b Postovsky Institute of Organic Synthesis of RAS(Ural Division), Yekaterinburg 620990, Russian Federation;
c Department of Chemistry, Visva-Bharati(A Central University), Santiniketan 731235, India
Compounds containing the 2, 2'-bipyridine moieties are the most common ligands [1] for the transition metal cations. Moreover, 2, 2'-bipyridines having an extended conjugation system, for instance aryl-substituted ones, are of special interest. In this case it is possible to achieve the more desirable values for the both absorption and the emission maxima, namely red shifting [2-4], compare to the "naked" 2, 2'-bipyridine for which these two parameters are observed in a short-wave region [5].
In this article, the objects of the study are 5, 5'-diarylsubstituted 2, 2'-bipyridines. The main advantage of these compounds is the absence of aryl substituents in the a-position of the pyridine core which favors the more efficient chelation of metal cations. Similar compounds are widely used as electron transport materials for organic electronics [6] including the organic electroluminescent devices [7], as components for dye-sensitized solar cells [8], as ligands for transition metal cations [9], as well as building blocks for some macrocycles [10]. In addition, the properly situated aromatic substituents in 2, 2'-bipyridine core can be employed as spacers for the connection to other molecules like porphyrins [11].
2. Results and discussion 2.1. ChemistryThe most common approaches to synthesize 5, 5'-diaryl-2, 2'-bipyridines are the Pd-catalyzed cross-coupling reactions of 5, 5'-dibromo-2, 2'-bipyridine [12] or various 2-bromopyridines [13]. Very few other synthetic strategies were also used, such as Co(Ⅱ)-catalyzed cycloaddition reactions between nitriles and diynes [14] or Ni-catalyzed dimerization of 3-phenylpyridine [15]. Furthermore, some 1, 1'-phenanthroline analogues of 2, 2'-bipyridines were prepared via the concerted building up of two edge pyridine rings [16]. It is worth noting that most of the reported methods are devoted to obtain 5, 5'-diaryl-2, 2'-bipyridines bearing same aromatic substituents. Only in rare cases the preparation of non-symmetric diaryl-derivatives of 5, 5'-diaryl-2, 2'-bipyridines or their analogues were reported, for example, by the step-wise cross-coupling reactions involving two bromine atoms in 1, 1'-phenanthrolines [17] or 2, 2'-bipyridines [18].
On the other hand, some new approaches to synthesize different pyridine ligands have been previously reported by using the "1, 2, 4-triazine" methodology, by which it is possible to prepare a wide range of oligopyridines [19]. The possibility of getting wide variation of additional substituents of different types in the oligopyridine core which is important for tuning both photophysical and metal chelation properties of the obtained ligands is the main advantage of such methodology. In addition, the direct annulation of a cycloalkane core on the aryl-functionalized pyridine ring by means of aza-Diels-Alder reaction between 1, 2, 4-triazines and enamines is beneficial for increasing the solubility of the oligopyridine ligands and their metal complexes in organic solvents [20]. Apart from that the "1, 2, 4-triazine" methodology does not require any hardly available reagents, complicated and tedious synthetic procedures or special catalysts.
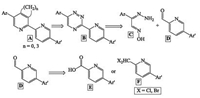
Previously we have reported a step-wise construction method to build up the target 2, 2'-pyridine core bearing aromatic substituents in unique positions by means of the sequence of several heterocyclization reactions [4, 21]. The obtained oligopyridines are hardly available via the common approaches. In the current manuscript we wish to report the use of "1, 2, 4-triazine" methodology for the synthesis of non-symmetrically functionalized 5, 5'-diaryl-2, 2'-bipyridines. Scheme 1 represents the retrosynthetic approach to obtain these ligands (compounds A). Thus, initially we proposed the use of 1, 2, 4-triazine analogues B as starting materials. Their syntheses, in turn, can be accomplished via the cyclocondensation reaction between isonitrosoacetophenone hydrazones C and 5-arylpyridine-2-carbaldehyde D. Next, two possible pathways can be used for obtaining aldehyde D: (ⅰ) the synthetic conversion of the corresponding carboxylic acid derivatives E or (ⅱ) the transformation of the corresponding dibromo-or dichloromethyl moiety in 5-arylpyridines F. The second approach seems to be the most logical one because of the smaller number of reaction steps. Moreover, this method has previously been reported for some similar aldehydes [22]. However, the synthetic approaches towards dibromo-or dichloromethyl-substituted arylpyridines F are limited by only direct chlorination [23] or bromination [24] reaction of the methyl group in the pyridine core. From the other hand the synthesis of 1, 2, 4-triazine precursor of the pyridine F (i.e. 3-dichloromethyl-6-phenyl-1, 2, 4-triazine) has been reported previously by means of the reaction between isonitrosoacetophenone hydrazone and the corresponding iminoether obtained in situ from dichloroacetonitrile [25].
