b Chengdu Institute of Biology, Chinese Academy of Sciences, Chengdu 610041, China;
c College of Life Science, Sichuan University, Chengdu 610064, China
The Chinese endemic genus Gymnotheca (Saururaceae) com prises two species, namely Gymnotheca chinensis Decne and Gymnotheca involucrata Pei, which are distributed mainly in the territory of Southwest China. Plants of this genus have long been used as traditional medicinal herbs to treat contusions and strains [1]. Our previous investigation on Gymnotheca had isolated a few of new lignans, including the dibenzocyclooctene, eupomatilone, eupodienone, norlignan, and polycyclic spiro lignan series [2-9]. In our continuing endeavor to discover novel and new structures from this unique Chinese endemic plant, two new biphenyl butyl neolignan derivatives, named gymnothebutyllignans A (1) and B (2) (Fig. 1), were isolated from the whole plants of G. involucrata. This type of compound has not previously been identified from a natural resource, to the best of our knowledge [10, 11]. Herein, we describe the isolation and structure elucidation of gymnothebu tyllignans A (1) and B (2) (Fig. 2).

|
Download:
|
| Figure 1. Chemical structures of compounds 1 and 2. | |
|
Download:
|
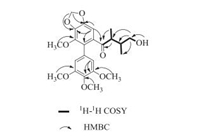
| Figure 2. Selected 1H-1H COSY and HMBC correlations of 1. | |
2. Results and discussion
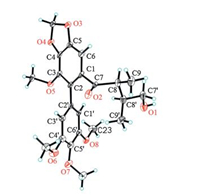
Compound 1 was obtained as a white crystal. Its molecular formula, C23H28O8 which indicated 10° of unsaturation, was established from the quasi-molecular ion peak at m/z 433.18570 [M+H]+ (calcd. for C23H29O8+: 433.18569) in the HR-ESIMS. Its IR spectrum exhibited strong absorption bands at 3358 and 1675 cm-1 due to hydroxyl and carbonyl functional groups. The 13C NMR spectrum of 1 displayed 20 carbon resonances showed there are symmetry elements in the molecule. The 1H NMR, 13C NMR and HSQC spectra of 1 showed two methyl groups [δH 0.59, 0.64 (d, each 3H, J = 7.0 Hz); δC 9.6, 11.5], two methine moieties [δH 1.75 (m, 1H), 2.55 (qd, 1H, J = 6.8, 3.5 Hz); δC 37.6, 46.0], one oxygenated methylene [δH 3.12-3.22 (m, 2H); δC 65.7], four methoxy groups [δH 3.75, 3.79, 3.79, 3.84 (s, each 3H); δC 56.4, 56.4, 60.2, 60.6], one methylenedioxy group [δH 6.12 (d, 2H, J = 5.5 Hz); δC 102.8], one carbonyl (δC 209.0), and two aromatic ring moieties [δH 6.64 (s, 1H), 6.30-6.65 (br., 2H); δC 153.9, 153.9, 149.5, 141.4, 139.6, 138.7, 137.2, 132.2, 127.9, 109.2, 109.2, 103.3]. Its 1H-1H COSY revealed the existence of a HOCH2CH(CH3)CH(CH3) fragment. The HSQC data and the δC 153.9, 109.2, and 56.4 signals enhancement means the presence of a 3, 4, 5-trimethoxy phenyl moiety [9]. The methylenedioxy group was assigned by the HMBC correlations of OCH2O to C-4 and C-5, as well as H-6 to C-4 and C-5. Similarly, the methoxy group at C-3 was also assigned. The connection between carbonyl and C-8 was established based on the HMBC correlations of H-8 to C-7, H-9 to C-7 and H-80' to C-7. The connection of C-1-C-7 was achieved by HMBC correlations of H-6 to C-7 and H-8 to C-1. The 3, 4, 5-trimethoxy phenyl attached to C-2 was deduced by the analysis of the HMBC correlations of H-6 to C-2, H-6 to C-7, and H-6 to C-5. Accordingly, the planar structure of compound 1 was established. Fortunately, a single crystal of 1 was obtained from MeOH, and X-ray crystallographic analysis with copper radiation was successfully performed (CCDC 1503704), which unambigu ously determined the absolutely structure (8S, 8'S) of 1 as deduced (Fig. 3). In summary, the structure of compound 1 was established and named gymnothebutyllignan A.
