b Institute of Microbiology and Biochemical Pharmacy, School of Pharmacy, Lanzhou University, Lanzhou 730000, China
Natural enzymes have been extensively investigated and widely applied because of their excellent properties, including high substrate specificity and high catalytic efficiency under mild conditions. However, their catalytic activity can be easily affected by environmental conditions such as acidity, temperature and inhibitors. Nanomaterials, due to their large specific surface areas, lowcost, flexibility in structure design and composition, and tunable catalytic activities have been exploited in simulating horseradish peroxidase (HRP) [1]. In 2007, Fe3O4 magnetic nanoparticles were firstly discovered to exhibit an intrinsic peroxidase-like enzyme mimetic activity [2]. After that, several metal oxide nanomaterials such as Co3O4 nanoparticles (NPs) [3], CeO2 NPs [4], ZnFe2O4 NPs [5], V2O5 nanowires [6] were also found to possess intrinsic peroxidaselike activity. Besides metal oxide nanomaterials, carbon nanomaterials including carbon nanodots (C-Dots) [7], single-walled carbon nanotubes (SWCNTs) [8], helical carbon nanotubes (HCNTs) [9], graphene oxide (GO) [10], C3N4 [11] displayed intrinsic enzyme mimetic activity. Additionally, many kinds of hybrid materials and other materials were proved to be highly efficient catalyst in simulating natural enzyme activity, for example, GO–Fe3O4 [12], hemin–GO nanosheets [13], hemin-MoS2 nanosheets [14], heminWS2 nanosheets [15], WS2 nanosheets [16], Au NPs [17] have also been reported possessing intrinsic peroxidase-like activity in the presence of H2O2. These enzyme-like nanomaterials could be employed to detect immunoassay [2, 4], nucleotide 8, H2O2 [10, 18], glucose [10, 15], melamine [19], and so on. They were more stable against denaturation or protease digestion than HRP. Furthermore, their preparation and storage were relatively simple.
Metal-organic frameworks (MOFs) have generated great excitement in the last few years because of its novel properties with potential applications. As a type of zeolite-like crystalline porous materials, MOFs have been extensively exploited in applications for gas storage, separation and catalysis because of their extremely large specific surface areas, wide variety of shapes, tunable pore sizes and exposed active sites [20–26]. MOFs have so many advantages and applications, especially, they have been widely used to catalyze organic reactions, and few of them take advantages of either unsaturated coordination sites or redoxactive metal centers to achieve specially catalytic activities [27]. So, do MOFs also have HPR property? Indeed, mesoporous MOF PCN-222(Fe) with porphyrinic Fe(Ⅲ) centers [28], Fe(Ⅲ)-based metal-organic frameworks, such as MIL-53 [29], MIL-68 and MIL-100 [30] were found to be effective peroxidase mimics.
In this paper, one previously reported Fe-based MOF, MIL-88 [31], is found to be able to exhibit intrinsic peroxidase-like activity for colorimetric sensor for the first time. It catalyzes the oxidation of TMB as peroxidase mimics to provide a colorimetric assay for H2O2 with significant solution color change in the presence of H2O2. Ascorbic acid (AA) induces an inhibitory effect on the oxidation, thus it also can colorimetric detect AA.
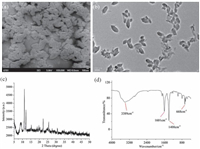
2. Results and discussion 2.1. Characterization of MIL-88Scanning electron microscopy (SEM) image indicates that the MIL-88 particles with sizes of about 250 mm have some extent of aggregation (Fig. 1a). Transmission electron microscopy (TEM) image clearly shows MIL-88 morphology as the shape of shuttle (Fig. 1b). The Powder X-ray diffraction (PXRD) pattern of MIL-53(Fe) (Fig. 1c) shows that the as-obtained sample was crystalline, and the diffraction peaks were coincident with the previously reported MOF MIL-88. SEM, TEM and PXRD proved that MIL-88 has been successfully made. MIL-88 was further confirmed by FTIR spectroscopy. In the spectrum of MIL-88, as shown in Fig. 1d, the band at 3389 cm-1 is attributed to the stretching of O-H. The peak at 1601 cm-1 is associated with stretching of the C=O bond of carboxyl groups. The peak at 1400 cm-1 can be assigned to the C=C bond. The weak peak at 668 cm-1 is associated with stretching of the C-H bond. The chemical composition determined by the EDX spectrum (Fig. S1 in Supporting information) reveals that the C, Fe, and O elements coexist in MIL-88. N2 sorption isotherms confirm their permanent porosity and the BET surface areas of MIL-88 is 99 m2/g (Fig. S2 in Supporting information).
