b Department of Chemistry, Rasht Branch, Islamic Azad University, Rasht, Iran
Multi-component reactions (MCRs) have gained significant attention due to the one-pot reaction, atom-economy, and the possibility of introducing maximum diversity in the product in one chemical operation. These types of reactions leading to interesting heterocyclic scaffolds, are particularly useful for the construction of diverse chemical libraries of drug-like molecules [1].
Heterocycles are a widespread structural motif found as key elements of numerous drugs and designed medicinal agents in medicinal chemistry. Among them, sulfur containing heterocycles such as, thiomorpholines, thiazepines and thiazines are ubiquitous compounds and they exhibit a variety of biological properties such as antiarrhythmic, antihypertensive, antibacterial and antidiabetic [2]. In particular, heterocycles comprising nitrogen, oxygen, and sulfur atoms in one ring are very important in view of their biological applications. Among these, the oxathiazines have been used for a long time in agricultural and biological applications. They act as nematicides [3], herbicides, fungicides, plant desiccants and defoliants [4], antifouling agents [5], and wood preservatives [6]. Due to these properties, many methods have been reported for the synthesis of oxathiazine compounds [7].
Spirocyclic frameworks represent an important class of naturally compounds which have some biological properties [8]. Synthesis of this framework has always been a challenging for organic chemists because it often requires synthetic design based on specific strategies. Moreover in some cases the products are rearranged to other organic framework [9]. Due to the enormous importance of this class of compounds, chemists have been much attention to these spiro derivatives and a range of method has been reported for their synthesis [10-12].
Recently, reactivity and properties of novel heterocyclic compounds were reported using tetramethyl guanidine [13]. Herein, in the course of our research goal into the synthesis of new heterocyclic compounds [14], according to some work on (O, S)-ketal synthesis and on-water catalysis-free synthesis [15], we have been interested in this strategy for the synthesis of hitherto unreported heterocycles including nitrogen, oxygen, and sulfur atoms in one ring with spiro skeleton in water without the catalysts.
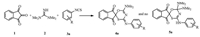
2. Results and discussionInitially, we planned to evaluate the reaction of ninhydrin (1), tetramethyl guanidine (2) and 4-chlorophenyl isothiocyanate (3a) in order to produce 4'-((4-chlorophenyl)amino)-6', 6'-bis(dimethylamino)-6'H-spiro[indene-2, 2'-[1, 3, 5]oxathiazine]-1, 3-dione (5a) at room temperature in ethanol. Surprisingly, it was found that, the spectral data of isolated product was not consistent with the expected derivative 5a. Indeed, the data was in good agreement with the structure of an unprecedented product 4'-((4-chlorophenyl)imino)-6'-(dimethylamino)-4'H-spiro[indene-2, 2'-[1, 3, 5] oxathiazine]-1, 3-dione 4a (Scheme 1).
|
Download:
|
| Scheme 1. The reaction leading to the synthesis of spiro[indene-oxathiazine] derivatives 4a. | |
The structure of compound 4a was elucidated by their mass, IR and high-field NMR and spectra and elemental analysis. The mass spectrum of 4a showed a molecular ion peak at m/z 399, which was consistent with the mass of a 1:1:1 condensation product of the three components having released HNMe2 molecule. The 1H NMR spectrum of the product exhibited a singlet integrating for six protons at δ 3.59 corresponding to the two methyl groups and clearly indicating that, there is only one -NMe2 group in the product. The aromatic region of the spectrum displayed separate signals for all the aromatic protons at the expected chemical shifts and with appropriate integral values. Further support for the structures of compounds 4a was obtained from the 13C NMR spectra of the product where a downfield resonance (e.g., 110.9 for 4a) was apparent corresponding to the presence of one spiro carbon in the molecule. Based on these features the product was identified as 4a.
