In recent years, focus on green chemistry using environmen tally benign reagents and reaction conditions is one of the most fascinating developments in synthesis of widely used organic compounds [1, 2]. The use of fluorinated solvents has recently received a great deal of attention as potential new media for organic synthesis [3]. Owing to their unique physicochemical properties (high hydrogen bonding donor's ability, nonvolatility, nonflammability, polarity, high ionizing power, and low nucleophilicity), fluorinated alcohols modify the course of reactions when they are used as solvent, allowing reactions which usually require the use of added reagents or metal catalysts to be carried out under neutral and mild conditions [4, 5]. The most commonly used and cheapest fluorinated alcohols are 2, 2, 2-trifluoroethanol (TFE) and 1, 1, 1, 3, 3, 3-hexafluoroisopropanol (HFIP) low toxicity [6]. Ultrasound, an efficient and virtually innocuous means of activation in synthetic chemistry, has been employed for decades with various successes. This high-energy induces new reactivity leading to the formation of unexpected chemical species. The remarkable phenomenon of cavitation makes sonochemistry unique. The effects of ultrasound observed during organic reaction are due to cavitation, a physical process that creates, enlarges, and implodes gaseous and vaporous cavities in an irradiated liquid. Cavitation induces very high local temperatures and pressures inside the bubbles, leading to turbulent flow of the liquid and enhances mass transfer. When compared to conventional methods, ultrasound-accelerated chemical reactions can give high yields in shorter reaction times and milder conditions [7]. Because of these advantages, ultrasound irradiation has been used for the synthesis of a wide variety of organic compounds [8].
Organic compounds containing five-membered aromatic heterocyclic rings form a wide range of compounds in the nature and often play an important role in diverse biochemical processes. Consequently, aromatic heterocycles such as thiophenes, benzo thiophene derivatives, and their reduced forms are important structural fragments in many pharmaceutical and chemical compounds. Thiophenes and thiazole compounds have been found to indicate nematocidal, insecticidal, antibacterial, antifungal, antiviral, and antioxidant activity [9–15]. Tetrahydrothiophene is an important building block of a large quantity of compounds that are very interesting from the point of view of biological activity. Its derivatives have exhibited antisecretory and antiulcer activities [16]. The copper nanoparticles in an ionic liquid were employed as a recyclable catalyst for the synthesis of bis-(4-hydroxy-2-oxothiazolyl)methanes in excellent yields and in short reaction time [17]. In particular, it is found in structures of nucleoside analogs and certain compounds where the sulfur atom is in the ring, such as sulfimides, salicinol, and kotalanol, which are excellent glycosidase inhibitors [18–20]. We report here a novel method for preparation of 5, 5'-(arylmethylene)bis(4-hydroxythia zole-2(3H)-one) via one-pot pseudo five-component condensation of monochloroacetic acid (2 equiv.), ammonium thiocyanate (2 equiv.), and aromatic aldehydes (1 eq) in trifluoroethanol (TFE)/water (1:1) under ultrasound irradiation at room tempera ture.
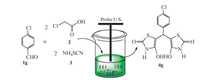
2. Results and discussionTo find out the suitable conditions for the reaction, a series of experiments were performed with the standard reaction of monochloroacetic acid, ammonium thiocyanate, and 4-chloroben zaldehyde (4g) as a model reaction (Scheme 1).
|
Download:
|
| Scheme 1. One-pot pseudo five-component Synthesis of 50-(chlorophenyle)bis(4-hydroxythiazole-2(3H)-one). | |
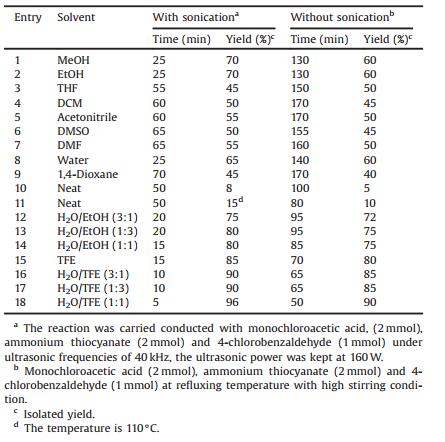
To find an appropriate reaction medium for the synthesis of 5, 5'-(arylmethylene)bis(4-hydroxythiazole-2(3H)-one), one-pot pseudo-five component reaction of monochloroacetic acid (2 mmol), ammonium thiocyanate (2 mmol) and 4-chlorobenzal dehyde (1 mmol) as a model reaction by detecting the efficiency of several classic solvents chosen as the for comparison with sonication conditions. When the reaction was carried out under solvent free conditions at room temperature and 110 ℃, we found 5% and 10% product (4g) (entries 10 and 11, Table 1). Nevertheless, the most dramatic improvement was observed when the solvent were switched from H2O to H2O/EtOH and TFE to TFE/EtOH. Therefore, the volume ratio of H2O/EtOH and TFE/EtOH were examined and the best results were obtained by carrying out the reaction in H2O/EtOH and TFE/EtOH with a ratio of 1:1 (v/v) (entries 12-18, Table 1). In order to verify the effect of ultrasound irradiation, the reaction was also performed in mentioned solvents by high stirring alone under silent condition (Table 1). As shown in Table 1, in all cases, the experimental results show that the yields of the products are lower than sonication within same reaction times. Based on the results of this study, it's clear that the ultrasound improves the yields of products. In our opinion, TFE act as Bronsted acid and play a significant role in increasing the electrophilic character of the electrophiles.
