Sialic acid as a characteristic terminal monosaccharide of glycoconjugate plays many pivotal roles in signal transduction, cellular communication, and antigen-antibody recognition [1–3]. Negatively charged sialic acid has endowed sialoglycoproteins with many important biochemical properties [4–6]. Previous studies have revealed that aberrant sialoglycoproteins are closely associated with cancers [3, 7–10]. thyroid lesions [11], cardiac arrhythmias [12], and acute myocardial infarction [13]. Given that the biomedical importance of sialoglycoproteins, the development of economic and efficient approach for sialoglycoprotein analysis with less or no desialylation is very important. More recently, mass spectrometry (MS) has become a central tool to identify and characterize sialoglycoproteins. Although, in some MS identifications of sialic acid-containing glycans or glycopeptides, sialic acid residues are usually removed prior to MS identification to obtain better MS signal, the information on protein sialylation, a key factor for revealing the pathogenesis of disease, is missing. Therefore, it is necessary for the integrity study of glycoprotein without desialylation. However, due to their low abundance and microhetergeneity, sialoglycoproteins or sialoglycopeptides are very difficult to be detected directly by mass spectrometry. Despite an enormous progress in the enrichment of glycoproteins or glycopeptides from complex biological samples (e.g., bodily fluids, cells, and tissues) based on chemical derivatization [14–16], hydrazine chemistry [17–19], affinity chromatography [20, 21], and hydrophilic interaction liquid chromatography [22, 23], graphitized carbon [24–26], and titanium dioxide (TiO2) [27–30], loss of water molecule and desialylation always accompany with the above-mentioned processes. Moreover, some drawbacks of these strategies prevent them from being applied to a comprehensive profiling of sialoglycoproteome. Although widely-used lectin-affinity approach benefits from its specificity for binding of different glycan types, each lectin only recognizes one subset of glycan species. To some extent, time-consuming is the main limitation of lectin-affinity chromatography for a scale-up enrichment of sialoglycoproteins. Boronate affinity marterials [31] and molecularly imprinted polymers (MIPs) were recently reported as two types of synthetic materials for the recognition and enrichment of sialic acid-containing proteins, peptides, and glycans. In previous studies, a hydrophilic boronate affinity monolithic capillary coupled with 4-vinylphenylboronic acid (VPBA, a boronate affinity marterial) was shown as the cheaper and more stable competitors to lectins. When the binding pH < the pKa value of the VPBA (ΔpH > 1), the boronate affinity marterial specifically bind to sialylated glycoproteins due to the binding affinity of the boronic acid towards sialic acid residues [32]. Many studies reported that the prepared MIPs could specifically recognize an intact sialic acid-containing glycoprotein and its characteristic fragments, even within a complex sample matrix [33, 34]. Except for synthetic materials, natural materials have been discovered and used in glycoprotein study. Recently, a natural biomaterial, cotton wool, has exhibited an advantage in the enrichment of N-linked glycans and glycopeptides [35]. However, it shows a poor specificity for the capturing of sialoglycans and sialoglycopeptides. To our knowledge, apart from Sambucus nigra lectin, Maackia amurensis lectin, and kapok fiber [36], no other natural materials has been reported for selective enrichment of sialoglycopeptides or sialoglycoproteins.
Poplar catkin has been regarded as a kind of nasty "pollution" worldwide during past hundred years because it often causes respiratory infection [37]. In this study, this natural poplar catkin derived from white poplar tree (Populus tomentosa Carr.) is employed to develop a novel capturing microtip, popcat-tip, for selective enrichment of sialoglycopeptides, showing a new and valuable perspective of poplar catkin.
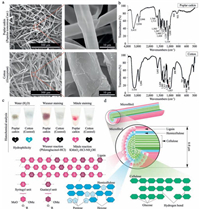
2. Results and discussion 2.1. Structural characterization of poplar catkinPrevious study has indicated that kapok fiber derived from the kapok tree (Bombax ceiba L.) has highly specific and efficient capability to enrich sialoglycopeptides [36]. In the present study, we have examined another natural biomaterial, poplar catkin derived from white poplar tree (Populus tomentosa Carr.), whether it has similar capability to selectively enrich sialoglycopeptides compared with kapok fiber. First, we performed the morphological analysis and structural component analysis of the poplar catkin based on a combination of SEM and FT-IR spectroscopy to determine its surface components. The SEM analysis was first performed to characterize the morphology of the poplar catkin. As shown in Fig. 1a, the surface morphology of the poplar catkin is significantly different from that of cotton wool which has been used for N-linked glycopeptide analysis [35]. The diameter of the poplar catkin (ca. 6–8 µm) is smaller than that of the cotton (ca. 20 µm). In addition, the smooth morphology could be observed on the surface of the poplar catkin relative to the fibrotic surface morphology of the cotton. Previous study indicated that the main component of the cotton is cellulose (ca. 100%) [35]. FT-IR spectroscopy analysis showed that the absorption spectrum of the poplar catkin with its characteristic absorption peaks at 1030, 1124, 1225, 1270, 1327, 1420, 1460, 1507, 1593, and 1715 cm-1 obviously differs from that of the cotton (Fig. 1b). A wide absorption peak at 3415 cm-1 is attributed to hydroxyl group stretching vibration in aromatic and aliphatic hydroxyl groups, while the peaks at 2930 cm-1 and 2846 cm-1 are predominantly originated from the C-H asymmetric and symmetrical vibrations in methyl and methylene groups, respectively [38, 39]. The shoulder peak at 1715 cm-1 arises from the unconjugated carbonyl or ketone stretching, while aromatic skeletal vibrations can result in three strong peaks at 1593 cm-1, 1507 cm-1, and 1420 cm-1. The peak at 1460 cm-1 is indicative of the C-H deformation on aromatic ring. Syringyl (S) and condensed guaiacyl (G) absorption was obviously observed at 1327 cm-1. The peak at 1270 cm-1 is resulted from the G-ring breathing with C=O stretching, S-ring breathing with C-C, C-O, and C=O stretching appears at 1225 cm-1. In addition, the strongest absorption peak at 1124 cm-1 shows S unit's aromatic C-H in-plane deformation, and the peak at 1030 cm-1 arises from the aromatic C-H in-plane deformation plus C=O stretching. Taken together, these peaks indicate that the outer layer component of the poplar catkin is GStype lignin.
