b Shanghai Institute of Ceramics, Chinese Academy of Sciences, Shanghai 200050, China;
c University of Chinese Academy of Sciences, Beijing 100049, China
Amyloid has long been thought to be intimately related with neurodegenerative diseases like Alzheimer's disease and Parkin son's disease [1, 2]. However, in recent decades, an increasing number of studies suggest that there exists a special type of amyloid structures in nature, usually referred to as "functional amyloids", that can play normal biological functions, including but not limited to adhesive biofilms of bacteria, catalytic scaffolds and hormones reservoirs [3-5]. Amyloid has thus been extensively utilized as nanomaterials to construct functional materials [6-8]. In particular, design of functional amyloid-based biomaterials with well-defined sequence-functionality relationship via genetic engineering has attracted great interest. Such modular genetic strategy provides a new avenue to rationally design multi functional molecular biomaterials with several features including self-assembling fibril structures, outstanding stability and excel lent mechanical properties. In addition, these amyloid structures can be further elaborated by introducing multiple functional domains into amyloid backbones to achieve multi-functionalities [9-11]. Despite great advances, no systematic studies have been done regarding how varied functional domains, positions and subunits affect fibril self-assembly and how they will affect the final morphology of assembled structures.
Herein, we used a functional amyloid system based upon CsgA, which is the subunit of culi—the major protein components of Escherichia coli biofilm [3, 12], to study the impacts of varied fusion domains, fusion positions as well as different subunits on amyloid fibril assembly and morphological changes.
2. Results and discussionCurli is the major extracellular proteinaceous fibers produced by E. coli and Salmonella enterica that is related to biofilm formation, immune activation, host colonization and cell invasion [13] (Fig. 1a). Curli fibril typically generates characteristic X-ray fiber diffraction pattern of amyloid proteins [14, 15], in which the meridional and equatorial reflection is 4.6 Å and 8.7 Å, respectively (Fig. 1b). Purified CsgA can self-assemble into single amyloid fibrils at early stage with diameter of 1-2 nm (Fig. 1c) and eventually aggregate to form multi-strand fibrils or fiber bundles with diameter from several nanometers to tens of nanometers (Fig. 1d).

|
Download:
|
| Figure 1. Characterization of curli fibrils produced by E. coli (a) Scanning electron microscope (SEM) image of E. Coli biofilms, in which curli fibrils form around the bacteria. (b) Xray fiber diffraction of CsgA fibrils. d1 and d2 stands for the equatorial reflection and meridional reflection, respectively. (c) Transmission electron microscope (TEM) image of CsgA fibrils collected at early stage of self-assembly. (d) TEM image of CsgA fibrils collected at later stage of self-assembly. | |
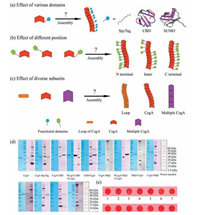
As the major subunit for curli fibers, CsgA is composed of five imperfect repeating loops [13]. Due to its remarkable properties, CsgA has been applied as the scaffold to construct a variety of multi-functional biomaterials [9-11]. In this study, we constructed several series of multi-component proteins based on CsgA. In the first group, SpyTag [16] (Streptococcus pyogenes peptide tag), CBD [17] (chitin binding domain) and SUMO [18] (small ubiquitin related modifier) were respectively added at the C terminus of CsgA, intending to study the influence of side domains on fibril assembly. In addition, we are interested in probing whether morphology of these two-component amyloid fibrils would change by fusing the same domains, including CBD, Mfp3 (mussel foot protein 3) and Mfp5 (mussel foot protein 5), at different positions. Finally, we selected diverse subunits—single loop of CsgA, CsgA, 2 tandem repeats of CsgA (2 × CsgA) and 4 tandem repeats of CsgA (4 × CsgA) and determined how they vary in morphology (Fig. 2a-c).