|
Download:
|
| Figure 1. (a) SEM image of MIL-88, (b) TEM image of MIL-88, (c) PXRD patterns of MIL-88, (d) FTIR spectrum of MIL-88. | |
2.2. Peroxidase-like activity of MIL-88
To demonstrate the peroxidase-like activity of MIL-88, the peroxidase-like activity of MIL-88 was evaluated by the catalytic oxidation of peroxidase substrate TMB in the presence of H2O2. As shown in Fig. 2a, the color of NaAc-HAc buffer was colorless. In the absence of H2O2 (Fig. 2b), a slight blue color solution was observed. But, in other papers which was colorless. In this paper the color of the solution was slight blue which was colorless as previously reported [29, 30], which means the concentration of TMB was higher than in others papers. In Fig. 2c, the color of TMB+H2O2 solution was darker than TMB solution, although there was no catalyst, TMB could partially be oxidized. The reason was the same for TMB solution, concentration of TMB+H2O2 was larger than reported literature. In contrast, the MIL-88+TMB+H2O2 system produced a deep blue color (Fig. 2d). These results supported that the MIL-88 behaves like peroxidase toward typical peroxidase substrates such as TMB. The reaction scheme is described in Fig. 2e as follows: in the presence of H2O2, TMB is catalyzed by the MIL-88 which acts as peroxidase, the absorbance of converted TMB provides a way to monitor the catalytic reaction at 652 nm, and the adsorption bands could be attributed to the charge-transfer complexes derived from the one-electron oxidation of TMB [32]. The phenomenon is similar to that observed for the commonly used horseradish peroxidase (HRP) enzyme [2].

|
Download:
|
| Figure 2. Color evolution of TMB in different reaction systems: (a) NaAc buffer, (b) NaAc buffer+TMB, (c) NaAc buffer+TMB+H2O2, (d) NaAc buffer+TMB+H2O+MIL-88 at 35℃ for 30 min (the pH of NaAc-HAc buffer is 4.0). (e) Corresponding reaction scheme for H2O2 reduction with TMB. | |
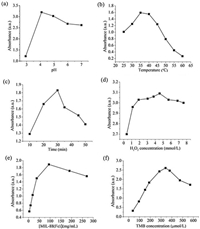
As previously reported, similar to horseradish peroxidase (HRP), the catalytic activity of MIL-88 and its enzyme mimics is also dependent on pH, temperature and substrate concentration [33, 34]. Thus, the pH, temperature, time, quantity of MIL-88, concentration of H2O2 and TMB dependent-activity of the MIL-88 have been also investigated in the current study. According to literatures [2–23, 27–29], most simulate catalytic activity of HRP were carried out in pH 4 buffers, so, firstly, a series of different pH solutions were investigated. Catalytic activity of MIL–88 pH was evaluated from 3 to 7, as shown in Fig. 3a, result showed maximum catalytic activity at pH 4.0. The catalytic oxidation of TMB with H2O2 using MIL-88 was much faster in acidic solutions than neutral or basic solutions, thus, 20 mmol/L NaAc-HAc with pH of 4.0 was selected as the optimal reaction medium to get high catalytic activity. The catalytic activity of MIL-88 was also dependent on temperature, time, quantity of MIL-88, and concentration of TMB and H2O2 (Fig. 3). The maximum catalytic activity of the nanocomposites was obtained under the following optimal conditions: pH 4.0, 35℃, 30 min, 94.5 µg/mL of MIL-88, 324 µmol/L TMB and 4.56 mmol/L H2O2. These results are close to the values obtained with other NP-based peroxidase mimetics and HRP [6, 18, 19].