|
Download:
|
| Figure 1. Structural characterization of poplar catkin. (a) Scanning electron micrographs of the poplar catkin derived from white poplar trees (Populus tomentosa Carr.) and cotton wool (Gossypium); (b) FT-IR spectra of the poplar catkin and the cotton wool; (c) Histochemical analysis of the poplar catkin. Mäule and Wiesner staining were employed for syringyl (S) and guaiacyl (G) lignin visualization on the surface of the poplar catkin; (d) Schematic diagram of structural components of the poplar catkin. | |
In order to further confirm the outer layer components of the poplar catkin, histochemical analysis based on Mäule and Wiesner (phloroglucinol-HCl) staining assays, which are commonly-used experiments to prove the presence of S-type lignin and G-type lignin, respectively, were performed [40, 41]. As shown in Fig. 1c, the colors of red-purple and purple were clearly developed on the surface of the poplar catkin by Mäule and Wiesner staining, respectively, and the negative results (colorless) are shown for the cotton, confirming the presence of lignin on the surface of the poplar catkin. To examine whether the lignin is the main outer layer component of the poplar catkin, a three-step sequential treatment (96:4 dioxane:water, 50:50 dioxane:water, and 80:20 dioxane:water with 1% NaOH) was also performed (Fig. 2a), followed by the FT-IR analysis (Fig. 2b–d). The residues of L1, H2, H3, and R3 extracted by different dioxane-water solutions were identified to be lignin, hemicellulose, and cellulose, respectively. In 96% dioxane treatment, the residue of L1 (H1 were not observed) was only detected, indicating that lignin is the main component on the outer layer of the poplar catkin. Meanwhile, hemicellulose (H2 and H3) and cellulose (R3) could be found in the other dioxanewater treatments, revealing that hemicellulose and cellulose are the internal structural components of the poplar catkin. Taken together, we conclude that the structure of poplar catkin is composed of lignin, hemicellulose, and cellulose from the outer layer to the inside (Fig. 1d). Based on this structural model, the hydrophobicity of poplar catkin can be attributable to the lignin located on the outer layer of poplar catkin, which has highly selective capability to enrich sialoglycopeptides [36].

|
Download:
|
| Figure 2. Component analysis of poplar catkin from white poplar trees (Populus tomentosa Carr.). (a) Strategy for the isolation of lignin, hemicellulose, and cellulose from poplar catkin; (b–d) FT-IR spectra of lignin, hemicellulosic, and cellulosic fractions isolated from the poplar catkin. | |
2.2. Poplar catkin for selectively enriching sialoglycopeptides
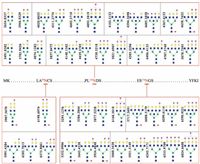
First, about 10 mg of poplar catkin was packed into a 200 µL pipet tip to make a popcat-tip. In this study, a widely used bovine fetuin as a model sialoglycoprotein was selected to evaluate the performance of the popcat-tip for selective enrichment of sialoglycopeptide. Five equal amounts of the enzymatic digest of bovine fetuin (8.0 pmol) were used to optimize eluting conditions for the popcat-tip enrichment using different eluting solutions, such as 0.1% TFA, 0.2% FA, H2O, NH4HCO3, and 0.25% NH3·H2O, as previously described [36]. Mass spectra of the enriched sialoglycopeptides of 8.0 pmol of bovine fetuin using the above-mentioned eluting solutions show that H2O as an eluting solution has the highest capability to elute large sialoglycopeptides in mass relative to 0.25% NH3·H2O (Fig. S1 in Supporting information), which is the best eluting solution for kapok fiber to enrich sialoglycopeptides [36], indicating that the surface components of the poplar catkin may be slightly different from those of kapok fiber. It is found that 4 pmol of bovine fetuin could generate more than 25 sialoglycopeptides over the high m/z region of 3500–7000 and that 8 pmol of bovine fetuin could produce more than 44 sialoglycopeptides with high intensities over the same m/z region (Fig. S2 in Supporting information). The proposed glycan structures are show in Fig. 3. The intra-and inter-day reproducibility of the popcat-tip enrichment experiments based on the enzymatic digest from 8.0 pmol of bovine fetuin was less than 5.4% (Fig. S3 in Supporting information) and 11.3% (Fig. S4), respectively.