|
|
Table 1 Synthesis of (4g) in different solvents under with/without ultrasound irradiation. |
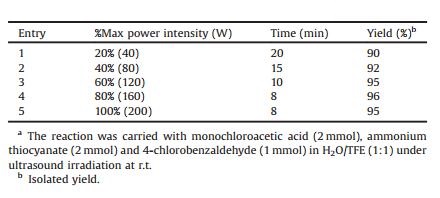
In order to investigate the effect of the ultrasonic intensity, power on the reaction, the reaction was also performed at 20%, 40%, 60%, 80% and 100% of the power rate of the ultrasonic probe (40, 80, 120, 160 and 200 W). The results are shown in Table 2. The intensity of sonication is proportional to the amplitude of vibration will lead to an increase in the intensity of vibration and to an increase in the sonochemical effects. Increasing the ultrasound power intensity up to 80% induces relatively higher yields ad shorter reaction times, beyond this level, the yield decrease slightly. Generally, the increase in the acoustic power could increase the number of active cavitation bubbles. Both increases can be expected to result in an increase in the maximum collapse temperature and the respective reaction could be accelerated. The results are summarized in Table 2. It can easily be seen that the condensation of a series of monochloroacetic acid, ammonium thiocyanate, and 4-chlorobenzaldehyde in H2O/TFE (1:1) under ultrasound leads to novel biologically important 5, 5'-(4-chlor ophenylmethylene)bis(4-hydroxythiazole-2(3H)-one) (4g).
|
|
Table 2 Effect of ultrasound intensity power on the synthesis of 4g.a |
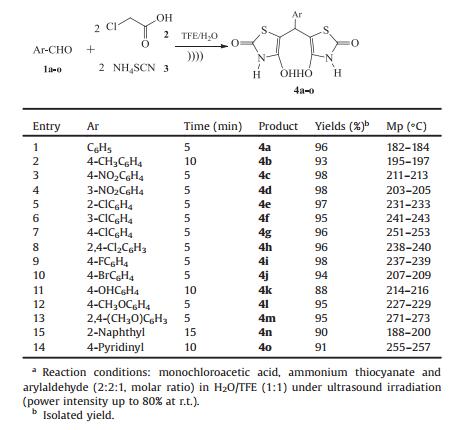
In the next step the scope and efficiency of the process was explored under the optimized conditions. For this purpose, monochloroacetic acid (2 mmol), ammonium thiocyanate (2 mmol) and a broad range of structurally diverse aromatic aldehydes (1 mmol) were condensed in the presence of ultrasonic irradiation at room temperature to achieve the desired product, and the results are displayed in Table 3. As shown in Table 3, products were obtained in excellent yields. It was found that aromatic aldehydes both with electron withdrawing and donating groups in reaction with other starting materials have excellent isolated yield.
|
|
Table 3 Pesudo five-component synthesis of 4a-o in H2O/TFE (1:1) under ultrasound irradiation at r.t.a |
The formation of compounds 4a-o could be explained by the reaction sequence in Fig. 1. On the basis of this mechanism, first, a condensation of monochloroacetic acid 2 with ammonium thiocyanate 3 is proposed to give the intermediate A to C and D. Then, the intermediate E is likely formed via condensation of aromatic aldehyde 1a-o with D which is tautomer of C. The next step is Michael addition of another intermediate of F to yield adduct G. Finally, after the tautomeric proton shift, the desired product 4a-o is formed (Fig. 1).

