0.3 at 10 Hz, and the tanδ is lower than 0.03 at 10, 000 Hz. Both the dielectric constant and tanδ decrease with increasing the frequency. The contribution of dipolar, electronic and ionic polarizations are proposed to drive the higher dielectric constant at lower frequencies.
|
Download:
|
| Figure 4. Plots of the dielectric constant and tanδ vs. temperature for P-BaTiO3. | |
3. Conclusion
Single-handed helical BaTiO3 nanotubes with optical activity were prepared using TiO2 through an impregnation approach. Chiral defects on the inner surfaces were proposed to be the origin of the optical activity of the BaTiO3 nanotubes. Both the dielectric constant and tanδ decrease with increasing the frequency. At 10 and 100 Hz, one dielectric constant peak at 9.6 ℃ and one tanδ peak at 5.0 ℃ are observed at -120 ℃ to 180 ℃.
4. ExperimentalCharacterization: Wide-angle X-ray diffraction (WAXRD) patterns were taken on an X'Pert-Pro MPD X-ray diffractometer using Cu Ka radiation with a Ni filter (1.542 Å). Raman spectra were obtained using a Renishaw Invia Raman microscope. The excitation wavelength was 532 nm. Field emission scanning electron microscopy (FE-SEM) images were obtained using a Hitachi 4800 instrument at 3.0 kV. Transmission electron microscopy (TEM) was performed using an FEI TecnaiG220 at 200 kV. The diffuse reflectance circular dichroism (DRCD) and diffuse reflectance ultraviolet-visible (DRUV-vis) spectra were obtained using a JASCO J-815 spectropolarimeter fitted with DRCD apparatus, with 10.0-nm bandwidth, 1.0-nm data pitch and 100-nm/min scan speed. Pellet of diameter 10 mm and thickness 1.18 mm are prepared from the BaTiO3 powder. The relative dielectric constant (εr) and the dielectric loss (tanδ) are measured using Novocontrol Concept 80 Broadband Dielectric Spectrometer.
Chemicals: The synthesis of the as-prepared TiO2 nanotubes has been reported previously [10]. Ba(OH)2·8H2O, ethanol and nitric acid were purchased from Sinopharm Chemical Reagent Co., Ltd. Deionized water was used in all the experiments.
Synthesis of single-handed twisted BaTiO3 nanotubes: The asprepared TiO2 nanotubes were put in a autoclave containing aqueous Ba(OH)2·8H2O solution. The sealed autoclave was then placed in an oven at 160 ℃ for 8 h. After cooling to room temperature, the solids were washed with dilute nitric acid, deionized water and ethanol and then dried overnight under vacuum at 40 ℃. The left-and right-handed helical BaTiO3 nanotubes were named as M-BaTiO3 and P-BaTiO3, respectively.
AcknowledgmentThis work was supported by the Priority Academic Program Development of Jiangsu High Education Institutions (PAPD).
| [1] | Eckert Jr. J.O., Hung-Houston C.C., Gersten B.L., Lencka M.M., Riman R.E.. Kinetics and mechanisms of hydrothermal synthesis of barium titanate. J. Am. Ceram. Soc. 79 (1996) 2929–2939. DOI:10.1111/jace.1996.79.issue-11 |
| [2] | Koka A., Zhou Z., Tang H.X., Sodano H.A.. Controlled synthesis of ultra-long vertically aligned BaTiO3 nanowire arrays for sensing and energy harvesting applications. Nanotechnology 25 (2014) 375603. DOI:10.1088/0957-4484/25/37/375603 |
| [3] | Koka A., Zhou Z., Sodano H.A.. Vertically aligned BaTiO3 nanowire arrays for energy harvesting. Energy Environ. Sci. 7 (2014) 288–296. DOI:10.1039/C3EE42540A |
| [4] | Lamberti A., Garino N., Bejtka K., et al., Synthesis of ferroelectric BaTiO3 tubelike arrays by hydrothermal conversion of a vertically aligned TiO2 nanotube carpet. New J. Chem. 38 (2014) 2024–2030. DOI:10.1039/C3NJ01138K |
| [5] | Li Y.L., Liao Z.Y., Fang F., et al., Significant increase of Curie temperature in nanoscale BaTiO3. Appl. Phys. Lett. 105 (2014) 182901. DOI:10.1063/1.4901169 |
| [6] | Goswami A.K.. Dielectric properties of unsintered barium titanate. J. Appl. Phys. 40 (1969) 619–624. DOI:10.1063/1.1657443 |
| [7] | Moreau J.J.E., Vellutini L., Man M.W.C., Bied C.. New hybrid organic-inorganic solids with helical morphology via H-bond mediated sol-gel hydrolysis of silyl derivatives of chiral (RR)-or (SS)-diureidocyclohexane. J. Am. Chem. Soc. 123 (2001) 1509–1510. DOI:10.1021/ja003843z |
| [8] | van Bommel K.J.C., Friggeri A., Shinkai S.. Organic templates for the generation of inorganic materials. Angew. Chem. Int. Ed. 42 (2003) 980–999. DOI:10.1002/anie.200390284 |
| [9] | Kobayashi S., Hamasaki N., Suzuki M., et al., Preparation of helical transitionmetal oxide tubes using organogelators as structure-directing agents. J. Am. Chem. Soc. 124 (2002) 6550–6551. DOI:10.1021/ja0260622 |
| [10] | Zhang C.Y., Wang S.B., Huo H.J., et al., Preparation of helical titania nanotubes using a sol-gel transcription approach. Mater. Lett. 88 (2012) 23–26. DOI:10.1016/j.matlet.2012.08.029 |
| [11] | Wang S.B., Li R., Zhang C.Y., et al., Formation and asymmetric catalysis of C-and 8-shaped titania tubes. Mater. Lett. 106 (2013) 71–74. DOI:10.1016/j.matlet.2013.05.009 |
| [12] | Wang S.B., Zhang C.Y., Li Y., Li B.Z., Yang Y.G.. Chirality of single-handed twisted titania tubular nanoribbons prepared through sol-gel transcription. Chirality 27 (2015) 543–550. DOI:10.1002/chir.v27.8 |
| [13] | Wang Y.Y., Huo H.J., Zhang C.Y., et al., Characterization of single-handed twisted lithium tantalate tubular nanoribbons with optical activity. Mater. Lett. 167 (2016) 125–127. DOI:10.1016/j.matlet.2015.12.148 |
| [14] | Ji B.T., Chen D.R., Jiao X.L., Zhao Z.Q., Jiao Y.X.. Preparation and electrical properties of nanoporous BaTiO3. Mater. Lett. 64 (2010) 1836–1838. DOI:10.1016/j.matlet.2010.05.026 |
| [15] | Zhang N., Li L.X., Yu J.Y.. High dielectric constant and good thermal stability from-55℃ to 450℃ in BaTiO3-based ceramics. Mater. Lett. 160 (2015) 128–131. DOI:10.1016/j.matlet.2015.07.106 |
 2017, Vol. 28
2017, Vol. 28 


