b College of Chemistry, Shahrood University of Technology, Shahrood, Iran
The indole moiety is a privileged structural motif in many biologically active and medicinally valuable molecules [1, 2]. Polycyclic frameworks lead to relatively rigid structures that might be expected to show substantial selectivity in their interactions with enzymes or receptors [3]. Construction of polycyclic indoles usually requires multistep reactions [4]. The preparation of polyfunctional indoles is therefore an important research field. On the other hand, indole and its derivatives are found in molasses tar and coal tar [5]. Indole nucleus is also found in amino acids such as tryptophan, plant hormones, and certain important alkaloids [6], as well as in liver, pancreas [7], brain [8] and bile [9]. Furthermore, indole is a powerful anti-oxidant and appears to be especially effective against breast and cervical cancer because of its ability to increase the break-down of estrogen in the human body [10]. Electrochemistry has recognized as a highpowered tool to develop environmentally friendly processes [11, 12]. Graphite electrode may be regarded as a catalyst (non-toxic and safe) that is easily separated from the obtained product. Hence, it can be concluded that electro-synthesis is a green and safe waste approach for synthesis of various organic compounds. Over the last two years, electro-oxidation of phenylamine derivatives such as p-phenylenediamines, 4-aminophenols and 2-aminophenols have been studied in some details, in both the presence and absence of various compound, based on different electrochemical mechanisms [9, 13–15]. According to the wide applications of indole derivatives, complexity of chemical synthesis of polycyclic indoles and importance of green chemistry in the recent years, we encouraged to develop a facile, non-catalyst, easy handling, clean, safe waste, one-pot and fast method for the electrochemical synthesis of new polycyclic indoles. In this way, the electrochemical oxidation of phenylamine derivatives (1a, b) has been investigated in the presence and absence of pyrazolidine-3,5-dione (3) as a nucleophile under mild conditions (no heat, no reflux, no pressure and without any chemical catalyst) in a mixture of phosphate buffer solution with ethanol as a green medium.
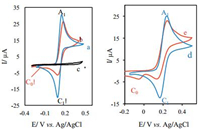
2. Results and discussionA cyclic voltammogram of 2 mmol/L of 4-aminophenol (1a) in a phosphate buffer solution (0.15 mol/L, pH 7.0) mixed with EtOH (70:30, v/v) shows one anodic (A1) and corresponding cathodic peak (C1), which are related to the transformation of 4-aminophenol (1a) to 4-iminocyclohexa-2,5-dienone (2a) and vice versa within a quasi-reversible 2e-process (Fig. 1, curve a). A peak current ratio (IpC1/IpA1) of nearly unity, particularly during the recycling of potential, can be considered as criteria for the stability of 4-iminocyclohexa-2,5-dienone (2a) formed at the surface of the working electrode (GC) under the optimum experimental conditions. In other words, any side reactions [9, 13–15] are too slow to be observed in the time scale of cyclic voltammetry. The electro-oxidation of 4-aminophenol (1a) in the presence of 3,5-pyrazolidinedione (3) was investigated in detail. As can be seen, curve b (Fig. 1) shows the cyclic voltammogram obtained for solution of 2 mmol/L of 1a in the presence of 4 mmol/L of 3,5-pyrazolidinedione (3) as a nucleophile. Under experimental conditions, the cathodic counterpart of anodic peak A1 decreases and a new cathodic peak (C0) appears at potentials more negative than cathodic peak (C1) which is related to electrochemical reduction of intermediate 6a to 5a. Furthermore, in Fig. 1, curve c shows the cyclic voltammogram obtained for a solution of 2 mmol/ L of 3,5-pyrazolidinedione (3) in the absence of 4-aminophenol (1a) under experimental condition for comparison. The same electrochemical behavior was seen (Fig. 1, curves d and e) for 1,4-diaminobenzene (1b) in the absence and presence of 3,5-pyrazolidinedione (3) in a phosphate buffer solution (0.15 mol/L, pH 6.0) mixed with EtOH (70:30, v:v).