|
Download:
|
| Figure 1. Proposed mechanism for the syntheis of 4a-o in in fluoro alcohols under ultrasound irradiation at r.t. | |
3. Conclusion
In summary, we have found an efficient and practical procedure for the synthesis of 5, 5'-(arylmethylene)bis(4-hydroxythiazole-2 (3H)-one) via condensation monochloroacetic acid, ammonium thiocyanate, and aromatic aldehyde in an aqueous fluoroalcohol solvent system under ultrasound irradiation at room temperature.
4. Experimental 4.1. Reagents and equipmentProducts were characterized by comparison with authentic samples and by spectroscopy data (IR, 1H NMR and 13C NMR spectra). The NMR spectra were recorded on a Bruker Avance DEX500 MHz instrument. The spectra were measured in DMSO-d6 relative to TMS as the internal standard. Melting points were determined using Barnstead-Electro thermal 9300 Melting Points. Mass spectra were recorded on a VG micromass 7070H and Finnigan mat 1020 B mass spectrometers operating at 70 eV. Elemental analyses were performed on Yanco-CHN CORDER elementary analyzer. IR spectra were recorded on a Perkin-Elmer 781 spectrophotometer from KBr pelletes. All reagents were purchased from Merck and Aldrich and were used without further purification. Analytical TLC was performed on DC-Alufolien Silica gel 60F254 Merck. All yields refer to isolated products after purification. All sonochemical reactions were carried out in a thermostated (25 ± 1 ℃) ultrasonic cleaning bath at 40 kHz. The ultrasonic cleaner had an output power of 200 W. The tank dimensions were 290 × 240 × 150 mm with a liquid (water) holding capacity of 9.5 L. The reactions were carried out in a RB flask of 10 mL capacity, suspended at the center of the cleaning bath, 5 cm below the surface of the liquid.
4.2. Typical experimental procedureA mixture of monochloroacetic acid (2 mmol), ammonium thiocyanate (2 mmol) and 4-chlorobenzaldehyde (1 mmol) were stirred in one-pot in H2O/TFE (1:1) (5 mL) at refluxing temperature and ultrasound irradiation at room temperature for appropriate time (Table 3). The ultrasonic apparatus used to show the temperature automatically so the temperature was controlled and fixed at room temperature by pouring cold water in the bath in the case of any elevation of temperature. The progress of the reaction is monitored by TLC. After completion of the reaction, the corresponding solid product was obtained through simple filter ing, and recrystallized from ethanol affording the highly pure products (4a-m).
5, 5'-(Phenylmethylene)bis(4-hydroxythiazole-2(3H)-one) (4a): 0.341 g (96%), pale yellow solid, mp: 182–184 ℃. IR (KBr, cm-1): 3556-3233 (br.), 3098, 2987, 1698, 1530, 1443, 712. 1H NMR (500 MHz, DMSO-d6): δ 3.54 (s, 1H, CH), 7–8 (m, 5H, Ar-H), 8.32 (s, 2H, NH), 10.14 (s, 2H, OH). 13C NMR (125 MHz, DMSO-d6): δ 77.9, 125.7, 127.8, 128.9, 129.6, 132.6, 136.8, 140.9, 142.8, 143.7, 146.8, 176.9, 196.5. MS (EI) m/z (%): 322 (M+, 80), 245 (15). HRMS (EI) Found: 322.0303 M+, calcd. 322.0103 M+. Anal. Calcd. for C13H10N2O4S2: C, 48.44; H, 3.13; N, 8.69; S, 19.89. Found: C, 48.047; H, 3.15; N, 8.63; S, 19.90.
5, 5'-(p-Tolylmethylene)bis(4-hydroxythiazole-2(3H)-one) (4b): 0.342 g (93%), yellow solid, mp: 195–197 ℃. IR (KBr, cm-1): 3543–3223 (br.), 3088, 2987, 2876, 1689, 1523, 1423, 732. 1H NMR (500 MHz, DMSO-d6): δ 1.95 (s, 3H, CH3), 5.20 (s, 1H, CH), 7–8 (m, 5H, Ar-H), 8.43 (s, 2H, NH), 12.06 (s, 2H, OH). 13C NMR (125 MHz, DMSO-d6): δ 17.45, 44.47, 122.7, 124.7, 128.2, 135.9, 139.7, 141.5, 146.7, 149.8, 151.6, 167.9, 176.2, 196.9. MS (EI) m/z (%): 336 (M+, 56), 244 (35). HRMS (EI) Found: 336.006 M+, calcd. 336.0204 M+. Anal. Calcd. for C14H12N2O4S2: C, 49.99; H, 3.60; N, 8.33; S, 19.06. Found: C, 49.97; H, 3.61; N, 8.32; S, 19.08.