|
Download:
|
| Figure 3. Effect of (a) pH, (b) temperature, (c) time, (d) H2O2 concentration, (e) quantity of MIL-88 and (f) TMB concentration on relative peroxidase-like activity. | |
2.3. Kinetic analysis
Like the natural enzyme catalyzed reaction, the MIL-88-catalyzed reaction was inhibited at high H2O2 concentration. Moreover, in a certain range of H2O2 concentrations, typical Michaelis–Menten curves can be obtained for MIL-88 (Fig. 4). The experiments were performed by changing the concentrations of TMB or H2O2 in this system while keeping the other as constant. Kinetic experiments were carried out using 15 µL of MIL-88 in 1.0 mL of NaAc–HAc buffer solution (20 mmol/L, pH 4.0) containing 0.1 mmol/L TMB as the substrate, and the H2O2 concentration was 1.14 mmol/L. The initial reaction rate was calculated by the Lambert–Beer law c=A/εb, while b is the thickness of the solution, A is the absorbance value, and c represents the substrate concentration. The absorbance data were back-calculated to give concentrations using a molar absorption coefficient, ε, which value is 39 000 L/mol/cm for the TMB derived oxidation products [7]. The Michaelis–Menten constant (Km) and maximum initial velocity (Vmax) were obtained from Lineweaver–Burk plots of the double reciprocal of the Michaelis–Menten equation,
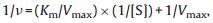
|

|
Download:
|
| Figure 4. Steady-state kinetic assay and catalytic mechanism of MIL-88. The velocity (ν) of the reaction was measured using MIL-88 (28.5 µg/mL) in HAc–NaAc buffer (20 mmol/L, pH 4.0) at 35℃. (a) The concentration of TMB was 0.1 mmol/L, and the H2O2 concentration was varied. (b) The concentration of H2O2 was 1.14 mmol/L, and the TMB concentration was varied. (c, d) Double reciprocal plots of activity of MIL-88 with the concentration of one substrate (TMB or H2O2) fixed and the other varied. | |
where ν is the initial velocity, Vmax represents the maximal reaction velocity, Km is the Michaelis–Menten constant and [S] is the concentration of the substrate. The results demonstrated that the oxidation reaction catalyzed by MIL-88 agreed with the typical Michaelis–Menten behavior towards both substrates TMB and H2O2. The double-reciprocal plots (Fig. 4c and d) revealed the characteristic parallel lines is a ping–pong mechanism and suggested that MIL-88 bound and reacted with the first substrate (TMB or H2O2), then released the first product before reacting with the second substrate (H2O2 or TMB). The Michaelis constant (Km) and reaction rate (Vmax) were obtained from Line weaver–Burk plots (Table S1 in Supporting information). The apparent Km value of MIL-88 with H2O2 as the substrate was significantly lower than HRP and other nanomaterials-based peroxidase mimics (Table S2 in Supporting information), showing that MIL-88 has higher catalytic activity to H2O2 than HRP and other materials. The apparent Km value of MIL-88 with TMB as the substrate was higher than HRP, consistent with the observation that a higher TMB concentration was required to achieve maximal activity for MIL-88.
2.4. Detection of H2O2 and ascorbic acidMIL-88 possesses intrinsic peroxidase property according to kinetic analysis. On the basis of the above optimum assay conditions, the sensitivity of MIL-88 for the detection of H2O2 and ascorbic acid were evaluated using the MIL-88-catalyzed blue color reaction. Since the catalytic activity of MIL-88 is H2O2-concentration-dependent, this can be used to detect H2O2. Fig. 5 depicts a typical H2O2 concentration-dependent absorbance change curve at 652 nm upon analyzing different concentrations of H2O2. The absorbance at 652 nm was proportional to H2O2 concentration from 2.0×10-6 mol/L to 2.03×10-5 mol/L (R2=0.981) (Fig. 5 insert) with a detection limit of 5.62×10-7 mol/L and the color variation was obvious on visual observation at low concentrations, which sensitivity is about the same as that reported in previous reports (Table S2).