|
Download:
|
| Figure 2. Investigation of varied fusion domains, fusion positions and subunits on amyloid fibril self-assembly and morphology. (a–c) Schematic illustration showing how different domains, varied fusion positions and subunits affect fibril self-assembly and morphology of CsgA-fusion proteins. (d) SDS-PAGE and western blotting of all recombinant proteins. (e) Congo red staining reveals that all recombinant proteins show amyloid features after incubating for 18 h. 1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14 respectively stands for CsgA, CsgA-SpyTag, CsgA-CBD, NCsgA-CBD-CCsgA, CBD-CsgA, CsgA-Mfp3, NCsgA-Mfp3-CCsgA, Mfp3-CsgA, CsgA-Mfp5, NCsgA-Mfp5-CCsgA, Mfp5-CsgA, CsgA-SUMO, 2 × CsgA and 4 × CsgA, respectively. Note: For NCsgA-CBD-CCsgA, CBD is fused between Loop 2 and Loop 3 of CsgA; For NCsgA-Mfp3-CCsgA, Mfp3 is fused between Loop 3 and Loop 4 and for NCsgA-Mfp5-CCsgA, Mfp5 is fused between Loop1 and Loop 2 of CsgA. | |
We started with genetic construction of several recombinant plasmids including pET22b/CsgA, pET22b/CsgA-SpyTag, pET22b/ CsgA-SUMO, pET22b/CsgA-CBD, pET22b/NCsgA-CBD-CCsgA, pET22b/CBD-CsgA, pET22b/CsgA-Mfp3, pET22b/NCsgA-Mfp3-CCsgA, pET22b/Mfp3-CsgA, pET22b/CsgA-Mfp5, pET22b/NCsgA Mfp5-CCsgA, pET22b/Mfp5-CsgA, pET22b/2 × CsgA and pET22b/ 4 × CsgA through Gibson assembly (Fig. S1-S12 in Supporting information). Plasmids were then transformed into the DE3 (BL21) competent strains. Target proteins were induced for 2 h by addition of 0.5 mmol/L IPTG and then were purified through cobalt resins.
All the protein bands were identified with expected molecular weights, as show in SDS-PAGE, and were further approved by western blotting (Fig. 2d). All freshly purified proteins tend to aggregate and form suspension after solution incubation for several days. In order to confirm if these aggregates are amyloid fibrils, we carried out Congo red staining, a dye assay that is often used to specifically recognize amyloid feature of proteins [12]. The results suggest that all protein aggregates could be stained by congo red, implying that these protein aggregates are indeed composed of amyloid fibrils (Fig. 2e).
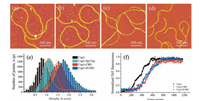
To study how varied fusion domains affect fibril assembly and fibril morphologies, we constructed CsgA, CsgA-SpyTag, CsgA-CBD and CsgA-SUMO, respectively, containing fusion domains with increased molecular weight (Fig. 2a). The AFM images for amyloid fibrils incubated on mica for 18 h were presented in Fig. 3a-d. All proteins are able to self-assemble into fibril structures. While CsgA-SpyTag fibril morphologically resembles CsgA fibril with continuous and non-branched feature, CsgA-CBD and CsgA-SUMO fibrils are either branched or much shorter compared with CsgA fibrils. Among the three side fusion domains, spytag has the lowest molecular weight (1.47 kDa) and seem to have the least influence on fibril assembly, possibly due to less spatial occupation and thereby less interference on the amyloid cores. We also conducted statistical analysis to compare the average diameter of these amyloid fibrils. The distribution of diameter of amyloid fibrils is shown in Fig. 3e. After fitting with Gaussian function, the average diameter is 0.87 ± 0.09 nm for CsgA, 1.12 ± 0.04 nm for CsgA SpyTag, 1.52 ± 0.05 nm for CsgA-CBD and 1.76 ± 0.08 nm for CsgA SUMO (Fig. 3e). Therefore, the average diameter of fibrils is in direct proportion to the molecular weight of the fused domains. When fused onto CsgA amyloid backbones, the additional functional domains tend not to participate in the core construction, but are exposed and randomly distributed around the core structures [19]. These domains occupy some extra spaces and thus contribute to the increased diameter of fibrils compared with CsgA. To assess if side domains will affect the dynamic self assembly of amyloid fibrils, we carried out THT kinetic assembly assay for CsgA, CsgA-CBD and CsgA-SUMO proteins. The results proved that CsgA-fusion proteins have elongated lag phase after introducing either CBD or SUMO domain compared with CsgA, while the lag phases between the two fusion proteins did not show much difference (Fig. 3f). We therefore confirmed that introduc tion of functional domains will retard fibril growth of fusion proteins.
