b Department of Chemistry, University of Louisville, Louisville, KY 40208, USA;
c Scientific Instrument Center, Shanxi University, Taiyuan 030006, China;
d College of Chemistry and Chemical Engineering, Shanxi University, Taiyuan 030006, China
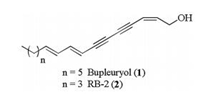
Polyacetylenes are a family of structurally diverse natural products [1], which show interesting biological activity, such as antitumor [2], antibacterial [3], anti-obesity [4], antidiabetic effect [5], cytoprotection [6], immunosuppressive activity [7, 8] and anti inflammatory effects [9]. Recently, 1, 3-diyne-type polyacetylenes have attracted broad attention from both chemists and biologists [10, 11]. Bupleurynol (1) and its analog (RB-2) (2) are natural products that were first isolated from the Bupleurum longiradiatum in 1987 [12] and 1999 [13], respectively (Fig. 1). We also isolated bupleurynol (1), RB-2 (2) and their isomers from Bupleuri Radix (Chaihu in Chinese) [14]. Our study further demonstrated that these natural products could increase the level of monoamines such as 5-hydroxytryptamine (5-HT) and norepinephrine (NE) in mice. Due to their low abundance in nature and exploring the relationship between the structural motifs and the interesting biological activities of bupleurynol (1) and RB-2 (2), a versatile and efficient route to synthesize them is required.
|
Download:
|
| Figure 1. The structures of bupleuryol and RB-2. | |
The first synthesis of bupleurynol was completed by the Organ and Antunes [15]. Ghasemi et al. further developed a single operation to synthesize bupleurynol using queued cross-coupling reactions [16]. However, these strategies suffered from low yields and lack of versatility for synthesizing other polyacetylene natural products. Hence, it remains as a challenge to develop a versatile and efficient strategy for the total syntheses of bupleurynol and its analogs. Herein, we reported a highly versatile route for the total syntheses of bupleurynol and RB-2 from a common intermediate.
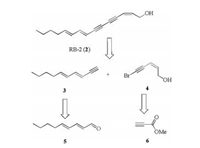
2. Results and discussionOur retrosynthetic analysis for RB-2 is depicted in Scheme 1. According to the previously reported method, RB-2 could be prepared from the terminal alkyne 3 and the enyne fragment 4 through the Cadiot–Chodkiewicz reaction [17]. The terminal alkyne 3 might be derived from the conjugated aldehyde 5 through a Corey–Fuchs reaction, while the enyne fragment 4 could be prepared from the methyl propionate 6 by a Sonogashira cross coupling and bromination.
|
Download:
|
| Scheme 1. Retrosynthetic analysis of RB-2 (2). | |
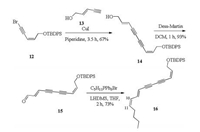
Our synthesis began with the preparation of the cis alkenyl iodide 7, which was obtained in 89% yield by heating methyl propiolate 6 and lithium iodide in acetic acid [18] (Scheme 2). The alkenyl iodide 7 was treated with ethynyltrimethylsilane in a Sonogashira cross-coupling reaction to give the enyne product 8 in 96% yield without isomerization. Compound 8 was converted to allyl alcohol 9 using diisobutylaluminum hydride at -78 ℃ in 92% yield [19].

|
Download:
|
| Scheme 2. The synthesis of intermediate 12. | |
Initially, the trimethyl chlorosilane (TMS) protecting group was removed using potassium carbonate to make the terminal alkyne, which included the free alcohol group. However, the allyl alcohol is extremely volatile and unstable. Therefore, it was protected with tert-butyl(chloro)diphenylsilane (TBDPS) group, which was fol lowed by the removal of TMS to give the compound 11 [20]. The bromoacetylene 12 was eventually obtained by the treatment of 11 with N-bromosuccinimide (NBS) in the presence of a catalytic amount of AgNO3 [21] and the absence of light.