|
Download:
|
| Figure 5. A dose-response curve for H2O2 detection using MIL-88 under the optimum conditions described. Inset: linear calibration plot for H2O2 with different concentrations of H2O2 in absorbance at 652 nm. | |
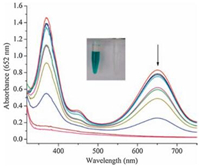
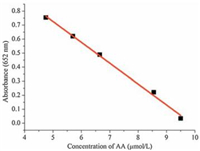
As is known to all, ascorbic acid has the function of antioxidation. If the oxidation process of peroxidase substrates-TMB can be effectively suppressed upon introducing a trace amount of AA to afford the solution color change from blue to colorless? With this in mind, a colorimetric biosensor system for AA based on MIL-88 can be readily developed. As shown in Fig. 6, upon the addition of AA, the typical UV–vis absorbance intensity of TMB at 652 nm became weaker. When the amount of added AA was enough, the peak of absorbance at 652 nm disappeared, which results in the corresponding calibration plot shown in the inset in Fig. 7. Antioxidation property of ascorbic acid caused the oxidation process of TMB was inhibited. This analytical method for AA detection was served to have a linear range from 2.57×10-6 mol/L to 1.01×10-5 mol/L (R2=0.989) with a detection limit of 1.03×10-6 mol/L (S/N=3).
|
Download:
|
| Figure 6. UV/vis spectra of TMB oxidation catalyzed by MIL-88 in the presence of ascorbic acid as inhibitor in a pH 4.0 acetate buffer at 35℃ for 30 min (TMB: 68 µmol/L, H2O2: 0.76 mmol/L, MIL-88: 1.9 mg/mL). | |
|
Download:
|
| Figure 7. Linear calibration plot for ascorbic acid with different concentrations of ascorbic acid in absorbance at 652 nm. | |
3. Conclusions
In conclusion, our experiment results prove that MIL-88 possesses intrinsic peroxidase-like activity and its catalysis ability is strongly dependent on time, pH value, temperature, and H2O2 concentration, the same as horseradish peroxidase. MIL-88 can accelerate oxidation of peroxidase substrate TMB in the presence of H2O2 with the solution color change from colorless to blue. Kinetic analysis suggests that the catalysis abides by a ping-pong mechanism, and MIL-88 has even higher catalytic activity to TMB than natural enzyme HRP. MIL-88, as a nano-enzyme, exhibits several advantages over natural enzymes, such as ease to prepare, low-cost, and predominant stability. In addition, compared to HRP and Fe3O4 nanoparticles, MIL-88 possesses high surface-to-volume ratios as well as high affinity to organic substrates-TMB. Based on this finding and inhibition effect of AA, we design and develop a simple, cheap, and highly selective and sensitive colorimetric assay to detect AA in buffer solution. The integration of a high density of catalytic centers, ultra-large open channels, and the extraordinary chemical stability of MIL-88 suggests a bright future for building MOF-based platforms for enzyme-mimic catalysis.
4. Experimental 4.1. Materials and apparatus3,3', 5,5'-Tetramethylbenzidine (TMB) were purchased from Sigma-Aldrich Chemical Co. (St. Louis, MO, USA). All other chemicals were purchased from commercial sources and used without further purification. Water was purified with a Milli-Q plus 185 equip from Millipore (Bedford, MA, USA) and used throughout all experiments. UV–vis absorption spectra and kinetic measurements were carried out on a Lambda 35 UV–vis spectrophotometer (Perkin-Elmer, USA). Transmission electron microscopy (TEM) images were recorded on a Tecnai G2 TF20 transmission electron microscope (FEI, USA). Scanning electron microscopy (SEM) images were recorded on a JSM-6701F field emission scanning electron microscope (Japan). X-ray diffraction (XRD) patterns were observed using an X'Pert PRO X-ray diffractometer (PANalytical, Netherlands). Fourier transform infrared (FTIR) spectrum was recorded on a Nicolet 360 FT-IR spectrometer using KBr pellets in the range of 4000–400 cm-1.