In order to find the optimum conditions, we carried out the above reaction in various solvents and temperatures (Table 1). As shown in Table 1, the best results were obtained in H2O at room temperature and with the increase of temperature, yield of the 4a decreased and more spots appeared in TLC, probably due to side reactions and/or conversion of product to other by-products.
|
|
Table 1 Optimization of the reaction conditions for the formation of 4a.a |
After optimizing the conditions, the generality of the method was evaluated by using various isothiocyanate 3 bearing electronwithdrawing and electron-donating groups. The reactions are performed as clean as the model reaction and the desired products 4a-g were obtained in good to excellent yields (Table 2).
|
|
Table 2 Prepared spiro[indene-2, 2'-[1, 3, 5]oxathiazine]-1, 3-dione derivatives (4a–g).a |
We have not established the exact mechanism for the formation of 4, however, a reasonable suggestion is offered in Scheme 2. At first, nucleophilic attack of tetramethyl guanidine to isothiocyanate (3) leads to the formation of intermediate 6. Then nucleophilic attack of sulfur atom to middle carbonyl group of ninhydrin can afford intermediate 7 (route a) or 8 (route b). It seems the route b is the main route of reaction due to the higher donating effect of the nitrogen atoms bearing two methyl groups compared to the one bearing an aromatic moiety. Finally, cyclization of them produced intermediate 5. There is a singlet for six protons at δ 3.59 in the 1H NMR spectra shows that the reaction has gone a step ahead to produce a more stable products 4.

|
Download:
|
| Scheme 2. Proposed mechanism for preparation of spiro[indene-2, 2'-[1, 3, 5] oxathiazine]-1, 3-dione derivatives (4a–g). | |
3. Conclusion
We have reported a novel, clean and efficient synthesis of new spiro[indene-2, 2'-[1, 3, 5]oxathiazine derivatives 4 by a threecomponent reaction. Simple procedure for product isolation, lack of problems connected with conventional solvent use, good yields are the main features of this method. The products described in this article have two important biological active moieties therefore, synthetic and biological applications of compounds 4 can be considered in the near future.
4. ExperimentalAll starting materials were purchased from Merck (Germany) and Fluka (Switzerland) and were used without further purification. All products were identified with the following methods: Melting points: Electrothermal 9100 apparatus; Elemental analyses for C, H, and N: Heraeus CHN-O-Rapid analyzer; Mass spectra: Finnigan-Matt 8430 mass spectrometer operating at an ionization potential of 70 eV; 1H NMR and 13C NMR spectra: at 500 and 125 MHz, respextively, on a Bruker Advance DRX-500 MHz in CDCl3; IR Spectra: in KBr on a Shimadzu IR-460 spectrometer.
General procedure for the synthesis of spiro[indene-2, 2'-[1, 3, 5] oxathiazine]-1, 3-diones 4a-g: A solution of tetramethyl guanindine (1 mmol) and isothiocyanate derivatives (3a-g) (1 mmol) in water (2 mL) were magnetically stirred for 5 min to obtained intermediate 6. Then, ninhydrin (1 mmol) was added to reaction mixture and the solution was stirred for appropriate time at room temperature. The reaction products were monitored using thin layer chromatography (TLC). After completion of the reaction, the mixture was filtered, and the precipitate was washed with ether (4 mL) to afford the pure product 4a-g.
4'-((4-Chlorophenyl)imino)-6'-(dimethylamino)-4'H-spiro [indene-2, 2'-[1, 3, 5]oxathiazine]-1, 3-dione (4a, Table 2, entry a): Yield: 94%. Yellow powder; mp 126-128 ℃. IR (KBr, cm-1): vmax 1171, 1241, 1374, 1496, 1533, 1723; 1H NMR (500 MHz, CDCl3): δ 3.59 (s, 6H, NMe2), 7.39 (d, 2H, 3J=7.5 Hz, Ar), 7.64 (t, 2H, 3J=7.5 Hz, Ar), 7.91 (d, 2H, 3J=7.9 Hz, Ar), 8.04 (d, 2H, 3J=7.9 Hz, Ar); 13C NMR (125 MHz, CDCl3): δ 44.0, 110.9, 127.4, 127.5, 130.1, 130.7, 130.9, 133.3, 135.7, 164.5, 167.6, 195.8; MS (EI, 70 eV): m/z (%)=399 (M+, 48), 354 (25), 255 (43), 191 (69), 144 (100), 111 (80), 77 (95); Anal. Calcd. for C19H14ClN3O3S: C, 57.07; H, 3.53; N, 10.51. Found: C, 57.01; H, 3.44; N, 10.87.