|
Download:
|
| Figure 3. Proposed structures of the detected glycans linked to three glycosites of bovine fetuin. The peptides containing three glycosites are RPTGEVYDIEIDTLETTCHVLDPTPLA99NCSVR, KLCPDCPLLAPL156NDSR, and VVHAVEVALATFNAES176N GSYLQLVEISR, respectively. Blue square, N-acetylglucosamine residue; green circle, mannose residue; yellow circle, galactose residue; red triangle, fucose residue; purple diamond, sialic acid residue. | |
Mass spectrum of the enzymatic digest from 8.0 pmol of bovine fetuin (Fig. 4a) is significantly different from that of the popcat-tipenriched glycopeptides of 8.0 pmol of bovine fetuin, which has more than 44 sialoglycopeptides detected (Fig. 4b). The zoomed Fig. 4b and the detailed m/z values are shown in Fig. S5 (Supporting information) and Table S1. Fig. 4c shows that the washing solution did not contain any glycopeptides. Mass spectrum of the popcat-tip-enriched peptides of α2-3,6,8 neuraminidase-treated enzymatic digestof8.0pmol of bovinefetuin shows a fewglycopeptides around the m/z region of 4000 (Fig. 4d), while these glycopeptides disappeared at the spectrum of the popcat-tip-enriched peptides of PNGase F-treated enzymatic digest of 8.0pmol of bovine fetuin (Fig. 4e). These findings suggest that the poplar catkin is a sialic acid-specific enrichment biomaterial. In addition, the signals observedataround m/z 4000in themassspectrum (Fig. 4d)maybe from glycopeptides without terminal sialic acid residues.

|
Download:
|
| Figure 4. Mass spectra of the glycopeptides derived from the enzymatic digest of 8.0 pmol of bovine fetuin. (a) the enzymatic digest; (b) the popcat-tip-enriched glycopeptides. (c) the washing solution; (d) the popcat-tip-enriched peptides of α2-3,6,8 neuraminidase-treated enzymatic digest; (e) the popcat-tip-enriched peptides of PNGase F-treated enzymatic digest. Purple diamond, sialic acid residue; star symbol, electric noise. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.) | |
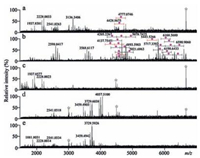
To confirm sialoglycopeptide enrichment performance of poplar catkin, less sialylated glycoprotein, transferrin, was selected as another example for sialoglycopeptide enrichment experiment. Mass spectrum of the enzymatic digest from 8.0pmol of transferrin (Fig. 5a) significantly differs from that of the popcattip-enriched glycopeptides from 8.0pmol of transferrin, which has more than 30 sialoglycopeptides detected (Fig. 5b). The zoomed Fig. 5b and the detailed m/z values areshownin Fig. S6 and Table S1 (Supporting information), respectively. The proposed glycan structures are shown in Fig. S7. It is found that a few of peptide signals were detected in the washing solution (Fig. 5c) and that no glycopeptides in the popcat-tip-enriched peptides of α2-3,6,8 neuraminidase-treated enzymatic digest from 8.0pmol of transferrin (Fig. 5d) and the popcat-tip-enriched peptides of PNGase Ftreated enzymatic digest from 8.0pmol of transferrin (Fig. 5e) was observed compared with Fig. 5b. Mass spectra of the enzymatic digest, the popcat-tip-enriched species, the washing solution, and the popcat-tip-enriched species of PNGase F-treated enzymatic digest from 8.0 pmol of RNase B, which is a high mannose glycoprotein without terminal sialic acid residues, have no significant difference over the m/z region of 3500–7000, along with no sialoglycopeptides detected (Fig. S8 in Supporting information). In addition, as compared with mass spectra of the enzymatic digest (Fig. 6a), the washing solution (Fig. 6c), the popcat-tip-enriched peptides of α2-3,6,8 neuraminidase-treated enzymatic digest (Fig. 6d), and the popcat-tip-enriched peptides of PNGase F-treated enzymatic digest (Fig. 6e) derived from the mixture of bovine fetuin (8.0 pmol), transferrin (8.0 pmol), RNase B (8.0 pmol), and albumin (8.0 pmol), mass spectrum of the popcattip-enriched glycopeptides from the enzymatic digest from the above-mentioned protein mixture has shown abundant signals of sialoglycopeptides (Fig. 6b). The zoomed Fig. 6b and the detailed m/z values are shown in Fig. S9 (Supporting information) and Table S1. In addition, we have performed a comparison of the popcat-tip approach with GA-ZipTip, TiO2, C18 disk, and cotton HILIC ZipTip using bovine fetuin as a model protein as described previously [36]. As a result, the poplar catkin has shown highly selective and efficient enrichment of sialoglycopeptides compared with other four approaches, which are similar to our previous study [36].