4.2. Preparation of MIL-88MIL-88 was prepared based on a previously reported method with minor modifications [32]. Briefly, iron (ⅲ) acetate, fumaric acid, sodium hydroxide, deionized water, and methanol were mixed in the following ratios: 1:3:1.5:50:1000. The resulting orange gels were kept in a Teflon-lined bomb at 100℃ for 3 days without stirring and cooled down to room temperature. The light orange solids were centrifuged at 3000 rpm for 2 min, washed with methanol and acetone, and dried at room temperature.
4.3. Detection of H2O2 and ascorbic acidKinetic measurements were carried out by monitoring the absorbance at various times with a spectrophotometer. Catalytic experiments were performed using 1.9 mg/mL MIL-88 in a reaction volume of 1 mL NaAc-HAc buffer solution (20 mmol/L pH 4.0) with 0.1 mmol/L TMB as substrate, or 1.14 mmol/L H2O2, unless otherwise stated. The Michaelis–Menten constant was calculated using Lineweaver–Burk plots of the double reciprocal of the Michaelis–Menten equation, 1/ν=Km/Vm (1/[S]+1/Km), where ν is the initial velocity, Vm represents the maximal reaction velocity, [S] corresponds to the concentration of substrate and Km is the Michaelis constant.
H2O2 detection was performed as follows: MIL-88(10 µL, 1.9 mg/mL), TMB (36 µL, 1.9 mmol/L) and HAc–NaAc buffer (20 mmol/L, pH 4.0) were added into a 1.5 mL Eppendorf tube. Then H2O2 with different concentrations was added. The volume of these four solutions added up to 1 mL, with volume change ignored. Subsequently, the mixed solutions were incubated for 30 min at 35℃. After reaction was finished, moving tubes into icewater bath to stop the catalytic reaction.
Ascorbic acid detection was realized as follows: MIL-88 (10 µL, 1.9 mg/mL), TMB (36 µL, 1.9 mmol/L), H2O2 (20 µL, 0.038 mmol/L) and HAc–NaAc buffer (20 mmol/L, pH 4.0) were added into a 1.5 mL Eppendorf tube. Then ascorbic acid with different concentrations was added. The volume of these five solutions added up to 1 mL, with volume change ignored. Subsequently, the mixed solutions were incubated for 30 min at 35℃. After reaction was finished, tubes were moved into ice-water bath to stop the catalytic reaction.
AcknowledgementsThe authors express their thanks to the support of the Funds for Distinguished Young Scientists of Gansu (No. 1506RJDA281) and the top priority program of "One-Three-Five" Strategic Planning of Lanzhou Institute of Chemical Physics, CAS, and the Foundation for Sci & Tech Research Project of Gansu Province (No. 1606RJYA307).
Appendix A. Supplementary dataSupplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.cclet.2017.02.011.
| [1] | L.Z. Gao, J.M. Wu, S. Lyle, et al., Magnetite nanoparticle-linked immunosorbent assay. J. Phys. Chem.C 112 (2008) 17357–17361. DOI:10.1021/jp805994h |
| [2] | L.Z. Gao, J. Zhuang, L. Nie, et al., Intrinsic peroxidase-like activity of ferromagnetic nanoparticles. Nat. Nanotechnol. 2 (2007) 577–583. DOI:10.1038/nnano.2007.260 |
| [3] | J.S. Mu, Y. Wang, M. Zhao, L. Zhang. Intrinsic peroxidase-like activity and catalaselike activity of Co3O4 nanoparticles. Chem. Commun. 48 (2012) 2540–2542. DOI:10.1039/c2cc17013b |