|
Download:
|
| Figure 3. Characterization of fibrils with various functional domains fused at C terminus of CsgA. (a) AFM image of CsgA fibrils. (b) AFM image of CsgA-SpyTag fibrils. (c) AFM image of CsgA-CBD fibrils. (d) AFM image of CsgA-SUMO fibrils. (e) Statistical analysis of average height of all fibrils. (f) Normalized ThT fluorescence kinetic assembly assay for CsgA, CsgA-CBD and CsgA-SUMO proteins. | |
Collectively, the above data suggest that fusion of functional domains does not interrupt fibril assembly, but retards fibril growth to certain extent. Introduction of functional side domain onto CsgA also tends to increase the average diameter of resultant fibrils, but often leads to shorter and branched fibrils possibly due to steric hinderance caused by fusion domains.
To assess how varied fusion position will affect fibril assembly, we constructed three similar groups of recombinant proteins, in which the same side domain (CBD, Mfp3 or Mfp5 domain) was fused at N terminus, intermediate position and C terminus of CsgA, respectively (Fig. 2b). Chitin binding domains (CBD) are C-terminus domain of Bacillus circulans chitinase that can bind specifically to insoluble chitin, which was reported to have rigid and compact twisted β-sandwich structures [17]. Mussel foot proteins are interfacial adhesion proteins of mussels and usually possess unstructured random coil structures [20].
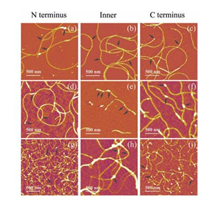
The AFM images of these series of fibrils were present in Fig. 4. The results indicate that all proteins are able to form self assembled nanofibrils, though morphologies of fibrils in each group vary among different samples. When side domain was fused at C or N terminus of CsgA, fibrils are morphologically similar between samples in each group no matter what the specific side domain is. However, if the side domains were fused at the position of CsgA, the morphological features of fibrils significantly differ from those fused either at C or N terminus. This observation appears more pronounced for Mfp3 and Mfp5 domains. For example, in the case of Mfp3 domain, the fibrils become extremely short, and white dots often appear in the middle part of fibrils (Fig. 4d-f). As far as Mfp5 domain is concerned, the fibrils are not homogeneous and tend to form large aggregates compared with those fusion proteins having C or N-terminus fusion domains (Fig. 4g-i). Such observations may highlight the impact of conformations of side domains. We hypothesized that, when they are fused to the amyloid core structures, rigid CBD may tend to retain its original conformation and would not interfere the backbone structure too much, while random coiled Mfps would randomly distribute over the cores, and thus may disturb the assembly of core structures to a larger degree.
|
Download:
|
| Figure 4. AFM images of CsgA-fusion fibrils functionalized with the same domain but at varied positions. (a–c) Morphologies of fibrils fused with CBD. (d–f) Morphologies of fibrils fused with Mfp3. (g–i) Morphologies of fibrils fused with Mfp5. The intermittent white dots, as indicated by black arrows, are possibly the results of random interactions among functional side domains, which might more or less interfere self-assembly of fibrils. The N-terminus, Inner and C-terminus stand for a specific functional domain that is fused at N-terminus, an intermediate position and C-terminus of CsgA, respectively. | |
CsgA is the major subunit of Curli and contains five imperfect repeating loops, which are predicted to form β strand-loop-β strand structure. Each repeating unit consists of several conserved amino acids whose side chains can form hydrogen-bonded network [21]. The stable hydrogen bonds, together with hydro phobic interactions, are the main forces that trigger self-assembly of CsgA and maintain the stability of self-assembled fibrils [22]. As such, CsgA proteins can self-assemble into amyloid fibrils both in vivo and in vitro (Fig. 1). As every repeating unit within CsgA has conserved amino acid sequence, an interesting and relevant question is to study if a single loop of CsgA is able to self-assemble into amyloid fibrils. Previous research proved that Loop1, Loop3 and Loop5 can all form amyloid fibrils respectively [23]. In addition, we are also interested to see if 2 × CsgA (sequence containing two repeating CsgA) and 4 × CsgA (sequence containing four repeating CsgA) can self-assemble to form amyloid fibrils or not.