|
Download:
|
| Scheme 1. Retrosynthetic approach of 5, 5'-diarylbipyridines A. | |
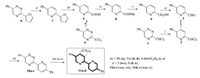
Therefore, we have employed this approach: 3-Dichloromethyl-1, 2, 4-triazine 1 was synthesized and the desired compound 2 was obtained in satisfied yields by aza-Diels-Alder reaction of 1 with 2, 5-norbornadiene (Scheme 2). At the next step, aiming to synthesize the desired aldehyde 3 we converted the a-dichloromethyl group in the pyridine core to the aldehyde one by means of either basic or acidic hydrolysis. However, the reaction failed to proceed in alkaline conditions. In acidic conditions, namely, after boiling in 80% propionic or aqueous hydrochloric acid no desired aldehyde 3 was detected in the reaction mixture. Only after boiling the compound 2 in 85% formic acid for 36 h product 3 was obtained in less than 30% yield according to the 1H NMR data, with marked tarring of the reaction mixture. Based on the reported above this synthetic approach was ruled out, and our further studies were directed to the realization of the alternative approach, starting from the corresponding pyridine carboxylic acid E.
|
Download:
|
| Scheme 2. Synthetic route for desired compounds. Reagents and conditions: i) 2, 5-norbornadiene, 1, 2-dichlorobenzene, reflux, 16 h; ii) 2, 5-norbornadiene, o-xylene, reflux, 16 h; iii) NaOH, KMnO4, water-pyridine (5:17), r.t., 2 h; then reflux, 10 min; iv) methanol, SOCl2, reflux, 15 h; v) NaBH4, ethanol, reflux, 2 h; vi) MnO2, 1, 2-dichloroethane, 50℃, 10 h; vii) corresponding hydrazone of isonitrosoacetophenone, ethanol, 10 h, r.t., then acetic acid, reflux, 5 min; viii) 1-morpholinocyclopentene, neat, 200℃, 3 h; then acetonitrile, 1 h, r.t.; ix) 2, 5-norbornadiene, o-xylene, reflux, 18 h. | |
5-Phenylpyridine-2-carboxylic acid 4 can be obtained from the 3-trichloromethyl-1, 2, 4-triazine 5 based on our previously reported method [4]. In addition, in this paper an alternative approach has been developed by starting from 3-(furan-2-yl)-6-phenyl-1, 2, 4-triazine 6 [26] by means of the transformation of the triazine ring into the pyridine one followed by conversion of the furan functionality into the carboxyl group.
The subsequent esterification reaction by means of refluxing 4 in methanol saturated with hydrogen chloride afforded the corresponding ester 8 quantitatively. After the following reduction and oxidation reactions the desired aldehyde 3 has been obtained in good yield. At the next step the heterocyclization reaction according to the previously published synthetic protocol [27] afforded the 1, 2, 4-triazine precursors 10 of target ligands in 55%-60% yields. The aza-Diels-Alder reaction between 10 and 1-morpholinocyclopentene or 2, 5-norbornadiene led to the target bipyridines 11, including the ones with annulated cyclopentene fragment (i.e. having the better solubility in organic solvents).
Structures of the obtained compounds were confirmed based on the 1H NMR, 13C NMR, mass-spectrometry and elemental analysis. In particular, in case of 1, 2, 4-triazine precursors 10 in the 1H NMR spectra the downfield singlets (9.44-9.49 ppm) of the protons at the C5 position of the 1, 2, 4-triazine cycle can be observed, along with the signals of protons of ABX system of pyridine rings and two sets of signals of protons of aromatic substituents. In case of products 11a-c the additional signals of protons of an annulated cyclopentene ring can be observed in the field corresponding to the resonance of aliphatic protons along with the signal of proton at the position of C3 of the 6, 7-dihydro-5H-cyclopenta[c]pyridine as a singlet in the area of 8.56-8.58 ppm. In case of compounds 11d, e two sets of proton signals of the two ABX systems of pyridine rings can be observed.