5, 5'-((4-Nitrophenyl)methylene)bis(4-hydroxythiazole-2(3H)-one) (4c): 0.391 g (98%), yellow solid, mp: 211–213 ℃. IR (KBr, cm-1): 3621-3265 (br.), 3102, 2899, 1693, 1522, 1465, 1342, 741. 1H NMR (500 MHz, DMSO-d6): δ 4.76 (s, 1H, CH), 7.49 (d, 2H, J = 7.5 Hz), 8.12 (d, 2H, J = 7.5 Hz), 8.09 (s, 2H, NH), 11.76 (s, 2H, OH). 13C NMR (125 MHz, DMSO-d6): δ 70.7, 121.8, 124.8, 125.8, 126.8, 128.7, 132.8, 142.8, 144.9, 149.0, 170.8, 175.9, 196.8. MS (EI) m/z (%) 367 (M+, 47), 244 (15). HRMS (EI) Found: 399.4903 M+, calcd. 366.9902 M+. Anal. Calcd. for C13H9N3O6S: C, 42.50; H, 2.47; N, 11.44; S, 17.46. Found: C, 42.52; H, 2.48; N, 11.45; S, 17.48.
5, 5'-((3-Nitrophenyl)methylene)bis(4-hydroxythiazole-2(3H)-one) (4d): 0.391 g (98%), pale yellow solid, mp: 203–205 ℃. IR (KBr, cm-1): 3601-3232 (br.), 3094, 2934, 1656, 1557, 1445, 1339, 708. 1H NMR (500 MHz, DMSO-d6): δ 4.97 (s, 1H, CH), 7.49–8.11 (m, 4H, Ar-H), 8.11 (s, 2H, NH), 12.86 (s, 2H, OH). 13C NMR (125 MHz, DMSO d6): δ 69.6, 126.8, 128.8, 146.6, 148.3, 144.8, 151.9, 152.7, 155.9, 165.8, 170.9, 175.0, 199.2. MS (EI) m/z (%) 367 (M+, 57), 244 (27). HRMS (EI) Found: 399.4903 M+, calcd. 366.9902 M+. Anal. Calcd. for C13H9N3O6S: C, 42.50; H, 2.47; N, 11.44; S, 17.46. Found: C, 42.52; H, 2.48; N, 11.45; S, 17.48.
5, 5'-((2-Chlorophenyl)methylene)bis(4-hydroxythiazole-2 (3H)-one) (4e): 0.376 g (97%), yellow solid, mp: 231–233 ℃. IR (KBr, cm-1): 3621-3165 (br.), 3096, 2812, 1683, 1520, 1456 1309, 7031. 1H NMR (500 MHz, DMSO-d6): δ 4.29 (s, 1H, CH), 7.20-7.87 (m, 4H, Ar-H), 8.61 (s, 2H, NH), 11.13 (s, 2H, OH). 13C NMR (125 MHz, DMSO d6): δ 77.9, 125.7, 127.8, 128.9, 129.7, 129.9, 132.7, 135.8, 141.8, 144.7, 174.7, 175.9, 194.9. MS (EI) m/z (%) 356 (M+, 19), 244 (16). HRMS (EI) Found: 355.9703 M+, calcd. 355.9704 M+. Anal. Calcd. for C13H9ClN2O4S2: C, 43.76; H, 2.54; N, 17.94; S, 17.97. Found: C, 43.74; H, 2.55; N, 17.96; S, 17.99.
5, 5'-((3-Chlorophenyl)methylene)bis(4-hydroxythiazole-2 (3H)-one) (4f): 0.368 g (95%), white solid, mp: 241–243 ℃. IR (KBr, cm-1): 3601-3112 (br.), 3089, 2821, 1690, 1565, 1485, 1328, 711. 1H NMR (500 MHz, DMSO-d6): δ 8.14 (s, 2H, NH), 4.62 (s, 1H, CH), 7.32–8.10 (m, 4H, Ar-H), 8.14 (s, 2H, NH), 11.76 (s, 2H, OH). 13C NMR (125 MHz, DMSO-d6): δ 76.9, 122.8, 123.8, 127.8, 130.6, 134.8, 141.9, 143.8, 145.9, 150.9, 151.0, 170.8, 196.5. MS (EI), m/z (%) 356 (M+, 32), 244 (21). HRMS (EI) Found: 355.9703 M+, calcd. 355.9704 M+. Anal. Calcd. for C13H9ClN2O4S2: C, 43.76; H, 2.54; N, 17.94; S, 17.97. Found: C, 43.74; H, 2.55; N, 17.96; S, 17.99.