|
Download:
|
| Figure 1. Typical cyclic voltammograms of 2 mmol/L 4-aminophenol (1a) in the absence (a), in the presence of 4 mmol/L 3,5-pyrazolidinedione (b), that of a 2 mmol/L 3,5-pyrazolidinedione (3) in the absence of 1a (c), cyclic voltammograms of 1,4-diaminobenzene (1b) in the absence (d) and in the presence of 4 mmol/L 3,5-pyrazolidinedione (e) at the glassy carbon electrode under experimental conditions at a scan rate of 50 mV/s. | |
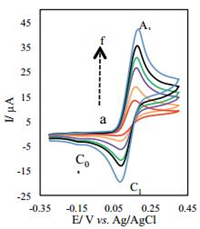
The cyclic voltammograms of 2 mmol/L of 4-aminophenol (1a) in the presence of 4 mmol/L of 3,5-pyrazolidinedione (3) are shown in Fig. 2, at various scan rates. It can be seen that proportional to the raising of the scan rate in parallel with the decrease in the height of C0 peak (cathodic peak of intermediate), the height of the cathodic (C1) peak of 4-aminophenol (1a) increases. A similar situation is also seen when the pyrazolidinedione (3) to 4-aminophenol (1a) concentration ratio is decreased (data not shown). In other words, increasing current ratio IpC1/IpA1 with the increasing scan rate is a good indication of the reactivity of 3 toward 4-iminocyclohexa-2,5-dienone (2a).
|
Download:
|
| Figure 2. Typical voltammograms of 2 mmol/L 4-aminophenol (1a) in the presence of 3,5-pyrazolidinedione (3) at the glassy carbon electrode, in 0.15 mol/L phosphate buffer solution (pH 7) mixed with ethanol (80:20 v:v) at different scan rates (a) 10, (b) 25, (c) 50, (d) 80, (e) 100 and (f) 150 mV/s. | |
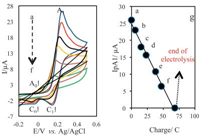
Controlled-potential coulometry was carried out in phosphate buffer solution (0.15 mol/L, pH 7) mixed with EtOH (70:30, v/v), containing 0.5 mmol of 4-aminophenol (1a) and 1 mmol of 3,5-pyrazolidinedione (3), at 0.3 V vs. Ag/AgCl (3 mol/ L) electrode. In the case of 1b, coulometry was carried out in phosphate buffer solution with pH 6 mixed with ethanol containing 0.5 mmol of 1b and 1 mmol of 3 at 0.3 V. The electro-synthesis progress was monitored by using cyclic voltammetry technique (Fig. 3). It is shown that, proportionally to the progress of coulometry, under constant potential, the anodic peak (A1) decreases and disappears when the charge consumption becomes about 4e-per molecule of 1b, and the new anodic peak (A0) is related to the electrochemical oxidation of intermediate 5b to 6b (Scheme 1).
|
Download:
|
| Figure 3. Cyclic voltammogram of 0.2 mmol 1,4-diaminobenzene (1b) in the presence of 0.4 mmol of 3,5-pyrazolidinedione (3), at glassy carbon electrode in 0.15 mol/L phosphate buffer solution (pH 6) mixed with ethanol (80:20, v:v) during controlledpotential coulometry at 0.3 V vs. Ag/AgCl (scan rate: 50 mV/s). After the consumption of (a) 0, (b) 6, (c) 15, (d) 23, (e) 38 and (f) 47 C. Progress of coulometry is associated with decreased anodic peak (A1) current. Curve g shows variation of anodic peak current (A1) versus charge consumed. | |
|
Download:
|
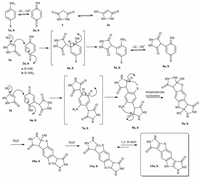
| Scheme 1. Proposed mechanism. | |
Regarding the results, it seems that the 1,4-Michael addition reaction of 3a to 2a, b is faster than the other side reactions and leads to the formation of intermediates 5a, b. The oxidation of these compounds (5a, b) is easier than the oxidation of the parentstarting molecule 1a, b by virtue of the presence of an electrondonating group [17–20]. Hence, 5a, b can be oxidized on the surface of electrode and produces 6a, b. This step causes the apparent numbers of transferred electrons to increase from the limit of n=2 to n=4 electrons per molecule of 1a, b. Then, 1,4-Michael addition reaction [21] of the second molecule of 3a to 6a, b is followed by two intramolecular cyclizations originating from two nucleophilic attacks of NH2 and X to C=O groups. Finally, elimination of two molecules of H2O leads to the final products 12a, b (Scheme 1). According to the coulometric results, voltametric and spectroscopic data, the ECECCCCC electrochemical mechanism is proposed for the electrochemical oxidation of phenylamine derivatives (1a, b) in the presence of 3,5-pyrazolidinedione (3), under experimental optimum condition (Scheme 1).