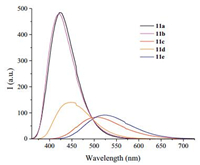
2.2. Photophysical propertiesIn order to estimate the influence of the nature of aromatic substituents the photophysical properties of the obtained 2, 2'-bipyridines 11 have been studied. Thus, in acetonitrile solutions all the compounds exhibited an intense emission in a range of ca. 422-521 nm, depending on the nature of the aromatic substituents and the presence of the annulated cyclopentene fragment (Table 1, Fig. 1, Fig. S1 in Supporting information). We compared the spectral data obtained for compounds 11 with those previously reported for some derivatives of 5-aryl-2, 2'-bipyridines and 5, 5'-disubstituted 2, 2'-bipyridines. In particular, it was demonstrated earlier [2] that both the absorption and emission maxima can be red-shifted due to the introduction of aryl substituents at the C5 position of 2, 2'-bipyridine core. The greater red-shift has been observed due to the introduction of the ester group at the C5' position [2].
|
|
Table 1 Photophysical properties of new bipyridines 11 and some previously reported analogues. |
|
Download:
|
| Figure 1. Emission spectra of bipyridines 11a–e in CH3CN at room temperature. | |
Even more significant red-shifts are observed for these 2, 2'-bipyridines 11, which are bearing an extra aryl substituent in the bipyridine core, especially for the ones not bearing the fused cyclopentene fragment. The largest bathochromic shift has been achieved for 4-methoxyphenyl-substituted bipyridine 11e: The bathochromic shift value in the emission maximum was up-to 122 nm compare to 5-monoaryl-substituted 2, 2'-bipyridine [2]. Moreover, in case of compounds 11a, b bearing the phenyl or ptolyl substituents the quantum yield values increased accordingly in comparison with monoaryl-substituted bipyridines [2].
Also in case of compounds 11, a significant bathochromic shift in the absorption maxima has been observed, for instance up 61 nm for the compound 11e.
In 2001, Loren and Siegel reported [28] the photophysical properties of mono-and dimanisyl-substituted 2, 2'-bipyridines (manisyl = 2, 6-dimethyl-4-methoxyphenyl), which are similar to these 2, 2'-bipyridines reported here. However, in our case the more significant bathochromic shift both the absorption and emission maxima is observed, while the fluorescence quantum yield values were comparable. Most probably, in case of manisylsubstituted bipyridines the conjugation of the aryl-bipyridine-aryl system is hampered due to the presence of bulky aromatic substituents in pyridine rings: the whole system is not flat and the conjugation is broken. On the other hand bis-(pyren-2-yl)-substituted 2, 2'-bipyridine [29] demonstrated the photophysical properties which are comparable to the properties of these reported compounds.
3. ConclusionIn summary, we have developed a convenient approach for the synthesis of 5, 5'-diaryl-2, 2'-bipyridines. This method does not require any special catalysts, complicated reaction conditions or expensive reagents. The notable advantages of this present methodology are the possibility of varying the aromatic substituents at the 5 and 50 positions of bipyridine core and the possibility for obtaining 5, 5'-diaryl-2, 2'-bipyridines bearing the fused cyclopentene cycle aiming the increasing of the solubility in organic solvents. The photophysical studies of the synthesized bipyridines have demonstrated the significant bathochromic shifts for the both absorption and emission maxima in comparison with a number of similar structures, which were previously described. In some cases the increasing of the fluorescence quantum yields is achieved.
4. Experimental 4.1. General method for the synthesis of triazines 10A solution of the corresponding hydrazone of isonitrosoacetophenone (1.36 mmol) in ethanol (25 mL) was added to a solution of aldehyde 3 (250 mg, 1.36 mmol) in ethanol (20 mL). The resulting mixture was stored at room temperature for 10 h. The resulting precipitate was filtered off, dried and suspended in glacial acetic acid (25 mL). The resulting mixture was heated to reflux for 5 times. After completion of the reaction, solvent was removed under reduced pressure. The residue was treated with ethanol. The resulting precipitate was filtered off, washed with ethanol and dried. Analytical samples were obtained by recrystallization from ethanol.