6'-(Dimethylamino)-4'-(p-tolylimino)-4'H-spiro[indene-2, 2'-[1, 3, 5]oxathiazine]-1, 3-dione (4b, Table 2, entry b): Yield: 85%. Yellow powder; mp 131-133 ℃. IR (KBr, cm-1): vmax 1170, 1257, 1367, 1461, 1529, 1723; 1H NMR (500 MHz, CDCl3): δ 2.49 (s, 3H, Me), 3.88 (s, 6H, NMe2), 7.10 (d, 2H, 3J=7.6 Hz, Ar), 7.30 (t, 2H, 3J=7.6 Hz, Ar), 7.40 (d, 2H, 3J=7.8 Hz, Ar), 7.93 (d, 2H, 3J=7.8 Hz, Ar); 13C NMR (125 MHz, CDCl3): δ 22.2, 45.7, 110.9, 125.1, 126.1, 127.5, 129.2, 130.0, 130.3, 130.6. 162.5, 166.0, 195.2; MS (EI, 70 eV): m/z (%)=379 (M+, 66), 335 (46), 235 (59), 187 (96), 144 (80), 91 (88), 77 (100); Anal. Calcd. for C20H17N3O3S: C, 63.31; H, 4.52; N, 11.07. Found: C, 63.45; H, 4.39; N, 10.83.
Other data of compounds 4c-g are deposited in Supporting information.
AcknowledgementWe gratefully acknowledge financial support from the Research Council of Science and Research Branch, Islamic Azad University.
Appendix A. Supplementary dataSupplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.cclet.2016.12.036.
| [1] |
(a) J. Zhu, H. Bienayme, Multicomponent Reactions, Wiley-VCH, Weinheim, Germany, 2006; (b) A. Dömling, Recent developments in isocyanide based multicomponent reactions in applied chemistry, Chem. Rev. 106(2006) 17-89; (c) P. Lu, Y. Wang, Strategies for heterocyclic synthesis via cascade reactions based on ketenimines, Synlett (2010) 165-173; (d) B. Ganem, Strategies for innovation in multicomponent reaction design, Acc. Chem. Res. 42(2009) 463-472; (e) D. J. Ramón, M. Yus, Asymmetric multicomponent reactions (AMCRs), Angew. Chem. Int. Ed. 44(2005) 1602-1634. |
| [2] |
(a) R. Fringuelli, L. Milanese, F. Schiaffella, Role of 1, 4-benzothiazine derivatives in medicinal chemistry, Mini Rev. Med. Chem. 5(2005) 1061-1073; (b) H. Li, G. J. Dryhurst, Irreversible inhibition of mitochondrial complex I by 7-(2-aminoethyl)-3, 4-dihydro-5-hydroxy-2H-1, 4-benzothiazine-3-carboxylic acid (DHBT-1): a putative nigral endotoxin of relevance to parkinson's disease, Neurochemistry 69(1997) 153'-1541. |
| [3] | C. Giordano, M. Ferraris, E. Barsuglia. , Methods of combatting nematodes using certain derivatives of 1, 3, 5-oxathiazine, Montedison Fibre SPA, U. S. Patent 4, 035, 496(1977). |
| [4] |
(a) W. G. Brouwer, A. R. Bell, A. R. Blem, R. A. Davis, 3-Aryl-5, 6-dihydro-1, 4, 2-oxathiazines and their oxides, U. S. Patent 4, 569, 690, issued February 11(1986). (b) W. G. Brouwer, A. R. Bell, A. R. Blem, R. A. Davis, 3-Aryl-5, 6-dihydro-1, 4, 2-oxathiazines and their oxides, U. S. Patent 4, 675, 044, issued June 23(1987). (c) W. G. Brouwer, A. R. Bell, A. R. Blem, R. A. Davis, 3-Aryl-5, 6-dihydro-1, 4, 2-oxathiazines and their oxides, Eur. Patent 0, 104, 940 A1(1984). |
| [5] |