|
Download:
|
| Figure 3. X-ray crystal structure of 1. | |
Compound 2 was obtained as white powder. It had the same molecular formula of C23H28O8 as 1 which was established by HRESIMS. The 1H, 13C NMR, HSQC and HMBC spectra (see Supporting information) indicated that it was a diastereoisomer of 1. By comparing the 13C NMR spectrum with those of 1, the chemical shift of C-9 was shifted (Δδ = +3.2), and the chemical shift of C-9' was shifted (Δδ = +4.2). Similarly, the signals of C-8 (δC 48.1), C-7' (δC 64.6) and C-80 (δC 38.8) were shifted (Δδ = +2.1, -1.1 and +1.2, respectively). This indicated that epimerization occurred at C-8 or C-80 in the molecule. In summary, the relative configuration of compound 2 was determined and named gymnothebutyllignan B.
3. ConclusionIn this paper, two new biphenyl butyl neolignan derivatives were isolated from the endemic medicinal plant of G. involucrata. To the best of our knowledge, gymnothebutyllignans A (1) and B (2), are the first examples of neolignans obtained from a natural resource bearing a biphenyl and a butyl moiety. This finding could shed new light on the further understanding of the family of lignan.
4. Experimental 4.1. General experimental proceduresUV spectra were recorded on a Perkin-Elmer Lambda 35 UV-vis spectrophotometer (Perkin-Elmer, Waltham, USA). IR spectra were measured on a Perkin-Elmer one FT-IR spectrometer (KBr) (Perkin-Elmer, Waltham, USA). 1D and 2D-NMR spectra were recorded on a Bruker-AVANCE Ⅲ-400 instrument using TMS as the internal reference (Bruker, Karlsruhe, Germany). HR-ESIMS spectra were measured on a Scientific LTQ Orbitrap XL (Thermo, Massachusetts, USA). Column chromatography (CC) was per formed using 300-400 mesh silica gel (Qingdao Marine Chemical Inc., Qingdao, China), 75-150 mm MCI CHP-20 gel (Mitsubishi, Kyoto, Japan). Semi-preparative HPLC was performed on LC3000 system (Beijing Chuang Xing Tong Heng Science and Technology Co., Ltd., Beijing, China) equipped with ODS column (5 mm, i.d. 10 mm × 250 mm, Kromasil) (Akzo Nobel, Amsterdam, Netherlands).
4.2. Plant materialG. involucrata Pei was collected from Emeishan in Sichuan Province, China, in September 2013. A voucher specimen was deposited at the herbarium of the Chengdu Institute of Biology, Chinese Academy of Sciences, No. 20131026. Prof. Shi-Jie Zhu, Chengdu University of Traditional Chinese Medicine, identified the plant species.
4.3. Extraction and isolationThe air-dried and powdered whole plants of G. involucrata (2.6 kg) were extracted with MeOH at room temperature. After filtration, the MeOH extract was concentrated to give a residue (374 g), which was suspended in H2O (2 L) and extracted successively with petroleum ether, AcOEt, and n-BuOH. The AcOEt extract (31 g) was subjected to column chromatography (CC) over MCI gel (85 mm × 100 mm), eluting with MeOH-H2O (90:10), to yield two fractions (A and B) based on TLC analysis results. Fraction A (18 g) was further isolated by medium pressure CC (SiO2 (49 × 460 mm), petroleum ether-acetone 100:1, 50:1, 20:1, 10:1, 5:1, 2:1, 1:1, 1:2, 1:5, 1:10) and to furnish six fractions (Fr.1-Fr.6). Compounds 1 (5 mg, tR = 16.9 min) and 2 (6 mg, tR = 15.8 min) were obtained from Fr.4 by semi-preparative HPLC (MeOH/H2O 75:25, flow rate 3.0 mL/min).
Gymnothebutyllignan A (1): White crystal (MeOH); [α]D20 = +37 (c = 0.11, MeOH); UV (MeOH) λmax (log ε) nm 297 (3.78); IR (KBr, cm-1): νmax 3358, 2971, 1677, 1629, 1444, 1380, 1332, 1244, 1041, 927; 1H NMR and 13C NMR data, see Table 1; HRESIMS m/z 433.18570 [M+H]+ (calcd. for C23H29O8+, 433.18569).
|
|
Table 1 1H (400 MHz) and 13C (100 MHz) NMR data of compounds 1 and 2 in acetone-d6 (δ in ppm, J in Hz). |
Gymnothebutyllignan B (2): White powder (MeOH); [α]D20 = -44 (c = 0.32, MeOH); UV (MeOH) λmax (log ε) nm 297 (3.77); IR (KBr, cm-1): νmax 3356, 2970, 1675, 1630, 1434, 1386, 1330, 1248, 1042, 917, 749; 1H NMR and 13C NMR data, see Table 1; HRESIMS m/ z 433.18570 [M+H]+ (calcd. for C23H29O8+, 433.18569).