4.2. General method for the synthesis of bipyridines 11a-cA mixture of the corresponding triazine 10 (0.4 mmol) and 1-morpholinocyclopentene (0.32 mL, 2 mmol) was stirred at 200 ℃ for 2 h under argon atmosphere. Then the additional portion of 1-morpholinocyclopentene (0.16 mL, 1 mmol) was added and the resulting mixture was stirred for additional 1 h at the same conditions. The reaction mass was cooled to room temperature. Acetonitrile (20 mL) was added and the resulting mixture was stored for 1 h at room temperature. The resulting precipitate was filtered off, washed with acetonitrile and dried. The analytical samples were obtained by recrystallization from acetonitrile.
4.3. General method for the synthesis of bipyridines 11d, eThe corresponding triazine 10 (0.4 mmol) was suspended in oxylene (20 mL). 2, 5-Norbornadiene (0.16 mL, 1.6 mmol) was added and the resulting mixture was stirred under reflux condition for 9 h. Then the additional portion of 2, 5-norbornadiene (0.16 mL, 1.6 mmol) was added and the resulting mixture was stirred at reflux for additional 9 h. After completion solvent was removed under reduced pressure. The residue was purified by flash chromatography (mixture of DCM and ethylacetate (10:1) as eluent). Analytical samples were obtained by recrystallization from acetonitrile.
The full description of experimental procedures and full characterization for all new compounds are presented in the Supporting information.
AcknowledgmentThis work was supported by the Russian Science Foundation (No. 15-13-10033).
Appendix A. Supplementary dataSupplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.cclet.2016.12.043.
| [1] | (a) A. von Zelewsky, Stereochemistry of Coordination Compounds, Wiley, Chichester, 1996. |
| [2] | Kozhevnikov V.N., Shabunina O.V., Kopchuk D.S., et al., Facile synthesis of 6-aryl-3-pyridyl-12, 4-triazines as a key step toward highly fluorescent 5-substituted bipyridines and their Zn(Ⅱ) and Ru(Ⅱ) complexes. Tetrahedron 64 (2008) 8963–8973. DOI:10.1016/j.tet.2008.06.040 |
| [3] |
(a) F. Xiong, S. Q. Wang, L. M. He, et al. , Different photophysical properties of aryl-bipyridine linked pyrene and anthracene, Chin. J. Chem. 23(2005) 811-815; (b) A. H. Younes, L. Zhang, R. J. Clark, L. Zhu, Fluorescence of 5-arylvinyl-5'-methyl-2, 2'-bipyridyl ligands and their zinc complexes, J. Org. Chem. 74(2009) 8761-8772. |
| [4] | Kopchuk D.S., Chepchugov N.V., Kim G.A., et al., Preparation of 56-diaryl-2, 2-bipyridines using a 1, 2, 4-triazine methodology. Russ. Chem. Bull. 64 (2015) 897–900. DOI:10.1007/s11172-015-0951-1 |
| [5] | Harriman A.. Photophysics of 22'-bipyridyl. J. Photochem. 8 (1978) 205–209. DOI:10.1016/0047-2670(78)80020-3 |
| [6] | M. Kimura, A. Oda, Bipiridine Derivative and organic electroluminescence element containing the sam, US 2010327265A1, (2010). |
| [7] | Yokoyama N., Hayashi S., Izumi S., Kusano S.. Compound having substituted pyridyl group and pyridoindole ring structure linked through phenylene group, and organic electroluminescent device. EP (2010) 2241568A1. |
| [8] | Dai F.R., Wu W.J., Wang Q.W., Tian H., Wong W.Y.. Heteroleptic ruthenium complexes containing uncommon 55'-disubstituted-2, 2'-bipyridine chromophores for dye-sensitized solar cells. Dalton Trans. 40 (2011) 2314–2323. DOI:10.1039/C0DT01043J |
| [9] | Constable E.C., Housecroft C.E., Neuburger M., Rösel P.J., Schaffner S.. Diversification of ligand families through ferroin-neocuproin metal-binding domain manipulation. J. Chem. Soc. Dalton Trans. 25 (2009) 4918–4927. |