|
Download:
|
| Figure 5. Mass spectra of the glycopeptides derived from the enzymatic digest of 8.0 pmol of transferrin. (a) the enzymatic digest; (b) the popcat-tip-enriched glycopeptides; (c) the washing solution; (d) the popcat-tip-enriched peptides of α2-3,6,8 neuraminidase-treated enzymatic digest; (e) the popcat-tip-enriched peptides of PNGase F-treated enzymatic digest. Purple diamond, sialic acid residue; star symbol, electric noise. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.) | |
|
Download:
|
| Figure 6. Mass spectra of the glycopeptides derived from the enzymatic digest of the mixture of 8.0pmol of bovine fetuin, 8.0pmol of transferrin, 8.0pmol of RNase B, and 8.0 of pmol albumin. (a) the enzymatic digest; (b) the popcat-tip-enriched glycopeptides; (c) the washing solution; (d) the popcat-tip-enriched peptides of α2-3,6,8 neuraminidase-treated enzymatic digest; (e) the popcat-tip-enriched peptides of PNGase F-treated enzymatic digest. Purple diamond, sialic acid residue; star symbol, electric noise. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.) | |
To assess the general applicability of popcat-tip for sialoglycopeptide enrichment, an albumin-removed serum was chosen for in-solution trypsin digestion, followed by the popcat-tip enrichment and MS analysis. More than 1000 peaks could be found in the mass spectrum over the m/z range of 2000–7000 (Fig. 7). Although most of these peaks were not identified, difference in mass of 291 Da between these peaks were observed, corresponding to typical sialic acid residues. These data demonstrate that the popcat-tip could enriched sialylated glycopeptides from a complicated protein pool. Taken together, the above-mentioned results significantly suggest that the poplar catkin has an excellent capability to selectively enrich sialoglycopeptides from complex peptide mixture.

|
Download:
|
| Figure 7. Mass spectrum of popcat-tip-enriched sialoglycopeptides from in-solution trypsin albumin-removed serum. | |
2.3. Lignin as a key component of poplar catkin for highly selective enrichment of sialoglycopeptides
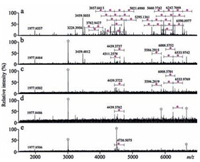
To prove whether lignin is a key component of the poplar catkin for highly selective enrichment of sialoglycopeptides, five equal amounts of the poplar catkin (about 10 mg) were delignified by 2% H2O2 at different time periods: 0, 5, 10, 20, and 30 min, respectively. The delignified poplar catkin was further used to enrich sialoglycopeptides, followed by mass spectrometric analysis. As shown in Fig. 8, the enrichment performance of the poplar catkin was significantly decreased with an increasing of the treatment time, and the signals of the enriched sialoglycopeptides almost disappeared at the treatment time of 30min. Taken together, we conclude that lignin of poplar catkin is a key component for the efficient capturing of sialoglycopeptides.
|
Download:
|
| Figure 8. Mass spectra of the enriched sialoglycopeptides derived from bovine fetuin using the delignified poplarcatkinwhich was treated with 2% H2O2 at different time periods: 0min (a), 5min (b), 10min (c), 20min (d), and 30min (e). Purple diamond, sialic acid residue; star symbol, electric noise. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.) | |
2.4. Enrichment mechanism of poplar catkin for sialoglycopeptides
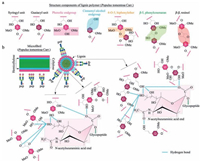
Based on the histochemical staining assays (Fig. 1c), it is found that the lignin distributed on the outer layer of poplar catkin is mainly composed of S and G units. Fig. 9a has listed mainly basic structure components of lignin with terminal hydroxyl groups and ethoxyl groups. Based on these structures of lignin, we speculate that three possible interactions exist between lignin and sialic acid residues terminated to glycopeptides (Fig. 9b). First, poplar catkin presents a relatively relaxing state when treated with a weak polar solution (i.e., 80% ACN in water) at washing step before sample loading. This weak polar solution would be helpful in exposing more surface anchored sites, such as oxygen atoms and hydroxyl groups. This stretched structure in the weak polar solution (80% ACN) enhances the formation of hydrogen bonds between these exposed oxygen atoms or hydroxyl groups of lignin and the sialic acid residues, as compared to the collapse status of the poplar catkin in a polar solution (e.g., H2O); Second, the long-chain tail of the sialic acid residues is helpful for the sialic acid residues inserting into the stretched structure of the poplar catkin to form hydrogen bonds, which might be a main reason for highly selective enrichment of sialoglycopeptides; Third, the hydrophobic microenvironment surrounding the poplar catkin may also tend to drive the sialic acid residues to form hydrogen bonds with the oxygen atoms or hydroxyl groups of lignin to reduce the surface tension potential energy between the poplar catkin and sialoglycopeptides. More importantly, the optimized weak polarity of the loading solution (80% ACN/20% H2O) is very important to the formation of these hydrogen bonds. Because this weak polar solution not only could let the poplar catkin to form the stretched structure, but also could reduce excessively hydrophobic shielding effect of lignin due to the presence of 20% H2O, resulting in the formation of hydrogen bonds between the hydroxyl groups or oxygen atoms of the poplar catkin and the sialic acid residues. Our findings indicate that this amphiprotic features of the washing and loading solutions are very important for poplar catkin to highly capture sialoglycopeptides. When sialoglycopeptides retained on the surface of poplar catkin were eluted with the strong polar solutions (e.g., 100% H2O or 0.25% NH3·H2O), the intermolecular interactions between sialoglycopeptides and the poplar catkin can be easily broken by the strong poplar molecules or ions (e.g., H2O and NH4+). In addition, the environment of strong polar phase would drive the poplar catkin to form a shrink status, resulting in hydrophilic groups of lignin being compressed into a smaller space and increasing the steric effect for the formation of hydrogen bonds. All these reasons might induce a complete release of the sialoglycopeptides from the poplar catkin.
