b Gansu Key Laboratory of Polymer Materials, College of Chemistry and Chemical Engineering, Northwest Normal University, Lanzhou 730070, China
Transition metal-catalyzed carbon-carbon and carbon-heteroatom cross-coupling reactions belong to the most powerful and flexible transformations known to organic chemists and have revolutionized the art and practice of synthesis in the last two decades [1]. The mild reaction conditions, high functional group tolerance, and broad availability of starting materials make this method more attractive. For example, Pd-catalyzed the formation of carbon-carbon and carbon-heteroatom bond has attracted remarkable attention [2-9].
In 2000, a novel and mechanistically unprecedented Pdcatalyzed C-C cross-coupling protocol for the synthesis of ketones from thiol esters and boronic acids under neutral, anaerobic conditions was reported by Liebeskind and Srogl [10-12]. Then, a lot of methods were developed for the C-C bond formation via the Pd and/or Cu, Ni-catalyzed the desulfurative cross-coupling reaction of thioethers or thioesters with arylboronic acids [13-15]. A key feature of these desulfitative carbon-carbon couplings [16] is the requirement of stoichiometric amounts of a Cu(Ⅰ) carboxylate, such as Cu(Ⅰ)-thiophene-2-carboxylate (CuTC) [17] or Cu(Ⅰ)-3-methylsalicylate as a metalcofactor. Because of the high thiophilicity of the Cu(Ⅰ) salt, it often used as a desulfurization reagents.
Recently, we developed a domino desulfitative coupling and acylation process through the three-component reaction of 3, 4-dihydropyrimidine-2-thiones, alkynes with copper(Ⅰ) Carboxylates using palladium acetate as catalyst (Scheme 1) [18]. Wherein, copper(Ⅰ) carboxylates act not only as desulfurative reagents but also as sources of nucleophilic carboxylates. In continuation of our interest in the synthesis of highly substituted and functionalized pyrimidines, we expanded the application scope of copper(Ⅰ) carboxylates in the intermolecular desulfitative and decarboxylative coupling reaction of two molecules of 3, 4-dihydropyrimidine-2-thione with one copper(Ⅰ) carboxylate (Scheme 1). Herein, copper(Ⅰ) carboxylates act not only as desulfurative reagents but also as sources of carbon nucleophiles. This novel transformation, involving desulfitative, aromatic and decarboxylative reactions in a one-pot process was very efficient.
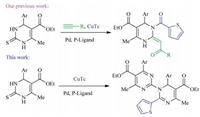
|
Download:
|
| Scheme 1. A desulfurative coupling arylation process. | |
In the last decade, a rapidly growing number of decarboxylative reactions have been discovered. Their key advantage over traditional cross-coupling reactions is that they draw on stable and readily available carboxylate salts as sources of carbon nucleophiles rather than expensive and sensitive organometallic reagents [19, 20]. In this type of reaction, a copper(Ⅰ) or silver(Ⅰ) catalyst mediates the extrusion of CO2 from the carboxylates while a palladium-complex catalyzes the coupling of the resulting carbon nucleophiles with carbon electrophiles.
2. Results and discussionInitially, the influence on the model reaction of various parameters such as catalysts, ligands, solvents was examined, and the results are summarized in Table 1. To optimize the reaction conditions, the cross-coupling reaction between DHPM (1a) with copper(Ⅰ)-thiophene-2-carboxylate (CuTC) (2a) was tested as the model reaction. Clearly, Pd catalyst and ligand are essential for the entire process (entries 1-3). Next, we tested the effect of different kinds of Pd catalyst. Obviously, Pd(acac)2 and Pd(PPh3)4 were not good for the reaction to form the product 3a in a low yield (entries 4 and 5). When PdCl2, PdCl2(dppf) and Pd(PPh3)2Cl2 were used as the catalyst, the yield of the product 3a was moderate (entries 6-8). Compared with the catalyst on the above, Pd(OAc)2 was the optimal catalyst to deliver the target product 3a in 75% yield (entry 9). Among the tested phosphine ligands such as DPE-Phos, PPh3, XPhos, PCy3 and dppp, DPE-Phos was the best one (entries 9-12). Finally, the effect of solvent was also investigated, and DMF was better than xylene and toluene (entries 14 and 15). To sum up, the optimal conditions are as follows: Pd(OAc)2 as a catalyst, DPE-Phos as a ligand, DMF as a solvent at 140 ℃ for 36 h, which afforded the desired product in the highest isolated yield.