With the requisite protection version of right fragment in hand, we developed a concise route to synthesize the (3E, 5E)-deca-3, 5-dien-1-yne 3 fragment from the commercially available aldehyde 5. However, the expected homologation product turned out to be more difficult to prepare. As summarized in Table 1, treatment of compound 5 via the Corey–Fuchs reaction gave only a trace amount of desired product [22]. Treatment of the aldehyde 5 with (bromomethyl)triphenylphosphonium bromide in the presence of potassium tert-butoxide afforded the bromo alkene compound [23]. The alkyne 3 was also detected based on the NMR of the crude product using the dimethyl-(1-diazo-2-oxopropyl) phosphonate (the Bestmann–Ohira reagent) [24], but the further purification of the alkyne turned out to be impractical due to the instability of compound 3.
|
|
Table 1 Conversion of aldehyde 5 to the terminal alkyne 3. |
In view of these results, a new approach was developed to synthesize the natural product, by coupling (E)-pent-2-en-4-yn-1-ol 13 and fragment 12 to form the C10–C11 double bond. Compound 13 is commercial available. Using copper iodide as the catalyst [25], coupling compound 12 with 13 under Cadiot– Chodkiewicz conditions provided the diyne intermediate 14 with 67% yield (Scheme 3). The free alcohol 14 was easily transformed into the key intermediate 15 in the presence of the Dess–Martin reagent which paved the way for the chain elongation through olefination [26]. With the main intermediate 15 in hand, we next focused on the olefination reaction to assemble the trans double bond. We first examined the feasibility of constructing the trans double bond under Wittig reaction conditions using commercially available pentyltriphenylphosphonium bromide [27]. Unfortu nately, we obtained only the cis double bond rather than the trans double bond.
|
Download:
|
| Scheme 3. The synthesis of the olefination reaction precursor 15 and the Wittig reaction. | |
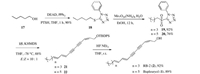
In our previous study [28], the one-pot Julia–Kocienski (J–K) olefination with a proper substrate pair proved be a powerful tool to prepare the trans conjugated double bond. Thus, the sulfide 18 was obtained in good yield upon the treatment of the primary alcohol 17 through a Mitsunobu thioetherification. The sulfide 18 underwent a molybdenum-catalyzed oxidation and smoothly afforded the sulfone 19 that could be the substrate for the J–K olefination (Scheme 4). Delightfully, the coupling of 15 and 19 through one-pot J–K olefination furnished the desired (E/E/Z)-triene substrate 21 in a good yield with excellent E/Z-selectivity (10:1) using the Barbier-type J–K protocol. Remarkably, the diyne and the cis double bond were stable even under strongly basic conditions. Finally, the removal of the TBDPS group with triethyl amine trihydrofluoride successfully yielded the natural product RB-2 (2) in 92% yield. The general applicability of this strategy was further demonstrated in the synthesis of bupleurynol 1.
|
Download:
|
| Scheme 4. Total synthesis of RB-2 (2) and bupleurynol (1). | |
Spectroscopic characterization (1H NMR, 13C NMR and high resolution mass spectrometry) confirmed that our synthetic bupleurynol and RB-2 and bupleurynol 1 were identical to the natural products [12, 13]. The spectral data of RB-2 and bupleurynol 1 are presented in the Supporting information. Consistent with the report for the natural isolate, a slow isomerization to the (Z/E/Z) isomer in neat form was observed in bupleurynol.
3. ConclusionIn summary, the concise total syntheses of RB-2 and bupleur ynol have been achieved in 10 linear steps with 26% and 22% yield, respectively. The synthesis relies on a Sonogashira cross-coupling, a Cadiot–Chodkiewicz coupling and a one-pot J–K olefination. Moreover, the access to the versatile intermediate 15, which is a common structural motif in the related polyacetylenes [29, 30], would facilitate synthetic endeavors toward other natural prod ucts. Further studies focusing on the biological evaluation of RB-2 and its derivatives are in progress and will be reported in due course.
4. ExperimentalAll reactions were carried out under an argon atmosphere with dry solvents. All reagents were obtained from commercial sources and used without further purification. NMR spectra were recorded on Bruker AV-600 spectrometers at ambient temperature, using TMS as an internal standard. High resolution mass spectra (HRMS) were recorded at a Mass Spectrometry Laboratory using a Thermo Scientific Q Exactive. Spectra of all the compounds are presented in the Supporting information.