(a) J. F. E. Van Gestel, Antibacterial and antifouling oxathiazines and their oxides, U. S. Patent 5, 712, 275, issued January 27(1998). (b) J. F. E. Van Gestel, Antibacterial and antifouling oxathiazines and their oxides, U. S. Patent 5, 922, 113, issued July 13(1999). |
| [6] |
(a) R. A. Davis, A. R. A Valcke, W. G. Brouwer, Uniroyal Chemical Company, Inc. and Uniroyal Chemical Ltd. /Ltee, Wood preservative oxathiazines, U. S. Patent 5, 777, 110(1998). (b) R. A. Davis, A. R. A Valcke, W. G. Brouwer, Oxathiazine holzschutzmittel, Eur. Patent Speci. 0, 715, 625 B1(1997). (c) R. A. Davis, A. R. A. Valcke, W. G. Brouwer, Wood preservative oxathiazines, U. S. Patent 6, 372, 297 B1(2002). (d) L. A. De Witte, A. R. A Valcke, M. A. Van der Flaas, W. M. Willems, Synergistic compositions comprising an oxathiazine and a benzothiophene-2-carboxamide-S, S-dioxide, U. S. Patent 6, 242, 440 B1(2001). |
| [7] |
(a) M. Van der Flaas, Preservative formulations comprising an oxathiazine and alkoxylated amines, Eur. Pat. Appl. 1273233 A1(2003). ; (b) G. L. Chee, B. Bhattarai, R. D. Gietz, et al. , Chemical reactivity and microbicidal action of bethoxazin, Bioorg. Med. Chem. 20(2012) 1494-1501; (c) S. Hoff, E. Zwanenbug, 6-acetamido-56-dihydro-1, 4, 2-oxathiazines: a novel class of compounds, Tetrahedron Lett. 13(1972) 5267-5268 |
| [8] | C. H. Heathcock, S. L. Graham, M. C. Pirrung, F. Plavac, C. T. White, Spirocyclic Systems in the Total Synthesis of Natural Products, vol. Ⅱ, John Wiley & Sons, New York, 1983, pp. p264. |
| [9] | Singh G.S., Desta Z.Y.. Isatins as privileged molecules in design and synthesis of spiro-fused cyclic frameworks. Chem. Rev. 112 (2012) 6104–6155. DOI:10.1021/cr300135y |
| [10] |
(a) A. Alizadeh, R. Ghanbaripour, M. Feizabadi, L. G. Zhu, M. Dusek, Synthesis of 3-[(coumarinyl)carbonyl]-3a, 8b-dihyroindeno[1, 2-b]pyrrole-4(1 H)-ones and their conversion to coumarin bearing spiro[isobenzofuran-1, 2'-pyrrole] moiety compounds via oxidative cleavage reaction, RSC Adv. 5(2015) 80518-80525; (b) H. Liu, J. Li, M. Xiong, J. Jiang, J. Wang, Cp* coⅢ-catalyzed C-H alkenylation/annulation to afford spiro indenyl benzosultam, J. Org. Chem. 81(2016) 6093-6099; (c) Z. Yuan, X. Fang, X. Li, et al. , 1, 6-Conjugated addition-mediated[2+1] annulation: approach to spiro[2. 5] octa-47-dien-6-one, J. Org. Chem. 80(2015) 11123-11130. |
| [11] |
(a) H. Peng, J. Li, F. Wang, B. Liu, B. Yin, Synthesis of spiro-lactams and polysubstituted pyrroles via ceric ammonium nitrate-mediated oxidative cyclization of N-furan-2-ylmethyl-beta enaminones, J. Org. Chem. 81(2016) 4939-4946; (c) J. Zheng, W. J. Cui, C. Zheng, S. L. You, Synthesis and application of chiral spiro Cp ligands in rhodium-catalyzed asymmetric oxidative coupling of biaryl compounds with alkenes, J. Am. Chem. Soc. 138(2016) 5242-5245. |