| [10] |
(a) D. M. Opris, A. Ossenbach, D. Lentz, A. D. Schlüter, A set of homologous hetarylenediyne macrocycles by oxidative acetylene-acetylene coupling, Org. Lett. 10(2008) 2091-2093; (b) J. R. Nitschke, S. Zu1rcher, T. Don Tilley, New zirconocene-coupling route to large, functionalized macrocycles, J. Am. Chem. Soc 122(2000) 10345-10352; (c) J. Sakamoto, A. D. Schlüter, Shape-persistent macrocycles: A synthetic strategy that combines easy and site-specific decorations with improved cyclization efficiency, Eur. J. Org. Chem. 16(2007) 270'-2712. |
| [11] | Bruce J.I., Chambron J.-C., Kölle P., Sauvage J.P.. Synthesis of a linear bisporphyrin with a Ru(phen)22+-complexed 2, 2'-bipyridine spacer. J. Chem. Soc. Perkin Trans. 10 (2002) 1226–1231. |
| [12] |
(a) H. J. Nie, J. Y. Shao, J. Wu, J. Yao, Y. W. Zhong, Synthesis and reductive electropolymerization of metal complexes with 5, 5'-divinyl-2, 2'-bipyridine, Organometallics 31(2012) 6952-6959; (b) B. N. Briggs, F. Durola, D. R. McMillin, J. P. Sauvage, Luminescence studies of copper(I)-containing[2] pseudorotaxanes, Can. J. Chem. 89(2011) 98-103. |
| [13] |
(a) S. Ladouceur, D. Fortin, E. Zysman-Colman, Role of substitution on the photophysical properties of 5, 5'-diaryl-2, 2'-bipyridine (bpy*) in[Ir (ppy)2(bpy*)]PF6 complexes: A combined experimental and theoretical study, Inorg. Chem. 49(2010) 5625-5641; (b) O. Henze, U. Lehmann, A. D. Schlüter, Synthesis of 5, 5ü-disubstituted 2, 2ü-bipyridines for modular chemistry, Synthesis 4(1999) 683-687. |
| [14] |
(a) A. Goswami, K. Ohtaki, K. Kase, T. Ito, S. Okamoto, Synthesis of substituted 2, 2'-bipyridines and 2, 2': 6', 2-terpyridines by cobalt-catalyzed cycloaddition reactions of nitriles and α, ω-diynes with exclusive regioselectivity, Adv. Synth. Catal. 350(2008) 143-152; (b) Y. Sugiyama, S. Okamoto, Regioselective syntheses of substituted pyridines and 2, 2'-bipyridines by cobalt-catalyzed[2+2+2] cycloaddition of α, ω-diynes with nitriles, Synthesis 14(2011) 2247-2254. |
| [15] | Donohoe R.J., Tait C.D., Dearmond M.K., Wertz D.W.. A spectroscopic study of some substituted tris(diimine) complexes of ruthenium(Ⅱ) and their reduction products. Spectrochim. Acta 42 (1986) 233–240. DOI:10.1016/0584-8539(86)80185-4 |
| [16] | Schmittel M., Ammon H.. A short synthetic route to 47-dihalogenated 1, 1'-phenanthrolines with additional groups in 3, 8-position:soluble precursors for macrocyclic oligophenanthrolines. Eur. J. Org. Chem. 5 (1998) 785–792. |
| [17] |
(a) J. Voignier, J. Frey, T. Kraus, et al. , Transition-metal-complexed cyclic[3]-and[4] pseudorotaxanes containing rigid ring-and-filament conjugates: Synthesis and solution studies, Chem. Eur. J. 17(2011) 5404-5414; (b) B. Champin, V. Sartor, J. P. Sauvage, A phen-terpy conjugate whose chelate coordination axes are orthogonal to one another and its zinc complex, New J. Chem. 30(2006) 22-25. |
| [18] | You Y.C., Tzeng M.C., Lai C.C., Chiu S.H.. Using oppositely charged ions to operate a three-station. Org. Lett. 14 (2012) 1046–1049. DOI:10.1021/ol203401d |
| [19] |
(a) A. M. Prokhorov, D. N. Kozhevnikov, Reactions of triazines and tetrazines with dienophiles, Chem. Heterocycl. Compd. 48(2012) 1153-1176; (b) G. R. Pabst, O. C. Pfüller, J. Sauer, The new and simple 'LEGO' System: Synthesis and reactions of ruthenium(Ⅱ) complexes, Tetrahedron 55(1999) 8045-8064; (c) A. Rykowski, D. Branowska, J. Kielak, A novel one-pot synthesis of annulated 2, 2'-bipyridine ligands by inverse electron demand Diels-Alder reaction of 5, 5'-bi-1, 2, 4-triazines, Tetrahedron Lett. 41(2000) 3657-3659; (d) I. S. Kovalev, D. S. Kopchuk, A. F. Khasanov, et al. , The synthesis of polyarenemodified 5-phenyl-2, 2'-bipyridines via the methodology and aza-Diels-Alder reaction, Mendeleev Commun. 24(2014) 117-118. |