AcknowledgmentsThis work was financially supported by National Natural Science Foundation of China (Nos. 21402111, 81473415), Basic research program of Shanxi Province (No. 2015021039), Shanxi Scholarship Council of China (No. 2015-020) and science and technology innovation project of Shanxi Province (No. 2016115).
Appendix A. Supplementary dataSupplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.cclet.2016.11.032.
| [1] | R. Negri, Polyacetylenes from terrestrial plants and fungi: recent phytochemical and biological advances, Fitoterapia 106(2015) 92-109. |
| [2] | W. Heydenreuter, E. Kunold, S.A. Sieber. Alkynol natural products target ALDH2 in cancer cells by irreversible binding to the active site. Chem. Commun. 51 (2015) 15784–15787. DOI:10.1039/C5CC06424D |
| [3] | H. Matsuura, G. Saxena, S. Farmer, et al., Antibacterial and antifungal polyine compounds from Glehnia littoralis ssp. leiocarpa. Planta Med. 62 (1996) 256–259. DOI:10.1055/s-2006-957872 |
| [4] | L.F. Liang, T. Wang, Y.S. Cai, et al., Brominated polyunsaturated lipids from the Chinese sponge Xestospongia testudinaria as a new class of pancreatic lipase inhibitors. Eur. J. Med. Chem. 79 (2014) 290–297. DOI:10.1016/j.ejmech.2014.04.003 |
| [5] | H. Kim, J. Chin, H. Choi, et al., Phosphoiodyns A and B, unique phosphoruscontaining iodinated polyacetylenes from a Korean sponge Placospongia sp. Org. Lett. 15 (2012) 100–103. |
| [6] | T.C. Chou, H. Dong, X. Zhang, et al., Multifaceted cytoprotection by synthetic polyacetylenes inspired by the ginseng-derived natural product panaxytriol. Proc. Natl. Acad. Sci. U. S. A. 108 (2011) 14336–14341. DOI:10.1073/pnas.1111332108 |
| [7] | B. Zhang, Y. Wang, S.P. Yang, et al., Ivorenolide A, an unprecedented immunosuppressive macrolide from Khaya ivorensis:structural elucidation and bioinspired total synthesis. J. Am. Chem. Soc. 134 (2012) 20605–20608. DOI:10.1021/ja310482z |
| [8] | Y. Wang, Q.F. Liu, J.J. Xue, et al., Ivorenolide B, an immunosuppressive 17-membered macrolide from Khaya ivorensis:structural determination and total synthesis. Org. Lett. 16 (2014) 2062–2065. DOI:10.1021/ol500667d |
| [9] | H. Dong, L. He, M. Huang, et al., Anti-inflammatory components isolated from Atractylodes macrocephala Koidz. Nat. Prod. Res. 22 (2008) 1418–1427. DOI:10.1080/14786410801931629 |
| [10] | Z.F. Zhou, M. Menna, Y.S. Cai, et al., Polyacetylenes of marine origin:chemistry and bioactivity. Chem. Rev. 115 (2015) 1543–1596. DOI:10.1021/cr4006507 |
| [11] | A.L. Shi Shun. Tykwinski, Synthesis of naturally occurring polyynes. Angew. Chem. Int. Ed. 45 (2006) 1034–1057. DOI:10.1002/(ISSN)1521-3773 |
| [12] | J. Zhao, Y.Z. Guo, X.S. Meng. The toxic principles of Bupleurum longiradiatum. Acta Pharm. Sin. 22 (1987) 507–511. |
| [13] | H.Q. Huang, X. Zhang, Y.H. Shen, et al., Polyacetylenes from Bupleurum longiradiatum. J. Nat. Prod. 72 (2009) 2153–2157. DOI:10.1021/np900534v |
| [14] | J. Liu, Y. Fang, L. Yang, et al., A qualitative and quantitative determination and pharmacokinetic study of four polyacetylenes from Radix Bupleuri by UPLCPDA-MS. J. Pharm. Biomed. Anal. 111 (2015) 257–265. DOI:10.1016/j.jpba.2015.04.002 |