The result suggests that one single loop of CsgA, CsgA itself, 2 and 4 repeating CsgA as monomer subunit can all form fibrils while morphologies vary among fibrils. CsgA tends to form continuous and extremely long fibrils (Fig. 5b), 2 × CsgA and 4 × CsgA self-assemble into much shorter fibrils (Fig. 5c-d), while Loop5 of CsgA has the shortest fibrils with intermittent feature (Fig. 5a). In addition, the diameters of fibrils seemed to increase with the increase of molecular weight of subunit, with an average diameter of 0.6 nm, 0.8 nm, 1.0 nm and 1.2 nm for Loop5, CsgA, 2 × CsgA and 4 × CsgA, respectively (Fig. 5).

|
Download:
|
| Figure 5. Morphological characterization of fibrils assembled with different monomer subunits. (a) Morphology of R5 fibril. (b) Morphology of CsgA fibril. (c) Morphology of 2 × CsgA fibril. (d) Morphology of 4 × CsgA fibril. (e) Height curve of R5 fibril. (f) Height curve of CsgA fibril. (g) Height curve of 2CsgA fibril. (h) Height curve of 4CsgA fibril. | |
In the case of fibrils formed by single loop of CsgA, the intramolecular and intermolecular hydrogen bonds and hydro phobic interactions are weaker than those of the other fibrils. The monomers thus seem not be able to stably stack to each other and form compact amyloid fibrils [21]. In contrast, 2 × CsgA and 4 × CsgA proteins tend to form straight nanofibrils. However, self assembly of those proteins containing multiple tandem repeats of CsgA seems to become more difficult compared with CsgA control, as indicated by short fibrils surrounded by white-dotted structures (Fig. 5d). This observation is consistent with previous studies, which revealed that packing of increasingly long polypeptides into fibril structures often lead to increasing structural disorder possibly due to more difficulty in searching for strong intermolec ular binding [24-26].
3. ConclusionUsing CsgA protein as a model system, we studied how varied fusion domains, fusion positions and fusion subunits affect self assembly and morphologies of amyloid fibrils. Although all CsgA fusion proteins tend to form self-assembling amyloid fibrils as indicated by Congo red, their morphologies indeed vary among samples based on AFM results. Specifically, the diameter of fibrils increases with the increase of the molecular weight of fusion domain possibly due to the extra space created by the side domains, which are thought to be external to the amyloid cores. Meanwhile, the dynamic assembly of recombinant proteins was delayed as a result of introduction of side domains. Moreover, fusion of the same functional domains at intermediate position seems to cause the most interference on fibril assembly compared with those fused at C or N-terminus, as mainly short and irregular fibrils were detected. This phenomenon appears more pronounced for Mfps than for CBD domains, possibly due to the difference in conformations between Mfps and CBD domains. In addition, the fibril diameter increases as the molecular weight of subunit increases. However, either reduction of the tandem repeats of CsgA to one single beta-sheet loop or increase in the number of tandem repeats of CsgAs from one to four produced shorter and intermittent fibrils compared with CsgA control protein. These results thus may imply that the hydrogen bonds and hydrophobic interactions in single loop of CsgA may not be as strong as those in CsgA fibrils. Meanwhile, packing of longer chains into fibril structures, as is the case for 2 × CsgA and 4 × CsgA, may lead to increasing structural disorder possibly due to more difficulty in searching for strong intermolecular binding. Our study will provide insights into the self-assembly of two compositional amyloid fibrils and lay the foundation for designing new multi-functional molecular biomaterials based upon amyloid proteins.
4. Experimental 4.1. Plasmid constructionTarget gene fragments were amplified by PCR with appropriate templates and then were cleaved by restriction enzymes Nde Ⅰ and Xho Ⅰ (Fermentas FastDigest) for 1 h at 37 ℃. The prepared fragments and the cleaved plasmid pET-22b treated with the same method were mixed and incubated with appropriate amount of Gibson Assembly Master Mix (NEB) for 1 h at 50 ℃, then transformed into DH5a E. coli competent cells. The strains were then smeared to the surface of LB media plates containing antibiotics to select the target strains. All the sequencing tests were carried out by Life Technology.