| [12] |
(a) G. I. Shakibaei, A. Bazgir, A highly efficient one-pot synthesis of indenopyridine-fused spirocyclic systems, RSC Adv. 6(2016) 22306-22311; (b) S. Hajra, S. Maity, S. Roy, Regioselective friedel-crafts reaction of electronrich benzenoid arenes and spiro epoxy oxindole at the spiro-centre: efficient synthesis of benzofuro indolines and 2H-spiro[benzofuran]33'-oxindoles, Adv. Synt. Catal. 358(2016) 230'-2306; (c) A. Alizadeh, J. Mokhtari, Novel four-component route to the synthesis of spiro[indoline-34'-pyridine]-3'-carboxylate derivatives, Tetrahedron 67(2011) 3519-3523. |
| [13] |
(a) I. Yavari, M. Nematpour, T. Damghani, Copper-catalyzed S-arylation of tetramethyl guanidine heterocumulene adducts, Tetrahedron Lett. 55(2014) 1323-1325; (b) I. Yavari, M. Nematpour, Copper-catalyzed tandem synthesis of tetrasubstituted pyrimidines from alkynes, sulfonyl azides, trichloro acetonitrile, and tetramethyl guanidine, Synlett 24(2013) 165-168; (c) I. Yavari, A. S. Shahvelayati, A. Malekafzali, Efficient synthesis of functionalized 24-diaminothiazoles from tetramethyl guanidine, isothiocyanates, and α-bromoketones, J. Sulf. Chem. 31(2010) 499-508; (d) I. Yavari, A. Sheikhi, M. Nematpour, Z. Taheri, Copper-catalyzed synthesis of pentasubstituted pyridines from N-sulfonyl ketenimines, 1, 1, 3, 3-tetramethyl guanidine, and acetylene dicarboxylates, Helv. Chim. Acta 98(2015) 534-538. |
| [14] |
(a) S. B. Azimi, J. Azizian, A green, one-pot synthesis of substituted 2, 3-dihydroquinazoline-4(1H)-ones in the presence of N-sulfonic acid pyridinium chloride, Synlett 27(2016) 1836-1839; (b) S. B. Azimi, J. Azizian, Reaction of benzyl alcohols, isatoic anhydride, and primary amines mediated by I2/K2CO3 in water: a new and green approach for the synthesis of 2, 3-dihydroquinazolin-4(1H)-ones, Tetrahedron Lett. 57(2016) 181-184; (c) P. Torabi, J. Azizian, J. Noei, Synthesis of benzimidazoles and benzoxazoles using TiCl3OTf in ethanol at room temperature, Tetrahedron Lett. 57(2016) 185-188. |
| [15] |
(a) J. S. Yu, Y. L. Liu, J. Tang, X. Wang, J. Zhou, Highly efficient on water catalystfree nucleophilic addition reactions using difluoroenoxysilanes: dramatic fluorine effects, Angew. Chem. Int. Ed. 53(2014) 9512-9516; (b) F. Zhou, X. P. Zeng, C. Wang, X. L. Zhao, J. Zhou, Organocatalytic asymmetric synthesis of 33-disubstituted oxindoles featuring two heteroatoms at the C3 position, Chem. Commun. 49(2013) 2022-2024; (c) J. S. Yu, H. M. Huang, P. G. Ding, et al. , Catalytic enantioselective construction of sulfur-containing tetr-asubstituted carbon stereocenters, ACS Catal. 6(2016) 5319-5344. |
 2017, Vol. 28
2017, Vol. 28