| [4] | A. Asati, S. Santra, C. Kaittanis, S. Nath, J.M. Perez. Oxidase-like activity of polymer-coated cerium oxide nanoparticles. Angew. Chem. Int. Ed. 48 (2009) 2308–2312. DOI:10.1002/anie.200805279 |
| [5] | L. Su, J. Feng, X.M. Zhou, et al., Colorimetric detection of urine glucose based ZnFe2O4 magnetic nanoparticles. Anal. Chem. 84 (2012) 5753–5758. DOI:10.1021/ac300939z |
| [6] | R. André, F. Natálio, M. Humanes, et al., V2O5 nanowires with an intrinsic peroxidase-like activity. Adv. Funct. Mater. 21 (2011) 501–509. DOI:10.1002/adfm.v21.3 |
| [7] | W.B. Shi, Q.L. Wang, Y.J. Long, et al., Carbon nanodots as peroxidase mimetics and their applications to glucose detection. Chem. Commum. 47 (2011) 6695–6697. DOI:10.1039/c1cc11943e |
| [8] | Y.J. Song, X.H. Wang, C. Zhao, et al., Label-free colorimetric detection of single nucleotide polymorphism by using single-walled carbon nanotube intrinsic peroxidase-like activity. Chemistry 16 (2010) 3617–3621. DOI:10.1002/chem.200902643 |
| [9] | R.J. Cui, Z.D. Han, J.J. Zhu. Helical carbon nanotubes:intrinsic peroxidase catalytic activity and its application for biocatalysis and biosensing. Chemistry 17 (2011) 9377–9384. DOI:10.1002/chem.v17.34 |
| [10] | Y.J. Song, K.G. Qu, C. Zhao, J.S. Ren, X.G. Qu. Graphene oxide:intrinsic peroxidase catalytic activity and its application to glucose detection. Adv. Mater. 22 (2010) 2206–2210. DOI:10.1002/adma.v22:19 |
| [11] | T.R. Lin, L.S. Zhong, J. Wang, et al., Graphite-like carbon nitrides as peroxidase mimetics and their applications to glucose detection. Biosens. Bioelectron. 59 (2014) 89–93. DOI:10.1016/j.bios.2014.03.023 |
| [12] | Y.L. Dong, H.G. Zhang, Z.U. Rahman, et al., Graphene oxide-Fe3O4 magnetic nanocomposites with peroxidase-like activity for colorimetric detection of glucose. Nanoscale 4 (2012) 3969–3976. DOI:10.1039/c2nr12109c |
| [13] | T. Xue, S. Jiang, Y.Q. Qu, et al., Graphene-supported hemin as a highly active biomimetic oxidation catalyst. Angew. Chem. Int. Ed. 51 (2012) 3822–3825. DOI:10.1002/anie.v51.16 |
| [14] | B.L. Li, H.Q. Luo, J.L. Lei, N.B. Li. Hemin-functionalized MoS2 nanosheets:enhanced peroxidase-like catalytic activity with a steady state in aqueous solution. RSC Adv. 4 (2014) 24256–24262. DOI:10.1039/c4ra01746c |
| [15] | Q. Chen, J. Chen, C.J. Gao, et al., Hemin-functionalized WS2 nanosheets as highly active peroxidase mimetics for label-free colorimetric detection of H2O2 and glucose. Analyst 140 (2015) 2857–2863. DOI:10.1039/C5AN00031A |
| [16] | T.R. Lin, L.S. Zhong, Z.P. Song, et al., Visual detection of blood glucose based on peroxidase-like activity of WS2 nanosheets. Biosens. Bioelectron. 62 (2014) 302–307. DOI:10.1016/j.bios.2014.07.001 |
| [17] | Y.J. Long, Y.F. Li, Y. Liu, et al., Visual observation of the mercury-stimulated peroxidase mimetic activity of gold nanoparticles. Chem. Commun. 47 (2011) 11939–11941. DOI:10.1039/c1cc14294a |
| [18] | H. Wei, E.K. Wang. Fe3O4 Magnetic nanoparticles as peroxidase mimetics and their applications in H2O2 and glucose detection. Anal. Chem. 80 (2008) 2250–2254. DOI:10.1021/ac702203f |
| [19] | N. Ding, N. Yan, C.L. Ren, X.G. Chen. Colorimetric determination of melamine in dairy products by Fe3O4 magnetic nanoparticles-H2O2-ABTS detection system. Anal. Chem. 82 (2010) 5897–5899. DOI:10.1021/ac100597s |