4.2. Protein expression and purification 4.2.1. ExpressionThe verified plasmids were transformed into DE3 (BL21) competent E. coli. The strains were then cultured in LB media containing 50 mg/mL carbenicillin at 37 ℃ for 2 h to reach OD600-1.5. 0.5 mmol/L IPTG was added into the culture to induce for protein expression for 2 h at 30 ℃. Strain pellets were then collected by centrifugation at 4000 × g for 10 min and then stored at -80 ℃ for later use.
4.2.2. Purification5 g cell pellets were lysed with 50 mL extraction solution (8 mol/L guanidine hydrochloride (GdnHCl), 300 mmol/L NaCl, 50 mmol/L K2HPO4/KH2PO4, pH 7.2) for 12 h at room temperature. Suspension was collected by centrifugation for 20 min at 15, 000 × g and incubated with 4 mL cobalt resins (Talon Metal Affinity Resin) for 1 h. Then cobalt resins binding with target proteins were collected by centrifugation at 4, 000 × g for 2 min and washed with 10 mL of 20 mmol/L potassium phosphate buffer (300 mmol/L NaCl, 20 mmol/L K2HPO4/KH2PO4, pH 7.2) twice. Resins were loaded on the gravity chromatography columns (Sangon) with 5 mL potassium phosphate buffer and further washed with excess 20 mmol/L potassium phosphate buffer. Then 5 mL elution buffer (300 mmol/L imidazole and 50 mmol/L potassium phosphate buffer, pH 7.2) were added to elute the target proteins. The purified proteins were collected in 1.5 mL tubes and stored in 4 ℃ fridge.
4.3. SDS-PAGE and western blotting 4.3.1. SDS-PAGE60 μL freshly eluted protein solutions were mixed with 20 μL 4 × LDS loading buffer (Life Technology) and then 20 μL mixed solutions were loaded into the lanes of mini gels (Life Technology) together with the standard protein ladder (Life Technology). After running at 165 volts for 50 min using Nupage MES running buffer (NOVEX), the gels were stained with Coomassie Blue solution (Amresco) for 1 h and then destained with washing solution (methyl alcohol, acetic acid and distilled water with a volume ratio of 4:1:5) for 1 h twice. The gels were then imaged using a Bio-Rad ChemiDoc MP system.
4.3.2. Western blottingProtein samples were run in mini gels and then blotted onto polyvinylidene difluoride membranes using iBlot systems (Invi trogen). The membranes were then blocked with 40 mL 20% (m/v) milk solution for 1 h and washed with 10 mL 1 × TBST for 10 min three times. After that, membranes were incubated with 30 mL anti-Higtag antibodies at a dilution of 1:5000 for 1 h and washed with 10 mL 1 × TBST for 10 min three times. They were then incubated with 30 mL secondary anti-mouse antibodies at a dilution of 1:5000 for 1 h. After treatment with 1 × TBST, the membranes were imaged through a Bio-Rad ChemiDoc MP system.
4.4. Sample preparation of synthetic peptidesThe loop5 peptide (R5) from CsgA monomer was synthesized by Pepmic Corporation. 1 mg peptides were dissolved in 1 mL mixed solution of trifluoroacetic acid and hexafluoroisopropanol at volume ratio of 1:1. After volatilization of mixed solution, the aggregation was suspended with 1 mL KPI (300 mmol/L NaCl, 20 mmol/L K2HPO4/KH2PO4, pH 7.2) solution and added on the surface of clean mica. The sample was then tested by AFM after incubating for 18 h.
4.5. Congo red staining50 μL amyloid fibrils suspensions were spotted onto Protran BA83 nitrocellulose membranes (Whatman) with a dot blot manifold (Schleicher & Schuell Minifold-Ⅰ Dot-Blot System). Membranes were incubated with 20 mL of 0.0025 (m/v%) Congo red solution for 1 h and then destained with distilled water for 1 h twice. The membranes were then imaged with GE imaging system.
4.6. Synchrotron X-ray fiber diffractionSuspension of mature protein fibrils was centrifuged to collect fibril pellets, followed by washing with copious distilled water several times to remove remaining salts. Fibril pellets were then lyophilized to protein powder. The samples were finally tested on beamline 14B on the Shanghai Synchrotron Radiation Facility, Shanghai Institute of Applied Physics, Chinese Academy of Sciences, China.