|
|
Table 1 Optimization of the reaction of DHPM (1a) with CuTC (2a).a |
With the optimized reaction conditions in hand, we used DHPMs and CuTC to test the reaction scope (Scheme 2). In general, moderate to good yields were obtained under the standard reaction conditions (3a-3p). The reaction tolerated a variety of dihydropyrimidine-2-thiones substituents and obtained a series of pyrimidine derivatives via C-N and C-C cross-coupling reaction. We also found that the reactions of dihydropyrimidine-2-thione bearing electron-donating groups (Me and OMe) on the phenyl ring provided higher yields than those containing electronwithdrawing groups (F, Br and Cl) on the phenyl ring. It is noteworthy that 4-(4-bromophenyl) substituted pyrimidine-2-thioneonly gave single-substituted product (3l), and the structure of 3l was confirmed by X-ray crystallographic analysis with CCDC No. 1475601 (Fig. 1). When electron-withdrawing group was a nitro group at the DHPM, the reaction almost did not take place (3q). Meanwhile, the para-substituted DHPMs gave the target products in higher yields compared with the meta or ortho-substituted DHPMs under the optimized reaction conditions (3b-3k).
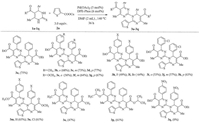
|
Download:
|
| Scheme 2. Scope of the DHPMs with copper(Ⅰ)-thiophene-2-carboxylate (CuTC). | |

|
Download:
|
| Figure 1. X-raycrystal structureof compound 3l. | |
To expand the scope of differentiated Cu(Ⅰ) carboxylates in the coupling reaction, we explored the copper(Ⅰ) furan-2-carboxylate (CuFC) with DHPMs in the standard reaction conditions. The reaction also can proceeded smoothly and obtained the satisfactory results (Scheme 3).
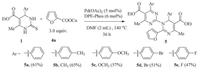
|
Download:
|
| Scheme 3. Scope of the DHPMs with copper(Ⅰ) furan-2-carboxylate (CuFC). | |
Next, on the basis of literatures in the last few years [21, 22], We explored a variety of copper(Ⅰ) carboxylates. Including, either electron-with drawing or electron copper(Ⅰ) Benzoic-carboxylate series or copper(Ⅰ) picolinate-2-carboxylate and copper(Ⅰ) aliphatic carboxylicacids, the reactions of Cu(Ⅰ) carboxylates with DHPM (1a) did not take place. However, when the cross-coupling reaction of copper(Ⅰ) picolinate with DHPM (1a), a new product was detected and the new product was 7a, the structure of which was confirmed by the 1H NMR, 13C NMR and MS. Next, in order to further explore experiments, we examined the possibility of the coupling of 7a with copper(Ⅰ)-thiophene-2-carboxylate (CuTC). To our delight, the desired product 3a was obtained in a satisfactory yield (Scheme 4). Thus, we hypothesized that the 7a possible an intermediate in this reaction process. Unfortunately, the proposed mechanism of the protocol not has a reliable explanation in the present and the follow-up work needs to further exploration in our laboratory.

|
Download:
|
| Scheme 4. Synthesis of compound 3a. | |
3. Conclusion
In conclusion, we have developed a simple domino process between dihydropyrimidinthiones (DHPMs) and copper(Ⅰ) carboxylates (CuTC, CuFC) in the presence of palladium acetate to synthetize a series of highly substituted and functionalized pyrimidines. In this reaction, we successfully achieved constructing the carbon-nitrogen bond and carbon-carbon bond in one pot. Besides, the copper(Ⅰ) carboxylate is not only as desulfurative reagents but also as a sources of carbon nucleophile.