Crystal data for 1: C23H28O8, M = 432.45, triclinic, a = 10.6222 (6) Å, b = 14.5924 (8) Å, c = 14.8889 (9) Å, α = 95.368 (3)°, β = 99.501 (2)°, γ = 105.234 (2)°, V = 2173.3 (2) Å3, T = 100 (2) K, space group P1, Z = 4, μ(CuKα) = 0.832 mm-1, 30613 reflections measured, 10729 independent reflections (Rint = 0.0494). The final R1 values were 0.0985 (I > 2σ(I)). The final wR (F2) values were 0.2771 (I > 2σ (I)). The final R1 values were 0.1166 (all data). The final wR (F2) values were 0.3112 (all data). The goodness of fit on F2 was 1.405. Flack parameter = 0.0(2). The Hooft parameter is -0.17(9) for 3832 Bijvoet pairs.
AcknowledgmentThis work was financially supported by grants from the National Natural Sciences Foundation of China (No. 31560102).
Appendix A. Supplementary dataSupplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.cclet.2016.12.011.
| [1] | Y. Q. Cheng, Flora Republicae Popularis Sinicae (Zhongguo Zhiwu Zhi), Science Press, Beijing, 1982 p. 9. |
| [2] | D.H. He, L.S. Ding, H.X. Xu, et al., Gymnothelignans A-O:conformation and absolute configuration analyses of lignans bearing tetrahydrofuran from Gymnotheca chinensis. J. Org. Chem. 77 (2012) 8435–8443. DOI:10.1021/jo301225v |
| [3] | S.J. Xiao, X.X. Lei, B. Xia, et al., Two novel norlignans from Gymnotheca chinensis. Tetrahedron Lett. 55 (2014) 2869–2871. DOI:10.1016/j.tetlet.2014.03.091 |
| [4] | S.J. Xiao, F. Chen, L.S. Ding, Y. Zhou. Two new eupodienone lignans from Gymnotheca chinensis. Chin. Chem. Lett. 25 (2014) 463–464. DOI:10.1016/j.cclet.2013.11.053 |
| [5] | S.J. Xiao, Y.Z. Lao, F. Chen, et al., Two new lignans from Gymnotheca chinensis Decne. Phytochem. Lett. 8 (2014) 38–40. DOI:10.1016/j.phytol.2014.01.007 |
| [6] | S.J. Xiao, D.M. Fang, B. Xia, et al., Biphenyl lignans with a tetrahydrofuran moiety from Gymnotheca chinensis Decne. Chin. J. Org. Chem. 34 (2014) 1677–1681. DOI:10.6023/cjoc201403017 |
| [7] | S.J. Xiao, D.H. He, D.M. Fang, et al., Further biphenyl lignans with a tetrahydrofuran moiety from Gymnotheca chinensis. Helv. Chim. Acta 97 (2014) 499–506. DOI:10.1002/hlca.v97.4 |
| [8] | S.J. Xiao, X.X. Lei, B. Xia, et al., Two novel polycyclic spiro lignans from Gymnotheca involucrata. Tetrahedron Lett. 55 (2014) 5949–5951. DOI:10.1016/j.tetlet.2014.09.044 |
| [9] | S.J. Xiao, D.L. Guo, B. Xia, et al., Polycyclic spiro lignans and biphenyl tetrahydrofuranone lignans from Gymnotheca involucrata. Planta Med. 82 (2016) 723–728. DOI:10.1055/s-00000058 |
| [10] | S. Mitra, S.R. Gurrala, R.S. Coleman. Totalsynthesis of the eupomatilones. J. Org. Chem. 72 (2007) 8724–8736. DOI:10.1021/jo701415m |
| [11] | R.S. Coleman, S.R. Gurrala. Total synthesis of eupomatilones 4 and 6:structurally rearranged and atropisomerically fluxional lignan natural products. Org. Lett. 6 (2004) 4025–4028. DOI:10.1021/ol048333e |
 2017, Vol. 28
2017, Vol. 28 