3. ConclusionsThe prominent features of this work such as the one-pot, simple electrochemical synthesis of polycyclic indole derivatives in aqueous/ethanol mixture instead of toxic solvents, at room temperature, high energyefficiencyand using the graphite electrode as an electron source instead of toxic catalysts, areinaccordancewith the principle of green chemistry. Cyclic voltammetry, controlledpotential coulometry and spectroscopic data indicated that the electrochemical oxidation of phenylamine derivatives (1a and 1b) in the presence of 3,5-pyrazolidinedione (3) were adopted with ECECCCCC mechanism (Scheme 1). Four-electron process of the electrochemical mechanism reaction was confirmed by coulometry, under constant potential data. In the present study, the obtained results explained that the electrochemistry can be applied as a green method for facile, high yield, safe waste, catalyst-free, rapid and one-pot synthesis of organic compounds, under mild conditions. In addition, this work introduces electrochemistry as a "powerful tool" for the synthesis of new supra heterocyclic compounds such as polycyclic indole derivatives.
4. Experimental 4.1. Apparatus and reagentsThe reaction equipment was used as described in the Supporting information. All chemical materials were purchased from Merck (Darmstadt, Germany). These chemicals were used without further purification.
4.2. Typical procedure for the chemical synthesis of pyrazolidine-3,5-dione (3)As an important starting material in this work, 3,5-pyrazolidinedione (3) was prepared according to the mechanism proposed by Metwally et al. (Scheme 2) via cyclization of ethoxycarbonylacetohydrazide using sodium methoxide [16]. The spectroscopic data (1H and 13C NMR) and melting point confirmed synthesis of this compound (3), according to Ref. [16] (data not shown).

|
Download:
|
| Scheme 2. Synthesis of 3,5-pyrazolidinedione. | |
4.3. Typical procedure for the electrochemical synthesis of indoles (12a, 12b)
In the proposed method, 100 mL of phosphate buffer solution (0.15 mol/L) mixed with ethanol (80:20, v/v), as supporting electrolyte (in the case of 12a pH 7 and 12b pH 6), was preelectrolyzed at the 0.3 V vs. Ag/AgCl in an undivided cell. Then, 0.2 mmol of 4-aminophenol (1a) or 1,4-diaminobenzene (1b) and 0.4 mmol of 3,5-pyrazolidinedione (3) were added to the electrochemical cell. Finally, the electrochemical synthesis under constant potential was performed using the 0.3 V vs. Ag/AgCl. The electrolysis was finished when the current decreased more than 95%. The process was interrupted several times during the electrosynthesis (for ensuring to complete the reaction), and the working electrodes (five carbon anodes) were washed in ethanol to reactivate (to clear the surface of working electrode from formed side products such as polymers). At the end of electrolysis, the electrochemical cell was placed in the refrigerator (T=4±1℃) for 24 h. The precipitated solid was collected by filtration and washed with warm etanol/acetonitrile (1:1, v/v) to separate the remained 4-aminophenol (1a) or 1,4-diaminobenzene (1b). Then, it was washed several times with cold water to more purification. After purification, the products (12a and 12b) were characterized using FT-IR, mass spectroscopy (MS), elemental analysis (CHN), NMR.