4. ExperimentalGeneral procedure for the synthesis of (3a-3q): Under argon atmosphere, DHPMs 1 (0.2 mol, 55.2 mg), CuTC (3.0 equiv, 0.6 mol, 114 mg), Pd(OAc)2 (5 mol%, 0.01 mol, 2.2 mg), DPE-Phos (6 mol%, 0.012 mol, 7.0 mg) were added into a Schlenk tube dried by Hotgun. The tube was stopped and degassed with Nitrogen for three times. Then DMF (2 mL) were added by syringe. The mixture was stirred under Nitrogen atmosphere at 140 ℃ for 36 h. Then the mixture was cooled down to room temperature with 4 mL saturated solution of NH4Cl added to quench the reaction and extracted with ethyl acetate (3×10 mL). The organic layers were combined and dried with anhydrous MgSO4 and extracted by acetic ether and evaporated in vacuum and further purified by column chromatography on silica gel with ethyl acetate/Petroleum ether (1:10-1:3) to give the corresponding compounds (3a-3q). When CuTC (3.0 equiv., 0.6 mol, 114 mg) is replaced by CuFC (3.0 equiv., 0.6 mol, 104 mg) in the above conditions, products (5a-5e) can be obtained.
Compound 3a: Yellow solid (yield: 75%), m.p. 145-146 ℃. 1H NMR (600 MHz, CDCl3): δ 7.54 (d, 2H, J = 7.2 Hz), 7.39 (t, 2H, J=6.4 Hz), 7.33-7.27 (m, 4H), 7.23 (t, 3H, J = 7.8 Hz), 7.06 (d, 1H, J = 2.8 Hz), 6.96 (s, 1H), 6.93, (t, 1H, J = 4.8 Hz), 4.25-4.12 (m, 4H), 2.56 (s, 3H), 2.54 (s, 3H), 1.25(t, 3H, J = 1.2 Hz), 1.06 (t, 3H, J = 1.2 Hz). 13C NMR (150 MHz, CDCl3): δ 168.10, 167.01, 166.17, 164.17, 158.84, 153.97, 149.06, 140.76, 139.72, 137.40, 130.08, 129.58, 129.53, 128.36, 128.31, 128.25, 127.86, 127.50, 127.22, 119.96, 112.31, 61.67, 60.41, 54.81, 22.88, 21.26, 14.24, 13.63; HRMS: calcd. for C32H31N4O4S+ [M+H]+: 567.2066; found 567.2063.
Single crystal X-ray diffraction for 3l: Colorless crystal sample with the size of 0.34 mm×0.33 mm×0.27 mm of 3l was chosen under a microscope. Then crystal data was collected on a Bruker SMART ApexII CCD diffractometer using graphite-monochromated Mo Kα radiation (λ= 0.71073 Å) at 300.79 K. The structure was solved using a direct method and refined by full-matrix least squares on F2 using the SHELXTL crystallographic software package.
AcknowledgmentsFinancial support from the National Natural Science Foundation of China (Nos. 21362032 and 21362031), the NSF of Gansu Province (No. 1208RJYA083).
Appendix A. Supplementary dataSupplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.cclet.2016.12.034.