4.7. Atomic force microscopy (AFM)Freshly purified protein solution was diluted to a concentra tion of 1 mmol/L and then incubated on mica surfaces for 18 h. After rinsing with excess deionized water and blow-drying using nitrogen flow, samples were then tested by AFM (Asylum MFP-3D) on AC air tapping mode using Veecoprobes Sb-doped Si cantilevers (ρ = 0.01-0.025 Ω-cm, k = 40 N/m, ν~300 kHz).
4.8. Transmission electron microscopy (TEM)20 μL sample was dropped onto a carbon coated nickel TEM grid (EM sciences). After incubating for 30 s, the sample was rinsed with 30 μL distilled water for three times and then stained with 2% uranyl acetate (Zhong Jing Ke Yi Technology). TEM images were obtained on a Tecnai G2 F20 TEM operated at 200 kV accelerating voltage.
4.9. Scanning electron microscopy (SEM)Biofilm samples on carbon paper were coated with Au sputtered to ~10 nm with a SBC12 sputter coater. The samples were then imaged with a Zeiss SUPRA 55 SAPPHIRE scanning electron microscope operated at 5 kV accelerating voltage. Images were obtained in secondary electron imaging (SEI) mode.
AcknowledgmentsWe thank Xin-Yu Wang for providing plasmids containing 2 × CsgA or 4 × CsgA and for assisting in SEM and TEM testing. We thank Ke Li for assisting in analyzing the results of Synchrotron X ray fiber diffraction. Synchrotron X-ray fiber diffraction was performed on beamline 14 B on Shanghai Synchrotron Radiation Facility, Shanghai Institute of Applied Physics, Chinese Academy of Sciences, China. TEM was performed by using Tecnai G2 F20 TEM at National Center for Protein Science (Shanghai), Institute of Biochemistry and Cell Biology, Shanghai Institutes for Biological Sciences, Chinese Academy of Sciences. This work was primarily supported by the Joint Funds of the National Natural Science Foundation of China (No. U1532127). This work was also supported in part by National Natural Science Foundation of China (No. 31570972). C.Z. acknowledges start-up funding support from ShanghaiTech University and 1000 Youth Talents Program, granted by the Chinese Central Government.
Appendix A. Supplementary dataSupplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.cclet.2016.12.008.
| [1] | F. Chiti, C.M. Dobson. Protein misfolding, functional amyloid and human disease. Annu. Rev. Biochem. 75 (2006) 333–366. DOI:10.1146/annurev.biochem.75.101304.123901 |
| [2] | C.M. Dobson. Protein folding and misfolding. Nature 426 (2003) 884–890. DOI:10.1038/nature02261 |
| [3] | M.R. Chapman, L.S. Robinson, J.S. Pinkner, et al., Role of Escherichia coli curli operons in directing amyloid fiber formation. Science 295 (2002) 851–855. DOI:10.1126/science.1067484 |
| [4] | D.M. Fowler, A.V. Koulov, C. Alory-Jost, et al., Functional amyloid formation within mammalian tissue. PLoS Biol. 4 (2006) e6. |
| [5] | S.K. Maji, M.H. Perrin, M.R. Sawaya, et al., Functional amyloids as natural storage of peptide hormones in pituitary secretory granules. Science 325 (2009) 328–332. DOI:10.1126/science.1173155 |
| [6] | Z.F. Wu, P. Yang. Simple multipurpose surface functionalization by phase transited protein adhesion. Adv. Mate. Interfaces 2 (2015) 1400401. DOI:10.1002/admi.201400401 |
| [7] | D.H. Wang, Y. Ha, J. Gu, et al., 2D protein supramolecular nanofilm with exceptionally large area and emergent functions. Adv. Mater. 28 (2016) 7413–7423. DOI:10.1002/adma.201670239 |
| [8] | A.T. Gao, Q. Wu, D.H. Wang, et al., A superhydrophobic surface templated by protein self-assembly and emerging application toward protein crystallization. Adv. Mater. 28 (2016) 579–587. DOI:10.1002/adma.v28.3 |