| [15] | L.M. Antunes, M.G. Organ. Metal-catalyzed coupling reactions on an olefin template:the total synthesis of bupleurynol. Tetrahedron Lett. 44 (2003) 6805–6808. DOI:10.1016/S0040-4039(03)01753-2 |
| [16] | H. Ghasemi, L.M. Antunes, M.G. Organ. Use of olefin templates in queued chemical transformations using late transition metal catalysis. Total synthesis of cis and trans bupleurynol via a single multireaction sequence. Org. Lett. 6 (2004) 2913–2916. DOI:10.1021/ol0489853 |
| [17] | K.S. Sindhu, A.P. Thankachan, P.S. Sajitha, et al., Recent developments and applications of the Cadiot-Chodkiewicz reaction. Org. Biomol. Chem. 13 (2015) 6891–6905. DOI:10.1039/C5OB00697J |
| [18] | S. Ma, X. Lu, Z. Li. A novel regio-and stereospecific hydrohalogenation reaction of 2-propynoic acid and its derivatives. J. Org. Chem. 57 (1992) 709–713. DOI:10.1021/jo00028a055 |
| [19] | X.Y. Jiang, X.H. Xu, F.L. Qing. Design and concise synthesis of gemdifluoromethylenated analogue of 7-epi-castanospermine. Chin. Chem. Lett. 25 (2014) 1115–1120. DOI:10.1016/j.cclet.2014.04.018 |
| [20] | M. Wan, M. Yao, J.Y. Gong, et al., Synthesis of the tetracyclic core of chlorospermines. Chin. Chem. Lett. 26 (2015) 272–276. DOI:10.1016/j.cclet.2015.01.037 |
| [21] | F. Bellina, A. Carpita, L. Mannocci, et al., First total synthesis of naturally occurring (-)-nitidon and its enantiomer. Eur. J. Org. Chem. 12 (2004) 2610–2619. |
| [22] | E.J. Corey, P.L. Fuchs. A synthetic method for formyl→ethynyl conversion (RCHO→RCCH or RC=CR'). Tetrahedron Lett. 13 (1972) 3769–3772. DOI:10.1016/S0040-4039(01)94157-7 |
| [23] | P. Kiliçkiran, H. Hopf, I. Dix, et al., Preparation of highly hindered polyenynes. Eur. J. Org. Chem. 21 (2010) 4035–4045. |
| [24] | J. Carreras, G. Gopakumar, L. Gu, et al., Polycationic ligands in gold catalysis:synthesis and applications of extremely p-acidic catalysts. J. Am. Chem. Soc. 135 (2013) 18815–18823. DOI:10.1021/ja411146x |
| [25] | D.J. Galler, K.A. Parker. Five easy pieces. The total synthesis of phosphoiodyn A (and placotylene A). Org. Lett. 17 (2015) 5544–5546. DOI:10.1021/acs.orglett.5b02642 |
| [26] | D. Dess, J. Martin. Readily accessible 12-I-5 oxidant for the conversion of primary and secondary alcohols to aldehydes and ketones. J. Org. Chem. 48 (1983) 4155–4156. DOI:10.1021/jo00170a070 |
| [27] | D.L. Chen, F.P. Wang, X.Y. Liu. A convergent approach to the tetracyclic core of atisane diterpenes. Chin. Chem. Lett. 27 (2016) 59–62. DOI:10.1016/j.cclet.2015.09.005 |
| [28] | K. Ma, D. Liao, S. Yang, et al., Studies towards the synthesis of the functionalized C3-C14 decalin framework of alchivemycin A. Org. Chem. Front. 3 (2016) 251–258. DOI:10.1039/C5QO00343A |
| [29] | Z. Zhang, C. Lu, X. Liu, et al., Global and targeted metabolomics reveal that bupleurotoxin, a toxic type of polyacetylene, induces cerebral lesion by inhibiting GABA receptor in mice. J. Proteome Res. 13 (2013) 925–933. |
| [30] | B. Schmidt, S. Audorsch. Stereoselective total synthesis of atractylodemayne A, a conjugated 2(E), 8(Z), 10(E)-triene-4, 6-diyne. Org. Lett. 18 (20016) 1162–1165. |
 2017, Vol. 28
2017, Vol. 28 