5, 5'-((4-Chlorophenyl)methylene)bis(4-hydroxythiazole-2 (3H)-one) (4g): 0.383 g (96%), pale yellow solid, mp: 251–253 ℃. IR (KBr, cm-1): 3565-3167 (br.), 3088, 2805, 1663, 1512, 1433, 1323, 721. 1H NMR (500 MHz, DMSO-d6): δ 4.56 (s, 1H, CH), 7.18 (d, 2H, J = 7.5 Hz), 7.65 (d, 2H, J = 7.5 Hz), 8.41 (s, 2H, NH), 12.83 (s, 2H, OH). 13C NMR (125 MHz, DMSO-d6): δ 74.8, 129.2, 130.8, 131.6, 134.9, 141.8, 142.4, 143.6, 147.8, 150.8, 172.8, 175.2, 197.2. (EI) m/z (%)356 (M+, 43), 244 (28). HRMS (EI) Found: 355.9703 M+, calcd. 355.9704 M+. Anal. Calcd. for C13H9ClN2O4S2: C, 43.76; H, 2.54; N, 17.94; S, 17.97. Found: C, 43.74; H, 2.55; N, 17.96; S, 17.99.
5, 5'-((2, 4-Dichlorophenyl)methylene)bis(4-hydroxythiazole-2 (3H)-one) (4h): 0.406 g (96%), yellow solid, mp: 238–240 ℃. IR (KBr, cm-1): 3683-3111 (br.), 3079, 2834, 1676, 1555, 1459, 1389, 741. 1H NMR (500 MHz, DMSO-d6): δ 4.78 (s, 1H, CH), 7.15 (d, 1H, J = 7.5 Hz), 7.31 (d, J = 7.5 Hz, 1H), 7.65 (d, 2H, J = 7.5 Hz), 8.32 (s, 2H, NH), 11.80 (s, 2H, OH). 13C NMR (125 MHz, DMSO-d6): δ 69.8, 128.8, 130.7, 132.6, 134.8, 157.8, 140.8, 141.8, 146.8, 151.6, 171.8, 178.9, 198.5. MS (EI), m/z (%) 391 (M+, 30), 244 (16). HRMS (EI) Found: 389.9306 M+, calcd. 389.9304 M+. Anal. Calcd. for C13H8Cl2N2O4S2: C, 39.91; H, 2.06; N, 7.16; S, 16.39. Found: C, 39.93; H, 2.03; N, 7.15; S, 16.40.
5, 5'-((4-Flouro-4-fluorophenyl)methylene)bis(4-hydroxythia zole-2(3H)-one) (4i): 0.364 g (98%), pale yellow solid, mp: 237–239 ℃. IR (KBr, cm-1): 3620–3094 (br.), 3065, 2865, 1676, 1512, 1424, 1354, 731. 1H NMR (500 MHz, DMSO-d6): δ 4.25 (s, 1H, CH), 7.11 (d, 2H, J = 7.5 Hz), 7.65 (d, 2H, J = 7.5 Hz), 8.18 (s, 2H, NH), 12.01 (s, 2H, OH). 13C NMR (125 MHz, DMSO-d6): δ 72.9, 118.2, 123.7, 127.9, 131.8, 132.9, 133.6, 137.9, 159.2, 161.8, 171.0, 175.2, 198.8. 19F NMR (470 MHz, DMSO-d6): δ 73.44 (s, 1F); MS (EI) m/z (%) 340 (M+, 18), 244 (15). HRMS (EI) Found: 340.0002 M+, calcd. 340.0001 M+. Anal. Calcd. for C13H9FN2O4S2: C, 45.88; H, 2.67; N, 8.23; S, 18.84. Found: C, 45.86; H, 2.66; N, 8.25; S, 18.46.