| [1] | Kataoka N., Shelby Q., Stambuli J.P., Hartwig J.F.. Air stable, sterically hindered ferrocenyl dialkylphosphines for palladium-catalyzed C-C, C-N, and C-O bondforming cross-couplings. J. Org. Chem. 67 (2002) 5553–5566. DOI:10.1021/jo025732j |
| [2] | Miyaura N., Suzuki A.. Palladium-catalyzed cross-coupling reactions of organoboron compounds. Chem. Rev. 95 (1995) 2457–2483. DOI:10.1021/cr00039a007 |
| [3] | Hassan J., Sevignon M., Gozzi C., Schulz E., Lemaire M.. Aryl-aryl bond formation one century after the discovery of the Ullmann reaction. Chem. Rev. 102 (2002) 1359–1470. DOI:10.1021/cr000664r |
| [4] | Ley S.V., Thomas A.W.. Modern Synthetic Methods for Copper-Mediated C (aryl)-O, C (aryl)-N, and C (aryl)-S Bond Formation. Angew. Chem. Int. Ed 42 (2003) 5400–5449. DOI:10.1002/(ISSN)1521-3773 |
| [5] |
(a) N. E. Leadbeater, M. Marco, palladium catalysis of the Suzuki reaction in water using microwave heating, Org. Lett. 4(2002) 2973-2976; (b) D. Nöteberg, W. Schaal, E. Hamelink, L. Vrang, M. Larhed, High-speed optimization of inhibitors of the malarial proteases plasmepsin I and Ⅱ, J. Comb. Chem. 5(2003) 456-464. |
| [6] |
(a) G. Burton, P. Cao, G. Li, R. Rivero, Palladium-catalyzed intermolecular coupling of aryl chlorides and sulfonamides under microwave irradiation, Org. Lett. 5(2003) 4373-4376; (b) A. Jensen, X. Liang, D. Tanner, N. Skjaerbaek, Rapid and efficient microwaveassisted synthesis of aryl aminobenzophenones using Pd-catalyzed amination, J. Org. Chem. 69(2004) 4936-4947. |
| [7] |
(a) P. Walla, C. O. Kappe, Microwave-assisted Negishi and Kumada crosscoupling reactions of aryl chlorides, Chem. Commun. 5(2004) 564-565; (b) I. Mutule, E. Suna, A convenient microwave assisted arylzinc generationNegishi coupling protocol, Tetrahedron Lett. 45(2004) 3909-3912. |
| [8] | Kaval N., Bisztray K., Dehaen W., et al., Microwave-enhanced transition metalcatalyzed decoration of 2(1H)-pyrazinone scaffolds. Mol. Divers. 7 (2003) 125–134. DOI:10.1023/B:MODI.0000006807.43408.d5 |
| [9] | Y. J. Wu, H. He, A. L'Heureux, Copper-catalyzed coupling of (S)-1-(3-bromophenyl)-ethylamine and N-H containing heteroarenes using microwave heating, Tetrahedron Lett. 44(203) (2017) 4217-4218. |
| [10] |
(a) L. S. Liebeskind, J. Srogl, Thiol ester-boronic acid coupling. A mechanistically unprecedented and general ketone synthesis, J. Org. Chem. 122(2000) 11260-11261; (c) H. Yang, H. Li, R. Wittenberg, et al. , Ambient temperature synthesis of high enantiopurity N-protected peptidyl ketones by peptidyl thiol ester-boronic acid cross-coupling, J. Am. Chem. Soc. 129(2007) 1132-1140. |
| [11] |
(a) L. S. Liebeskind, J. Srogl, Heteroaromatic thioether-boronic acid crosscoupling under neutral reaction conditions, Org. Lett. 4(2002) 979-981; (c) C. Savarin, J. Srogl, L. S. Liebeskind, Substituted alkyne synthesis under nonbasic conditions: Copper carboxylate-mediated, palladium-catalyzed thioalkyne-boronic acid cross-coupling, Org. Lett. 3(2001) 91-93. |
| [12] | Kusturin C., Liebeskind L.S., Rahman H., Sample K., Schweitzer B.. Switchable catalysis:Modular synthesis of functionalized pyrimidinones via selective sulfide and halide cross-coupling chemistry. Org. Lett. 5 (2003) 4349–4352. DOI:10.1021/ol035649y |