|
Download:
|
| Figure 9. Proposed mechanisms of specific enrichment of sialoglycopeptides using the popcat-tip. (a) Structural illustration of the main structural components of lignin (Populus tomentosa Carr.); (b) Schematic diagram of the intermolecular interactions between the poplar catkin and sialoglycopeptides. Oxygen atoms of methoxyl and hydroxyl groups of lignin are prone to form a number of hydrogen bonds with the sialic acid residues. | |
3. Conclusion
We introduce a simple, low-cost, eco-friendly, highly selective, and highly efficient approach to enrich sialoglycopeptides from complex peptide mixture on the basis of a natural biomaterial, poplar catkin derived from the white poplar tree (Populus tomentosa Carr.), under an optimized eluting condition. It should be noted that the outer layer components of the poplar catkin are GS-type lignins which play an essential role in isolating sialoglycopeptides, without losses of sialic acid residues and water molecules from sialoglycopeptides, which allow us to further comprehend the pathophysiological significance of glycoprotein sialylation. Most importantly, our findings strongly reveal that the eco-friendly investigations of natural phenomena based on natural biomaterials are possible as compared with humanmade materials.
4. Experimental 4.1. Materials2,5-Dihydroxylbenzoic acid (DHB), dithiothreitol (DTT), iodoacetamide (IAA), bovine fetuin, human transferrin, and bovine serum albumin were purchased from Sigma-Aldrich Chemicals (Steinheim, Germany). Bovine RNase B, PNGase F, and α2-3,6,8 neuraminidase were obtained from New England Biolabs (Massachusetts, USA). Sequencing-grade modified trypsin and alkaline phosphatase were purchased from Roche Diagnostics (Mannheim, Germany) and Promega (Madison, WI, USA), respectively. Poplar catkin was collected from white poplar tree (Populus tomentosa Carr.) in Beijing, China. HPLC-grade acetonitrile was from Fisher Scientific (Pittsburg, PA, USA) and the ultrapure water was purified by a Milli-Q system (Millipore, USA). All other reagents were of analytical grade.
4.2. Scanning electron microscopy (SEM) and Fourier-transform infrared (FT-IR) spectroscopyScanning electron microscope images of samples were obtained on a FEI Quanta 200 Environmental Scanning Electron Microscope (FEI Company, USA) operated at an accelerating voltage of 20kV. The sample was coated with a thin layer of gold. FT-IR spectra of poplar catkin and cotton wool, as well as lignin, hemicellulose, and cellulose isolated from the poplar catkin, respectively, were obtained using a Thermo Scientific Nicolet FT-IR microscope (Impact 400 system, Madison, WI, USA) equipped with a liquid nitrogen cooled MCT detector. Their spectra were recorded in the range of 4000–500cm-1.
4.3. Isolation of lignin, hemicelluloses, and cellulosesLignin, hemicellulosic, and cellulosic fractions were isolated with sequential three-step treatments with 96:4 dioxane:water, 50:50 dioxane:water, and 80:20 dioxane:water containing 1% NaOH. Poplar catkin was milled by Retsch MM400 mixer mill (Haan, Germany) with the aid of two 5-mm stainless steel balls, andthen theresultingpoplarcatkinwas directlysuspended in96:4 dioxane:water solution at a ratio of 1:20 (g:mL) and heated at 85℃ for 2h. The solution was filtered and washed with 96:4 dioxane: water until the filtrate was clear. The solid residue, namely R1, was dried at 50℃. The filtrate was concentrated to 3/5 volume using a speed vacuum concentrator (Thermo Electron Corporation, Waltham, MA, USA), followed by the addition of 3 volumes of 95% ethanol. After filtration through a 20 µm nylon membrane filter, the hemicellulosic pellets were washed with 70% ethanol and airdried to obtain hemicellulosic fraction H1 (H1 wasn't found in this study). The solubilised ligninwas obtained from the corresponding supernatants after concentration to approximately 1/3 volume via the evaporation of ethanol and remaining dioxane, followed by precipitation at pH 2.0 adjusted by 0.03% hydrochloric acid (HCl). The lignin was washed with acidified water (pH 2.0), and freezedried, namelyL1.The residue R1obtainedfrom96:4 dioxane:water treatment was successively treated with 50:50 dioxane:water solution at a ratio of 1:20 (g:mL) and heated at 85℃ for 2h. The mixture was filtered with a 20 µm nylon membrane filter. After filtration, the insoluble residue, denoted as R2, was washed with 50:50 dioxane:water and dried at 50℃. The filtrate was concentrated to 3/5 volume, followed by the addition of 3 volumes of 95% ethanol. The hemicellulosic fraction (H2) was filtered through a 20 µm nylon membrane filter, washed with 70% ethanol and air-dried. The solubilised lignin (L2) was obtained from the corresponding supernatant after evaporation of ethanol and remaining dioxane, followed by precipitation at pH 2.0 adjusted with 0.03% HCl, purified by washing with 0.03% HCl, and freezedried. The residue (R2) obtained from 96:4 and 50:50 dioxane: water treatments was further extracted with 80:20 dioxane:water containing 1% NaOH at a ratioof 1:20 (g:mL) and heatedat 85℃ for 2h. The residue was filtered via a 20 µm nylon membrane filter, washed with 80:20 dioxane:water, and dried at 50℃. After filtration, the insoluble cellulosic residue was denoted as R3. The filtrate was adjusted to pH 5.8 with HCl, concentrated to 1/3 volume using the SpeedVacuum concentrator, and then transferred into 3 volumes of 95% ethanol. The hemicellulosic fraction (H3) was removed via a 20 µm nylon membrane filter, washed with 70% ethanol, and dried at room temperature for further use. The lignin fraction (L3) was obtained from the corresponding supernatants after evaporation of ethanol and remaining dioxane. It was washed with 0.03% HCl and then freeze-dried for analysis. The cellulosic pellets were washed with 70% ethanol and air-dried to obtain cellulosic fraction.