4.4. Characterization of productsCompound 12a: Yield: 83%. Mp > 260℃ (dec). FT-IR (KBr, cm-1): 3417 (NH), 1660 (C=O, amide), 1570 and 1480 (C=C aromatic). 1H NMR (400 MHz, DMSO-d6): δ 7.63 (s, 2H, aromatic), 10.03 (s, broad, 2H, NH pyrazolidine ring), 10.52 (broad, 2H, NH, pyrazolidine ring), 11.28 (s, 2H, NH phenylamine ring). 13C NMR (100 MHz, DMSO-d6): δ 110.6, 112.2, 117.7, 122.6, 129.2, 166.6. MS (EI, m/z) (relative intensity): 269 (M+, 19), 186 (70), 156 (62), 108 (45), 54 (55). Anal. Calcd. for C12H8N6O2: C, 53.73; H, 3.01; N, 31.33. Found: C, 53.69; H, 3.07; N, 31.27.
Compound 12b: Yield: 78%. Mp > 260℃ (dec). FT-IR (KBr, cm-1): 3390 (NH), 1664 (C=O, amide), 1570 and 1443 (C=C aromatic). 1H NMR (400 MHz, DMSO-d6): δ 7.58 (s, 1H, aromatic), 7.88 (s, 2H, aromatic), 10.04 (s, broad, 2H, NH pyrazolidine ring), 10.38 (broad, 2H, NH, pyrazolidine ring), 11.24 (s, 1H, NH phenylamine ring). 13C NMR (100 MHz, DMSO-d6): δ 110.2, 110.3, 113.3, 117.6, 123.3, 124.6, 125.3, 131.5, 141.5, 143.3, 166.2, 166.9. MS (EI, m/z) (relative intensity): 270 (M+, 28), 187 (76), 161 (55), 94 (80), 54 (60). Anal. Calcd. for C12H7N5O3: C, 53.54; H, 2.62; N, 26.01. Found: C, 53.57; H, 2.57; N, 25.93.
AcknowledgmentThe authors thank from Semnan University Research Council for financial support of this work.
Appendix A. Supplementary dataSupplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.cclet.2016.11.022.
| [1] | R. Sundberg. The Chemistry of Indoles. Elsevier (2012) . |
| [2] | S.W. Pelletier. Alkaloids:Chemical and Biological Perspectives. Springer (1999) . |
| [3] | R.M. Shaheen, D.W. Davis, W. Liu, et al., Antiangiogenic therapy targeting the tyrosine kinase receptor for vascular endothelial growth factor receptor inhibits the growth of colon cancer liver metastasis and induces tumor and endothelial cell apoptosis. Cancer Res. 59 (1999) 5412–5416. |
| [4] | S.F. Vice, R.W. Friesen, G.I. Dmitrienko. C-2-side chain modification of 2-methyl-3-alkylindoles via 3-methylthioindolenines:a new approach to pyrrolo[1,2-a] indoles. Tetrahedron Lett. 26 (1985) 165–168. DOI:10.1016/S0040-4039(00)61870-1 |
| [5] | R. Weissgerber. Indole in coal tar. Chem. Ber. 43 (1910) 3520–3528. DOI:10.1002/(ISSN)1099-0682 |
| [6] | S. Ali, N. Ali, B. Ahmad Dar, V. Pradhan, M. Farooqui. Chemistry and biology of indoles and indazoles:a mini-review. Mini Rev. Med. Chem. 13 (2013) 1792–1800. DOI:10.2174/1389557511313120009 |
| [7] | M. Nencki. Ueber sulfoharnstoff-oxalsäureäther. Chemische Berichte 7 (1874) 779–780. |
| [8] | F. Stöckly. Zur Kenntniss der Fäulnissprodukte des Gehirns. J. Prakt. Chem. 24 (1881) 17–24. DOI:10.1002/prac.18810240102 |
| [9] | A. Asghari, M. Ameri, S. Radmannia, et al., None-catalyst and clean synthesis of symmetric and asymmetric indoles from electrochemical oxidation of 4-aminophenol and p-phenylenediamine in the presence of malononitrile in green media. J. Electroanal. Chem. 733 (2014) 47–52. DOI:10.1016/j.jelechem.2014.09.015 |