| [20] |
(a) A. P. Krinochkin, D. S. Kopchuk, D. N. Kozhevnikov, Luminescent neutral lanthanide complexes of 5-aryl-2, 2'-bipyridine-6-carboxylic acids, synthesis and properties, Polyhedron 102(2015) 556-561; (b) D. S. Kopchuk, A. P. Krinochkin, D. N. Kozhevnikov, P. A. Slepukhin, Novel neutral lanthanide complexes of 5-aryl-2, 2'-bipyridine-6'-carboxylic acids with improved photophysical properties, Polyhedron 118(2016) 3'-36. |
| [21] |
(a) D. S. Kopchuk, N. V. Chepchugov, G. V. Zyryanov, et al. , An efficient synthetic approach to 4', 5, 5"-triaryl-2, 2': 6', 2"-terpyridines, Tetrahedron Lett. 57(2016) 296-299; (b) D. S. Kopchuk, N. V. Chepchugov, G. A. Kim, et al. , Synthesis of unsymmetric 6, 6-diaryl-2, 2-bipyridines using a 1, 2, 4-triazine methodology, Russ. Chem. Bull. 64(2015) 695-698; (c) D. S. Kopchuk, N. V. Chepchugov, O. S. Taniya, et al. , Effective synthetic approach to 4', 5-Diaryl-2, 2': 6', 2"-terpyridines, Russ. J. Org. Chem. 51(2015) 1162-1165. |
| [22] |
(a) K. Miyata, D. Schepmann, B. Wünsch, Synthesis and ü receptor affinity of regioisomeric spirocyclic furopyridines, Eur. J. Med. Chem. 83(2014) 709-716; (b) J. Corte, J. Hangeland, M. Quan, J. M. Smallheer, T. Fang, Six-membered heterocycles useful as serine protease inhibitors, WO2005/123680 A1, (2005); (c) G. C. Condie, J. Bergman, Reactivity of β-carbolines and cyclopenta[b] indolones prepared from the intramolecular cyclization of 5(4H)-oxazolones derived from L-tryptophan, Eur. J. Org. Chem. 6(2004) 1286-1297. |
| [23] | V. Farina, G. Li, J. Liu, et al. , Process for making heteroaryl amine intermediate compounds, WO2007/044490 A2, (2007). |
| [25] | Kozhevnikov D.N., Kataeva N.N., Rusinov V.L., Chupakhin O.N.. Chloromethyl-, dichloromethyl-, and trichloromethyl-1, 2, 4-triazines and their 4-oxides:Method for the synthesis and tele-substitution reactions with C-nucleophiles. Russ. Chem. Bull. 53 (2004) 1295–1300. DOI:10.1023/B:RUCB.0000042289.07168.8f |
| [26] | Saraswathi T.V., Srinivasan V.R.. Syntheses and spectral characteristics of 6-mono-, 3, 6-di-and 35, 6-trisubstituted-1, 2, 4-triazines. Tetrahedron 33 (1977) 1043–1051. DOI:10.1016/0040-4020(77)80223-8 |
| [27] | Kozhevnikov V.N., Kozhevnikov D.N., Shabunina O.V., Rusinov V.L., Chupakhin O.N.. An efficient route to 5-(hetero)aryl-24'-and 2, 2'-bipyridines throughreadilyavailable3-pyridyl-1, 2, 4-triazines. Tetrahedron Lett. 46 (2005) 1791–1793. DOI:10.1016/j.tetlet.2005.01.135 |
| [28] | Loren J.C., Siegel J.S.. Synthesis and fluorescence properties of manisylsubstituted terpyridine, bipyridine, and phenanthroline, Angew. Chem. Int. Ed. 40 (2001) 754–757. DOI:10.1002/1521-3773(20010216)40:4<>1.0.CO;2-X |
| [29] | Constable E.C., Neuburger M., Rösel P., et al., Ligand-based charge-transfer luminescence in ionic cyclometalated iridium(Ⅲ) complexes bearing a pyrene-functionalized bipyridine ligand:A joint theoretical and experimental study. Inorg. Chem. 52 (2013) 885–897. DOI:10.1021/ic302026f |
| [30] | Melhuish W.H.. Quantum efficiencies of fluorescence of organic substances:Effect of solvent and concentration of the fluorescent solute. J. Phys. Chem. 65 (1961) 229–235. DOI:10.1021/j100820a009 |
 2017, Vol. 28
2017, Vol. 28