4.4. Mäule and Wiesner staining assaysMäule staining was employed for syringyl (S) lignin visualization. Briefly, poplar catkin was immersed in 1% (w/v) potassium permanganate (KMnO4) solution for 5min at room temperature and then washed with 16% HCl until the color turned from black/ dark brown to light brown. After a subsequent treatment with 20% ammonium hydroxide, red-purple color appeared. Wiesner staining was used for guaiacyl (G) lignin identification. Poplar catkin was incubated in a mixture of 2% (w/v) phloroglucinol (in 95% ethanol) and 12 mol/L HCl (2:1, v/v) for 1min for the Wiesner reaction.
4.5. Double-enzyme digestionProteins (i.e., bovine fetuin, human transferrin, bovine RNase B, and bovine serum albumin) were prepared, respectively, in 25mmol/L NH4HCO3/50mmol/L DTT (pH 7.8) and incubated at 56℃ for 60min. Subsequently, alkylation was performed in 25mmol/L NH4HCO3/100mmol/L IAA for 45min at room temperature, followed by DTT quenching reaction. The resulting protein was digested with 12 µL of 50ng/µL sequencing-grade modified trysinat 37℃ overnight, and then dephosphorylated with 1.5 units of alkaline phosphatase at 37℃ for 4h. The resulting enzymatic digest was lyophilized and then stored at -80℃ for further analysis.
4.6. Enrichment of sialoglycopeptides using popcat-tipPopcat-tip was prepared as described previousely [36]. Briefly, poplar catkin was washed with 50% acetonitrile (ACN) in water and then packed into a 200 µL pipet tip to make a popcat-tip, with about 2.0mm length of the poplar catkin. Before the enzymatic digest loading, the popcat-tip was treated subsequently with H2O, 100% ACN, and 80% ACN for activation. Then, the enzymatic peptides in 80% ACN was loaded into the popcat-tip, followed by centrifugation at 100g for 5min. 80% ACN solution as a washing solution was loaded and centrifuged. Finally, different eluting solutions such as 0.1% TFA, 0.2% FA, H2O, NH4HCO3, and 0.25% NH3·H2O were employed to elute sialoglycopeptides, respectively.
4.7. Reproducibility and stabilityFive different amounts of bovine fetuin (i.e., 0.5, 1, 2, 4, 8, and 16pmol) were used to evaluate the limit of detection. The intraday and interday assays were also used to evaluate the reproducibility and stability of the popcat-tip-enrichment of sialoglycopeptides.
4.8. Delignification of poplar catkinFive equal amounts (approximately 10mg each) of poplar catkin were treated with 2% H2O2 (pH 11.5 adjusted by NaOH solution) at 60℃ for the different time periods of delignification (i.e., 0, 5, 10, 20, and 30min), respectively. The treated poplar catkin was washed with water to remove H2O2 and NaOH.
4.9. Mass spectrometric analysisMass spectra were acquired using an 9.4T Apex-ultraTM hybrid Qh-FTICR MS (Bruker Daltonics, Billerica, MA) equipped with a 355nm Nd:YAG Smartbeam Ⅱ laser. Approximately 0.3 µL of sample solution was spotted onto a MALDI target plate and then air-dried at room temperature prior to the addition of 0.3 µL of 20 µg/µL DHB (50% ACN/0.1% TFA). The instrument resolution was 490, 000 at m/z 400 over the m/z range of 1500–7000 inpositive ion mode. GlycoMod tool (http://web.expasy.org/glycomod/) was employed to help structure confirmation of glycan based on the observed masses of glycopeptides and combined with previous published data [36].
Competing financial interestsThe authors declare the following competing financial interest (s): Dr. Zhi-Li Li and Xiao-Dong Wang are listed as the inventors on a patent (No. ZL201410035250.9) filed in 2014 for a method for enriching sialylated glycopeptides, sialylated glycans and sialylated glycosides. The patent is owned by the Institute of Basic Medical Sciences, Chinese Academy of Medical Sciences. No other potential conflict of interest relevant to this article was reported.
AcknowledgmentThis study was supported by the National Natural Science Foundation of China (No. 21575164) to Z. L.