| [20] | S. Ma. Gas adsorption applications of porous metal-organic frameworks. Pure Appl. Chem. 81 (2009) 2235–2251. |
| [21] | J.Y. Lee, O.K. Farha, J. Roberts, et al., Metal-organic framework materials as catalysts. Chem. Soc. Rev. 38 (2009) 1450–1459. DOI:10.1039/b807080f |
| [22] | J.L.C. Rowsell, O.M. Yaghi. Effects of functionalization catenation, and variation of the metal oxide and organic linking units on the low-pressure hydrogen adsorption properties of metal-organic frameworks. J. Am. Chem. Soc. 128 (2006) 1304–1315. DOI:10.1021/ja056639q |
| [23] | Z.Y. Gu, J. Park, A. Raiff, Z.W. Wei, H.C. Zhou. Metal-organic frameworks as biomimetic catalysts. ChemCatChem 6 (2014) 67–75. DOI:10.1002/cctc.v6.1 |
| [24] | Y.X. Sun, W.Y. Sun. Influence of temperature on metal-organic frameworks. Chin. Chem. Lett. 25 (2014) 823–828. DOI:10.1016/j.cclet.2014.04.032 |
| [25] | L. Hao, X.L. Liu, J.T. Wang, et al., Metal-organic framework derived magnetic nanoporous carbon as an adsorbent for the magnetic solid-phase extraction of chlorophenols from mushroom sample. Chin. Chem. Lett. 27 (2016) 783–788. DOI:10.1016/j.cclet.2016.01.021 |
| [26] | T. Wang, Q.H. Liu, Y. Gao, et al., A multi-responsive luminescent sensor towards Fe3+ and acetone based on a Cd-containing metal-organic framework. Chin. Chem. Lett. 27 (2016) 497–501. DOI:10.1016/j.cclet.2016.01.011 |
| [27] | J.W. Liu, L.F. Chen, H. Cui, et al., Applications of metal-organic frameworks in heterogeneous supramolecular catalysis. Chem. Soc. Rev. 43 (2014) 6011–6061. DOI:10.1039/C4CS00094C |
| [28] | D.W. Feng, Z.Y. Gu, J.R. Li, et al., Zirconium-metalloporphyrin PCN-222:mesoporousmetal-organic frameworks with ultrahigh stabilityas biomimetic catalysts. Angew. Chem. Int. Ed. 51 (2012) 10307–10310. DOI:10.1002/anie.201204475 |
| [29] | L.H. Ai, L.L. Li, C.H. Zhang, J. Fu, J. Jiang. MIL-53(Fe):a metal-organic framework with intrinsic peroxidase-like catalytic activity for colorimetric biosensing. Chemistry 19 (2013) 15105–15108. DOI:10.1002/chem.201303051 |
| [30] | J.W. Zhang, H.T. Zhang, Z.Y. Du, et al., Water-stable metal-organic frameworks with intrinsic peroxidase-like catalytic activity as a colorimetric biosensing platform. Chem. Commun. 50 (2014) 1092–1094. DOI:10.1039/C3CC48398C |
| [31] | C. Serre, F. Millange, S. Surblé, G. Férey. A route to the synthesis of trivalent transition-metal porous carboxylates with trimeric secondary building units. Angew. Chem. Int. Ed. 43 (2004) 6286–6289. |
| [32] | A. Fateeva, P. Horcajada, T. Devic, et al., Synthesis structure, characterization, and redox properties of the porous MIL-68(Fe) solid. Eur. J. Inorg. Chem. 2010 (2010) 3789–3794. DOI:10.1002/ejic.201000486 |
| [33] | K. Chattopadhyay, S. Mazumdar. Structural and conformational stability of horseradish peroxidase:effect of temperature and pH. Biochemistry 39 (2000) 263–270. DOI:10.1021/bi990729o |
| [34] | Y.J. Guo, L. Deng, J. Li, et al., Hemin-graphene hybrid nanosheets with intrinsic peroxidase-like activity for label-free colorimetric detection of singlenucleotide polymorphism. ACS Nano 5 (2011) 1282–1290. DOI:10.1021/nn1029586 |
 2017, Vol. 28
2017, Vol. 28 