| [10] | D. Nematollahi, V. Hedayatfar. Diversity in electrochemical oxidation of dihydroxybenzenes in the presence of 1-methylindole. J. Chem. Sci. 123 (2011) 709–717. DOI:10.1007/s12039-011-0132-1 |
| [11] | H. Salehzadeh, D. Nematollahi, H. Hesari. An efficient electrochemical method for the atom economical synthesis of some benzoxazole derivatives. Green Chem. 15 (2013) 2441–2446. DOI:10.1039/c3gc40954f |
| [12] | M. Ameri, A. Asghari, A. Amoozadeh, M. Bakherad, D. Nematollahi. Green and highly efficient synthesis of new bis-benzofurans via electrochemical methods under ECECCC mechanism. J. Electrochem. Soc. 161 (2014) G75–G80. DOI:10.1149/2.0371410jes |
| [13] | H. Beiginejad, D. Nematollahi, F. Varmaghani, M. Bayat. Efficient factors on the hydrolysis reaction rate of some para-aminophenol derivatives in acidic pHs. J. Electrochem. Soc. 160 (2013) H469–H473. DOI:10.1149/2.084308jes |
| [14] | H. Beiginejad, D. Nematollahi, F. Varmaghani. Electrochemical oxidation of some aminophenols in various pHs. J. Electrochem. Soc. 160 (2013) H41–H46. |
| [15] | A. Maleki, D. Nematollahi. Mechanism diversity in anodic oxidation of N, Ndimethyl-p-phenylenediamine by varying pH. J. Electroanal. Chem. 704 (2013) 75–79. DOI:10.1016/j.jelechem.2013.06.002 |
| [16] | S.A. Metwally, M.I.A. Moneim, Y.A. Elossely, R.I. Awad, K. Abou-Hadeed. Synthesis and crystal structure of some 3,5-pyrazolidinediones. Chem. Heterocycl. Compd. 46 (2010) 426–437. DOI:10.1007/s10593-010-0527-9 |
| [17] | M. Ameri, A. Asghari, A. Amoozadeh, M. Bakherad, D. Nematollahi. Facile and one-pot, electro-organic synthesis of a new bis-quinone by the ECCE mechanism in green media. Chin. Chem. Lett. 25 (2014) 1607–1610. DOI:10.1016/j.cclet.2014.06.022 |
| [18] | D. Nematollahi, A. Amani, E. Tammari. Electrosynthesis of symmetric and highly conjugated benzofuran via a unique ECECCC electrochemical mechanism:evidence for predominance of electrochemical oxidation versus intramolecular cyclization. J. Org. Chem. 72 (2007) 3646–3651. DOI:10.1021/jo062468b |
| [19] | M. Ameri, A. Asghari, A. Amoozadeh, M. Bakherad, D. Nematollahi. An efficient simple, non-catalytic electrosynthesis of new polycyclic benzofuran derivatives. Tetrahedron Lett. 56 (2015) 2141–2144. DOI:10.1016/j.tetlet.2015.03.049 |
| [20] | M. Ameri, A. Asghari, A. Amoozadeh, M. Bakherad. First electroorganic synthesis based on a metal-and amine-free sonogashira-type coupling reaction with an ECECECE mechanism. J. Electrochem. Soc. 162 (2015) G25–G28. DOI:10.1149/2.0961506jes |
| [21] | Y. Lu, Y. Zhao, S. Wang, et al., An efficient synthesis of 2-thio-5-amino substituted benzoquinones via KI catalyzed cascade oxidation/michael addition/oxidation starting from hydroquinone. RSC Adv. 6 (2016) . DOI:10.1039/c5ra26524j |
 2017, Vol. 28
2017, Vol. 28 