Appendix A. Supplementary dataSupplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.cclet.2017.02.001.
| [1] | G. Blix, E. Lindberg, L. Odin, I. Werner. Sialic acids. Nature 175 (1995) 340–341. |
| [2] | B.A. Cunningham, J.J. Hemperly, B.A. Murray, et al., Neural cell adhesion molecule:structure, immunoglobulin-like domains, cell surface modulation, and alternative RNA splicing. Science 236 (1987) 799–806. DOI:10.1126/science.3576199 |
| [3] | M. Fukuda. Possible roles of tumor-associated carbohydrate antigens. Cancer Res. 56 (1996) 2237–2244. |
| [4] | W.R. Alley Jr, B.F. Mann, M.V. Novotny. High-sensitivity analytical approaches for the structural characterization of glycoproteins. Chem. Rev. 113 (2013) 2668–2732. DOI:10.1021/cr3003714 |
| [5] | J.C. Paulson. Glycoproteins:what are the sugar chains for?. Trends Biochem. Sci. 14 (1989) 272–276. DOI:10.1016/0968-0004(89)90062-5 |
| [6] | P. Göröug, J.D. Pearson. Sialic acid moieties on surface glycoproteins protect endothelial cells from proteolytic damage. J. Pathol. 146 (1985) 205–212. DOI:10.1002/(ISSN)1096-9896 |
| [7] | E. Gorelik, U. Galili, A. Raz. On the role of cell surface carbohydrates and their binding proteins (lectins) in tumor metastasis. Cancer Metastasis Rev. 20 (2001) 245–277. DOI:10.1023/A:1015535427597 |
| [8] | T.F. Scanlin, M.C. Glick. Terminal glycosylation and disease:influence on cancer and cystic fibrosis. Glycoconj. J. 17 (2000) 617–626. DOI:10.1023/A:1011034912226 |
| [9] | Y.J. Kim, A. Varki. Perspectives on the significance of altered glycosylation of glycoproteins in cancer. Glycoconj. J. 14 (1997) 569–576. DOI:10.1023/A:1018580324971 |
| [10] | J. Romppanen, T. Haapalainen, K. Punnonen, I. Penttil. Serum sialic acid and prostate-specific antigen in differential diagnosis of benign prostate hyperplasia and prostate cancer. Anticancer Res. 22 (2002) 415–420. |
| [11] | A. Krzeslak, Z. Gaj, L. Pomorski, A. Lipinska. Sialylation of intracellular proteins of thyroid lesions. Oncol. Rep. 17 (2007) 1237–1242. |
| [12] | C.A. Ufret-Vincenty, D.J. Baro, L.F. Santana. Differential contribution of sialic acid to the function of repolarizing K+ currents in ventricular myocytes. Am. J. Physiol. Cell Physiol. 28 (2001) C464–474. |
| [13] | S.S. Gökmen, C. Kazezoglu, B. Sunar, et al., Relationship between serum sialic acids, sialic acid-rich inflammation-sensitive proteins and cell damage in patients with acute myocardial infarction. Clin. Chem. Lab. Med. 44 (2006) 199–206. |
| [14] | J. Amano, T. Nishikaze, F. Tougasaki, et al., Derivatization with 1-pyrenyldiazomethane enhances ionization of glycopeptides but not peptides in matrix-assisted laser desorption/ionization mass spectrometry. Anal. Chem. 82 (2010) 8738–8743. DOI:10.1021/ac101555a |
| [15] | S. Sekiya, Y. Wada, K. Tanaka. Derivatization for stabilizing sialic acids in MALDI-MS. Anal. Chem. 77 (2005) 4962–4968. DOI:10.1021/ac050287o |
| [16] | M. Toyoda, H. Ito, Y.K. Matsuno, H. Narimatsu, A. Kameyama. Quantitative derivatization of sialic acids for the detection of sialoglycans by MALDI MS. Anal. Chem. 80 (2008) 5211–5218. DOI:10.1021/ac800457a |
| [17] | H. Zhang, X.J. Li, D.B. Martin, R. Aebersold. Identification and quantification of N-linked glycoproteins using hydrazide chemistry, stable isotope labeling and mass spectrometry. Nat. Biotechnol. 21 (2003) 660–666. DOI:10.1038/nbt827 |
| [18] | Y. Tian, F.J. Esteva, J. Song, H. Zhang. Altered expression of sialylated glycoproteins in breast cancer using hydrazide chemistry and mass spectrometry. Mol. Cell. Proteom. 11 (2012) M111.011403.. DOI:10.1074/mcp.M111.011403 |
| [19] | J. Nilsson, U. Rüetschi, A. Halim, et al., Enrichment of glycopeptides for glycan structure and attachment site identification. Nat. Methods 6 (2009) 809–811. DOI:10.1038/nmeth.1392 |
| [20] | J. Hirabayashi. Lectin-based structural glycomics:glycoproteomics and glycan profiling. Glycoconj. J. 21 (2004) 35–40. DOI:10.1023/B:GLYC.0000043745.18988.a1 |
| [21] | Z. Yang, W.S. Hancock. Approach to the comprehensive analysis of glycoproteins isolated from human serum using a multi-lectin affinity column. J. Chromatogr. A 1053 (2004) 79–88. DOI:10.1016/S0021-9673(04)01433-5 |
| [22] | Y. Takegawa, K. Deguchi, H. Ito, et al., Simple separation of isomeric sialylated N-glycopeptides by a zwitterionic type of hydrophilic interaction chromatography. J. Sep. Sci. 29 (2006) 2533–2540. DOI:10.1002/(ISSN)1615-9314 |