| [13] |
(a) H. Prokopcova, C. O. Kappe, Copper-catalyzed C-C coupling of thiol esters and boronic acids under aerobic conditions, Angew. Chem. Int. Ed. 47(2008) 3674-3676; (b) H. Prokopcova, C. O. Kappe, The Liebeskind-Srogl C-C cross coupling reaction, Angew. Chem. Int. Ed. 48(2009) 2276-2286. |
| [14] | Henke A., Srogl J.. Pd2+ and Cu2+ catalyzed oxidative cross-coupling of mercaptoacetylenes and arylboronic acids. Chem. Commun. 47 (2011) 4282–4284. DOI:10.1039/c1cc10505a |
| [15] |
(a) A. C. Wotal, D. J. Weix, Synthesis of functionalized dialkyl ketones from carboxylic acid derivatives and alkyl halides, Org. Lett. 14(2012) 1476-1479; (b) C. I. Someya, M. Weidauer, S. Enthaler, Nickel-catalyzed C(sp2)-C(sp2) cross coupling reactions of sulfur-functionalities and grignard reagents, Catal. Lett. 143(2013) 424-431. |
| [16] | Dubbaka S.R., Vogel P.. Organosulfur compounds:electrophilic reagents in transition metal catalyzed carbon-carbon bond forming reactions. Angew. Chem. 44 (2005) 7674–7684. DOI:10.1002/(ISSN)1521-3773 |
| [17] | Oliver Kappe C.. Palladium (0)-catalyzed, copper (I)-mediated coupling of boronic acids with cyclic thioamides Selective carbon-carbon bond formation for the functionalization of heterocycles. J. Org. Chem. 72 (2007) 4440–4448. DOI:10.1021/jo070408f |
| [18] |
(a) Z.J. Quan, X.D. Jia, Z. Zhang, Y.X. Da, X.C. Wang, A domino desulfitative coupling/acylation/hydration process cocatalyzed by copper (I) and palladium (Ⅱ):synthesis of highly substituted and functionalized pyrimidines, Adv. Synth. Catal. 354(2012) 2939-2948; (d) B.X. Du, Z.J. Quan, X.C. Wang, Chemo controlled cross coupling of di(hetero) aryl disulfides with Grignard reagents:C-C vs. C-S bond formation, Adv. Synth. Catal. 357(2015) 1270-1276. |
| [19] |
(a) L. J. Gooßen, G. Deng, L. M. Levy, Synthesis of biaryls via catalytic decarboxylative coupling, Science 313(2006) 662-664; (b) L. J. Gooßen, N. Rodríguez, B. Melzer, et al. , Synthesis via Pd-catalyzed decarboxylative coupling of aromatic carboxylates with aryl halides, J. Am. Chem. Soc. 129(2007) 4824-4833. |
| [20] |
(a) J. M. Becht, C. Catala, L. D. Cedric, C. Le Drian, A. Wagner, Synthesis of biaryls via decarboxylative Pd-catalyzed cross-coupling reaction, Org. Lett. 9(2007) 1781-1783; (d) R. Shang, Q. Xu, Y. Y. Jiang, et al. , Pd-catalyzed decarboxylative cross coupling of potassium polyfluorobenzoates with aryl bromides, chlorides, and triflates, Org. Lett. 12(2010) 1000-1003. |
| [21] |
(a) L. N. Guo, H. Wang, X. H. Duan, Recent advances in catalytic decarboxylative acylation reactions via a radical process, Org. Biomol. Chem. 14(2016) 7380-7391; (e) L. J. Gooßen, N. Rodríguez, K. Gooßen, Carboxylic acids as substrates in homogeneous catalysis, Angew. Chem. Int. Ed. 47(2008) 3100-3120. |
| [22] |
(a) X. Li, D. Zou, F. Leng, et al. , Arylation of 2-substituted pyridines via Pdcatalyzed decarboxylative cross-coupling reactions of 2-picolinic acid, Chem. Commun. 49(2013) 312-314; (d) M. Nakano, H. Tsurugi, T. Satoh, M. Miura, Palladium-catalyzed perarylation of 3-thiophene and 3-furancarboxylic acids accompanied by C-H bond cleavage and decarboxylation, Org. Lett. 10(2008) 1851-1854. |
 2017, Vol. 28
2017, Vol. 28 