5, 5'-((4-Bromophenyl)methylene)bis(4-hydroxythiazole-2 (3H)-one) (4j): 0.407 g (94%), brown solid, mp: 207–209 ℃. IR (KBr, cm-1): 3643-3154 (br.), 3101, 2843, 1676, 1520, 1498, 1376, 711. 1H NMR (500 MHz, DMSO-d6): δ 4.16 (s, 1H, CH), 7.43 (d, 2H, J = 7.5 Hz), 7.89 (d, 2H, J = 7.5 Hz), 8.14 (s, 2H, NH), 12.13 (s, 2H, OH). 13C NMR (DMSO-d6, 125 MHz): δ 74.8, 120.8, 133.8, 134.9, 135.8, 141.8, 146.8, 147.9, 150.9, 152.9, 170.9, 175.9, 198.9. MS (EI) m/z (%) 401 (M+, 64), 244 (7). HRMS (EI) Found: 399.9201 M+, calcd. 399.9202 M+. Anal. Calcd. for C13H9BrN2O4S2: C, 38.91; H, 2.26; N, 6.98; S, 15.98. Found: C, 38.92; H, 2.23; N, 6.96; S, 15.95
5, 5'-((4-hydroxyphenyl)methylene)bis(4-hydroxythiazole-2 (3H)-one) (4k): 0.325 g (88%), pale yellow solid, mp: 214–216 ℃. IR (KBr, cm-1): 3601–3111 (br.), 3069, 2832, 1675, 1541, 1487, 1365, 710. 1H NMR (500 MHz, DMSO-d6): δ 4.18 (s, 1H, CH), 6.81 (d, 2H, J = 7.8 Hz), 7.18 (d, 2H, J = 7.8 Hz), 9.31 (s, 1H, OH), 8.22 (s, 2H, NH), 12.23 (s, 2H, OH). 13C NMR (125 MHz, DMSO-d6): δ 75.9, 118.9, 121.2, 131.4, 136.9, 139.9, 141.9, 148.9, 156.4, 157.9, 172.9, 177.2, 199.3. MS (EI) m/z (%) 338 (M+, 12), 244 (15) HRMS (EI) Found: 338.0004 M+, calcd. 338.0002 M+. Anal. Calcd. for C13H10N2O5S2: C, 46.15; H, 2.98; N, 8.28; S, 18.95. Found: C, 46.13; H, 2.99; N, 8.27; S, 18.94.
5, 5'-((4-Methoxyphenyl)methylene)bis(4-hydroxythiazole-2 (3H)-one) (4l): 0.364 g (95%), yellow solid, mp: 227–229 ℃. IR (KBr, cm-1): 3698-3119 (br.), 31018, 2827, 1660, 1554, 1487, 1367, 730. 1H NMR (500 MHz, DMSO-d6): δ 3.87 (s, 3H, CH3), 4.82 (s, 1H, CH), 6.89 (d, 2H, J = 7.5 Hz), 7.23 (d, 2H, J = 7.5 Hz), 8.31 (s, 2H, NH), 12.91 (s, 2H, OH). 13C NMR (125 MHz, DMSO-d6): δ 45.9, 67.3, 121.8, 130.2, 132.9, 134.5, 137.9, 139.9, 143.6, 147.9, 159.8, 160.9, 170.9, 177.2, 198.1. MS (EI) m/z (%) 352 (M+, 17), 244 (14) HRMS (EI) Found: 352.0303M+, calcd. 352.0202M+. Anal. Calcd. for C14H12N2O6S2: C, 47.72; H, 3.43; N, 7.95; O, 22.70; S, 18.20. Found: C, 43.71; H, 3.44; N, 7.396; S, 18.22.
5, 5'-((2, 4-Dimethoxyphenyl)methylene)bis(4-hydroxythia zole-2(3H)-one) (4m): 0.393 g (95%), yellow solid, mp: 271–273 ℃. IR (KBr, cm-1): 3504-3133 (br.), 3069, 2817, 1619, 1523, 1463, 1354, 732. 1H NMR (500 MHz, DMSO-d6): δ 3.94 (s, 6H, 2 × CH3), 4.54 (s, 1H, CH), 6.78 (d, 2H, J = 7.7 Hz), 7.85 (s, 2H, Ar-H), 8.09 (d, 1H, J = 7.7 Hz, Ar-H), 8.15 (s, 2H, NH), 12.63 (s, 2H, OH). 13C NMR (125 MHz, DMSO-d6): δ 21.9, 55.9, 67.8, 121.9, 122.8, 123.8, 134.9, 136.8, 139.8, 159.2, 159.6, 170.8, 178.9, 199.9. MS (EI) m/z (%) 382 (M+, 37), 244 (18). HRMS (EI) Found: 382.0303 M+, calcd. 382.0302 M+. Anal. Calcd. for C15H14N2O6S2: C, 47.11; H, 3.69; N, 7.33; S, 16.77. Found: C, 47.12; H, 3.70; N, 7.35; S, 16.72.