| [9] | C. Zhong, T. Gurry, A.A. Cheng, et al., Strong underwater adhesives made by self-assembling multi-protein nanofibres. Nat. Nanotechnol. 9 (2014) 858–866. DOI:10.1038/nnano.2014.199 |
| [10] | A.Y. Chen, Z.T. Deng, A.N. Billings, et al., Synthesis and patterning of tunable multiscale materials with engineered cells. Nat. Mater. 13 (2014) 515–523. DOI:10.1038/nmat3912 |
| [11] | P.Q. Nguyen, Z. Botyanszki, P.K. Tay, N.S. Joshi. Programmable biofilm-based materials from engineered curli nanofibres. Nat. Commun. 5 (2014) 4945. DOI:10.1038/ncomms5945 |
| [12] | L.P. Blanco, M.L. Evans, D.R. Smith, M.P. Badtke, M.R. Chapman. Diversity, biogenesis and function of microbial amyloids. Trends Microbiol. 20 (2012) 66–73. DOI:10.1016/j.tim.2011.11.005 |
| [13] | M.M. Barnhart, M.R. Chapman. Curli biogenesis and function. Annu. Rev. Microbiol. 60 (2006) 131–147. DOI:10.1146/annurev.micro.60.080805.142106 |
| [14] | J. Greenwald, R. Riek. Biology of amyloid:structure, function, and regulation. Structure 18 (2010) 1244–1260. DOI:10.1016/j.str.2010.08.009 |
| [15] | D.M. Fowler, A.V. Koulov, W.E. Balch, J.W. Kelly. Functional amyloid-from bacteria to humans. Trends Biochem. Sci. 32 (2007) 217–224. DOI:10.1016/j.tibs.2007.03.003 |
| [16] | B. Zakeri, J.O. Fierer, E. Celik. Peptide tag forming a rapid covalent bond to a protein, through engineering a bacterial adhesin. Proc. Natl. Acad. Sci. U. S. A. 109 (2012) E690–E697. DOI:10.1073/pnas.1115485109 |
| [17] | T. Ikegami, T. Okada, M. Hashimoto, et al., Solution structure of the chitinbinding domain of Bacillus circulans WL-12 chitinase A1. J. Biol. Chem. 275 (2000) 13654–13661. DOI:10.1074/jbc.275.18.13654 |
| [18] | C.M. Hickey, N.R. Wilson, M. Hochstrasser. Function and regulation of SUMO proteases. Nat. Rev. Mol. Cell Biol. 13 (2012) 755–766. DOI:10.1038/nrm3478 |
| [19] | S. Sambashivan, Y.S. Liu, M.R. Sawaya, G. Mari, E. David. Amyloid-like fibrils of ribonuclease A with three-dimensional domain-swapped and native-like structure. Nature 437 (2005) 266–269. DOI:10.1038/nature03916 |
| [20] | D.S. Hwang, J.H. Waite. Three intrinsically unstructured mussel adhesive proteins, mfp-1, mfp-2, and mfp-3:analysis by circular dichroism. Protein Sci. 21 (2012) 1689–1695. DOI:10.1002/pro.v21.11 |
| [21] | S.K. Collinson, J.M.R. Parker, R.S. Hodges, W.W. Kay. Structural predictions of AgfA, the insoluble fimbrial subunit of salmonella thin aggregative fimbriae. J. Mol. Biol. 290 (1999) 741–756. DOI:10.1006/jmbi.1999.2882 |
| [22] | P.F. Tian, W. Boomsma, Y. Wang, et al., Structure of a functional amyloid protein subunit computed using sequence variation. J. Am. Chem. Soc. 137 (2015) 22–25. DOI:10.1021/ja5093634 |
| [23] | X. Wang, D.R. Smith, J.W. Jones, M.R. Chapman. In vitro polymerization of a functional Escherichia coli amyloid protein. J. Biol. Chem. 282 (2007) 3713–3719. |
| [24] | T.P.J. Knowles, M.J. Buehler. Nanomechanics of functional and pathological amyloid materials. Nat. Nanotechnol. 6 (2011) 469–479. DOI:10.1038/nnano.2011.102 |
| [25] | T.P.J. Knowles, C.A. Waudby, G.L. Devlin, et al., An analytical solution to the kinetics of breakable filament assembly. Science 326 (2009) 1533–1537. DOI:10.1126/science.1178250 |
| [26] | A. Relini, S. Torrassa, R. Ferrando, et al., Detection of populations of amyloidlike protofibrils with different physical properties. Biophys. J. 98 (2010) 1277–1284. DOI:10.1016/j.bpj.2009.11.052 |
 2017, Vol. 28
2017, Vol. 28 