| [23] | C.D. Calvano, C.G. Zambonin, O.N. Jensen. Assessment of lectin and HILIC based enrichment protocols for characterization of serum glycoproteins by mass spectrometry. J. Proteom. 71 (2008) 304–317. DOI:10.1016/j.jprot.2008.06.013 |
| [24] | S. Yang, S.T. Eshghi, H. Chiu, D.L. DeVoe, H. Zhang. Glycomic analysis by glycoprotein immobilization for glycan extraction and liquid chromatography on microfluidic chip. Anal. Chem. 85 (2013) 10117–10125. DOI:10.1021/ac4013013 |
| [25] | L. Xin, H.J. Zhang, H. Liu, Z.L. Li. Equal ratio of graphite carbon to activated charcoal for enrichment of N-glycopeptides prior to matrix-assisted laser desorption/ionization time-of-flight mass spectrometric identification. Rapid Commun. Mass Spectrom. 26 (2012) 269–274. DOI:10.1002/rcm.5327 |
| [26] | M.R. Larsen, P. Højrup, P. Roepstorff. Characterization of gel-separated glycoproteins using two-step proteolytic digestion combined with sequential microcolumns and mass spectrometry. Mol. Cell. Proteom. 4 (2005) 107–119. |
| [27] | G. Palmisano, S.E. Lendal, K. Engholm-Keller, et al., Selective enrichment of sialic acid-containing glycopeptides using titanium dioxide chromatography with analysis by HILIC and mass spectrometry. Nat. Protoc. 5 (2010) 1974–1982. DOI:10.1038/nprot.2010.167 |
| [28] | W.J. Wang, H. Liu, Z.L. Li. Tandem mass spectrometric characterization of fetuin sialylated glycopeptides enriched by TiO2 microcolumn. Chin. J. Chem. 29 (2011) 2229–2235. DOI:10.1002/cjoc.v29.11 |
| [29] | G. Palmisano, S. E. Lendal, M. R. Larsen, Titanium dioxide enrichment of sialic acid-containing glycopeptides, in: K. Gevaert, J. Vandekerckhove (Eds. ), GelFree Proteomics, Humana Press, New York, 2011, pp. 309-322. |
| [30] | M.R. Larsen, S.S. Jensen, L.A. Jakobsen, N.H.H. Heegaard. Exploring the sialiome using titanium dioxide chromatography and mass spectrometry. Mol. Cell. Proteom. 6 (2007) 1778–1787. DOI:10.1074/mcp.M700086-MCP200 |
| [31] | Z.J. Bie, Y. Chen, H.Y. Li, R.H. Wu, Z. Liu. Off-line hyphenation of boronate affinity monolith-based extraction with matrix-assisted laser desorption/ionization time-of-flight mass spectrometry for efficient analysis of glycoproteins/glycopeptides. Anal. Chim. Acta 834 (2014) 1–8. DOI:10.1016/j.aca.2014.04.035 |
| [32] | Y. Lu, Z.J. Bie, Y.C. Liu, Z. Liu. Fine-tuning the specificity of boronate affinity monoliths toward glycoproteins through pH manipulation. Analyst 138 (2013) 290–298. DOI:10.1039/C2AN36048A |
| [33] | Z.J. Bie, Y. Chen, J. Ye, S.S. Wang, Z. Liu. Boronate-affinity glycan-oriented surface imprinting:a new strategy to mimic lectins for the recognition of an intact glycoprotein and its characteristic fragments. Angew. Chem. Int. Ed. Engl. 54 (2015) 10211–10215. DOI:10.1002/anie.201503066 |
| [34] | S.S. Wang, D.Y. Yin, W.J. Wang, et al., Targeting and imaging of cancer cells via monosaccharide-imprinted fluorescent nanoparticles. Sci. Rep. 6 (2016) 22757. DOI:10.1038/srep22757 |
| [35] | M.H.J. Selman, M. Hemayatkar, A.M. Deelder, M. Wuhrer. Cotton HILIC SPE microtips for microscale purification and enrichment of glycans and glycopeptides. Anal. Chem. 83 (2011) 2492–2499. DOI:10.1021/ac1027116 |
| [36] | Y.J. Liu, Y.J. Liu, D. Zhang, R.Q. Zhang, Z.L. Li. Kapok fiber:a natural biomaterial for highly specific and efficient enrichment of sialoglycopeptides. Anal. Chem. 88 (2016) 1067–1072. DOI:10.1021/acs.analchem.5b04014 |
| [37] | A.W. Frankland. Seasonal allergic rhinitis. Proc. R. Soc. Med. 64 (1971) 447–450. |
| [38] | T.Q. Yuan, S.N. Sun, F. Xu, R.C. Sun. Structural characterization of lignin from triploid of Populus tomentosa Carr. J. Agric. Food Chem. 59 (2011) 6605–6615. DOI:10.1021/jf2003865 |
| [39] | A.P. Zhang, C.F. Liu, R.C. Sun. Fractional isolation and characterization of lignin and hemicelluloses from Triploid of Populus tomentosa Carr. Ind. Crops Prod. 31 (2010) 357–362. DOI:10.1016/j.indcrop.2009.12.003 |
| [40] | J.K. Weng, T. Akiyama, N.D. Bonawitz, et al., Convergent evolution of syringyl lignin biosynthesis via distinct pathways in the lycophyte Selaginella and flowering plants. Plant Cell 22 (2010) 1033–1045. DOI:10.1105/tpc.109.073528 |
| [41] | L. Jouanin, T. Goujon, V. de Nadaï, et al., Lignification in transgenic poplars with extremely reduced caffeic acid O-methyltransferase activity. Plant Physiol. 123 (2000) 1363–1374. DOI:10.1104/pp.123.4.1363 |
 2017, Vol. 28
2017, Vol. 28 