5, 5'-(Naphthalen-2-ylmethylene)bis(4-hydroxythiazole-2 (3H)-one) (4n): 0.363 g (90%), pale yellow solid, mp: 188–200 ℃. IR (KBr, cm-1): 3678-3098 (br.), 3076, 2843, 1663, 1502, 1433, 1343, 711. 1H NMR (500 MHz, DMSO-d6): δ 4.85 (s, 1H, CH), 7.19-8.03 (m, 7H, Ar-H), 8.11 (s, 2H, NH), 12.10 (s, 2H, OH). 13C NMR (125 MHz, DMSO-d6): δ 84.9, 121.8, 125.9, 126.9, 127.0, 127.2, 127.6, 128.9, 131.9, 134.8, 135.8, 137.8, 138.9, 143.8, 171.9, 176.8, 196.7. MS (EI) m/z (%) 372 (M+, 35), 244 (21). HRMS (EI) Found: 372.0202 M+, calcd. M+, 372.0201. Anal. Calcd. for C17H12N2O2S2: C, 54.83; H, 3.25; N, 7.52; S, 17.22. Found: C, 50.82; H, 3.26; N, 7.53; S, 17.24.
5, 5'-(Pyridin-4-ylmethylene)bis(4-hydroxythiazole-2(3H)-one) (4o): 0.323 g (91%), pale yellow solid, mp: 255–257 ℃. IR (KBr, cm-1): 3532–3107 (br.), 3099, 2825, 1653, 1502, 1463, 1343, 711. 1H NMR (500 MHz, DMSO-d6): δ 4.45 (s, 1H, CH), 7.33 (d, 2H, J = 7.8 Hz), 7.75 (d, 2H, J = 7.5 Hz), 8.47 (s, 2H, NH), 11.87 (s, 2H, OH). 13C NMR (125 MHz, DMSO-d6): δ 67.7, 121.8, 124.8, 149.7, 150.7, 151.7, 152.8, 152.8, 153.7, 170.6, 175.9, 198.9. MS (EI) m/z (%) 323 (M+, 27), 244 (35). HRMS (EI) Found: 323.0001 M+, calcd. 323.0002 M+. Anal. Calcd. for C12H9N3O4S2: C, 44.57; H, 2.81; N, 13.00; S, 19.83. Found: C, 40455; H, 2.83; N, 13.02; S, 19.82.
AcknowledgmentWe are grateful for the research council of Payame Noor University.
| [1] | P. T. Anastas, J. C. Warner, Green Chemistry, 1998, Theory and Practice, Oxford University Press, Oxford, UK, 1998. |
| [2] | P. T. Anastas, T. Williamson, Green Chemistry, Frontiers in Benign Chemical Synthesis and Process, Oxford University Press, Oxford, UK, 1998. |
| [3] | J.P. Begv, B. Crousse, D. Bonnet, - Delpon. Fluorinated alcohols:a new medium for selective and clean reaction. Synlett (2004) 18–29. |
| [5] | M.O. Ratnikov, V.V. Tumanov, W.A. Smit. Lewis acid catalyst free electrophilic alkylation of silicon-capped π donors in 1, 11, 3, 3, 3-hexafluoro-2-propanol. Angew. Chem. Int. Ed. 47 (2008) 9739–9742. DOI:10.1002/anie.v47:50 |
| [6] | J.T.C. Wojtyk, P.M. Kazmaier, E. Buncel. Effects of metal ion complexation on the spiropyran-merocyanine interconversion:development of a thermally stable photo-switch. Chem. Commun (1998) 1703–1704. |
| [7] | G. Gravotto, P. Cintas. Power ultrasound in organic synthesis:moving cavitational chemistry from academia to innovative and large-scale applications. Chem. Soc. Rev. 35 (2006) 180–196. DOI:10.1039/B503848K |
| [8] | E.A. Muravyova, S.M. Desenko, V.I. Musatov, et al., Ultrasonic-promoted threecomponent synthesis of some biologically active 1, 2, 5, 6-tetrahydropyrimidines. J. Comb. Chem. 9 (2007) 797–803. DOI:10.1021/cc700089a |
| [9] |
(a) H. Pessoa-Mahana, K. C. Johann, R. H. Nadia, et al. , Solvent-free microwave synthesis of 3-(4-benzo[b]thiophene-2-carbonyl)-1-piperazinyl-1-benzo[b] thiophen-2-yl-1-propanones. New hetero bis-ligands with potential 5-HT1A serotonergic activity, Heterocycles 75(2008) 1913-1929; (b) J. Blagg, C. Mowbray, D. C. Pryde, et al. , Non-peptide C5 a receptor antagonists: part 1, Bioorg. Med. Chem. Lett. 18(2008) 5601-5604. |
| [10] |
(a) D. Simoni, R. Romagnoli, R. Baruchello, et al. , Novel A-ring and B-ring modified combretastatin A-4(CA-4) analogues endowed with interesting cytotoxic activity, J. Med. Chem. 51(2008) 6211-6215; (b) M. A. A. Radwan, M. A. Shehab, S. M. El-Shenawy, Synthesis and biological evaluation of 5-substituted benzo[b]thiophene derivatives as antiinflammatory agents, Monatsh. Chem. 140(2009) 445-450. |
| [11] |
(a) I. M. I. Fakar, M. A. A. Radwan, S. El-Batran, O. M. E. Abd El-Salam, S. M. ElShenawy, Synthesis and pharmacological evaluation of 2-substitutedbenzo[b] thiophenes as anti-inflammatory and analgesic agents, Eur. J. Med. Chem. 44(2009) 1718-1725; (b) R. M. V. Abreu, I. C. F. R. Ferreira, M. J. R. P. Queiroz, QSAR model for predicting radical scavenging activity of di (hetero) arylamines derivatives of benzo[b] thiophenes, Eur. J. Med. Chem. 44(2009) 1952-1958. |
| [12] | J.C. Aloup, J. Bouchaudon, D. Farge, et al., Synthesis and antisecretory and antiulcer activities of derivatives and analogs of 2-(2-pyridyl) tetrahydrothiophene-2-carbothioamide. Med. Chem. 30 (1987) 24–29. DOI:10.1021/jm00384a004 |
| [13] | N. Azizi, M. Hasani, M. Khajeh, M. Edrisi. A straightforward and sustainable one-pot, four-component synthesis of rhodanine derivatives. Tetrahedron Lett. 56 (2015) 1189–1192. DOI:10.1016/j.tetlet.2015.01.102 |
| [14] | V. Ramesh, B.A. Rao, P. Sharma, et al., Synthesis and biological evaluation of new rhodanine analogues bearing 2-chloroquinoline and benzo. Eur. J. Med. Chem. 83 (2014) 569–580. DOI:10.1016/j.ejmech.2014.06.013 |
| [15] | S.J. Singh, S.M.S. Chauhan. Potassium carbonate catalyzed one pot fourcomponent synthesis of rhodanine derivatives. Tetrahedron Lett. 54 (2013) 2484–2488. DOI:10.1016/j.tetlet.2013.03.004 |
| [16] | (a) D. V. Selwood, K. Carter, R. J. Young, K. S. Jandu, Synthesis and biological evaluation of the L-enantiomer of 2-deoxy-5-ethyl-4-thiouridine, Bioorg. Med. Chem. Lett. 8(1996) 991-994. |
| [17] | P. Singh, A. Katyal, R. Kalra, R. Chandra. Copper nanoparticles in an ionic liquid:an efficient catalyst for the synthesis of bis-(4-hydroxy-2-oxothiazolyl) methanes. Tetrahedron Lett. 49 (2008) 727–730. DOI:10.1016/j.tetlet.2007.11.106 |
| [18] | H. Yuasa, T. Kajimoto, W.C.H. Ong. Synthesis of iminothiasugar as a potential transition-state analog inhibitor of glycosyltransfer reactions. Tetrahedron Lett. 35 (1994) 8243–8246. DOI:10.1016/0040-4039(94)88293-2 |
| [19] |
(a) M. Yoshikawa, T. Murakami, H. Shimada, et al. , Salacinol, potent antidiabetic principle with unique thiosugar sulfonium sulfate structure from the Ayurvedic traditional medicine Salacia reticulata in Sri Lanka and India, Tetrahedron Lett 38(1997) 8367-8370; (b) M. Yoshikawa, T. Murakami, K. Yashiro, H. Matsuda, A potent α-glucosidase inhibitor with thiosugar sulfonium sulfate structure, from antidiabetic Ayurvedic medicine Salacia reticulate, Chem. Pharm. Bull. 46(1998) 1339-1340. |
| [20] | E.A. Muravyova, S.M. Desenko, V.I. Musatov, et al., Ultrasonic-promoted threecomponent synthesis of some biologically active 1, 2, 5, 6-tetrahydropyrimidines. J. Comb. Chem. 9 (2007) 797–803. DOI:10.1021/cc700089a |
 2017, Vol. 28
2017, Vol. 28