b Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan 430030, China;
c State Key Laboratory of Phytochemistry and Plant Resources in West China, Kunming Institute of Botany, Chinese Academy of Sciences, Kunming 650204, China
Polycyclic polyprenylated acylphloroglucinols (PPAPs) are a class of structurally intriguing and biologically active secondary metabolites from the family Guttiferae [1]. Homoadamantyl-PPAPs are subclass of PPAPs containing a homoadamantane core structure. Recently, many PPAPs with diverse structures were isolated and identified by natural product chemists around the world [2-5]. Most of those PPAPs are bicyclo[3.3.1]-type, however, homoadamantyl-PPAPs are rarely reported [6].
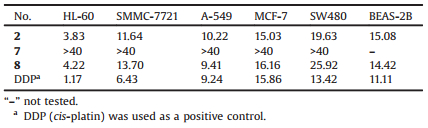
In our continuing efforts on bioactive PPAPs from the medicinal plants Hypericum sampsonii, scores of numerous novel PPAPs were previously identified [7-9], such as hyperisampsins A-D. Further chemical investigation on H. sampsonii has led to the isolation of two new PPAPs with a homoadamantyl framework, hyperisampsins N and O (1 and 2), along with 19 known analogs (3-21) (Fig. 1). The structures of these known compounds were determined by comparison of their NMR data with those reported in the literature, and identified as plukenetione C (3) [10], otogirinin B (4) [11], peroxysampsones A (5) [10] and B (6) [10], hypersampsonones G (7) [12] and F (8) [12], hypersampsone P (9) [13], attenuatumione D (10) [14], hypercohone A (11) [15], sampsoniones C (12) [16], F (13) [16], G (14) [16], and H (15) [16], plukenetione B (16) [17], hypersampsone Ⅰ (17) [18], 7b-H-1-benzoyl-5a-hydroxy-6, 6, 13, 13-tetramethyl-11-(3-methyl-2-butenyl)tetracyclo [7.3.1.1.03, 1103, 7] tetradecane-2, 12, 14-trione (then named as sampsonione R, 18) [19, 20], sampsoniones A (19) [21] and B (20) [21], and otogirinin C (21) [11]. Herein, we report the isolation, structural elucidation, and absolute configuration determination of these new PPAPs. In addition, cytotoxic activities of compounds 2, 7, and 8 against five human cancer cell lines were evaluated, and compounds 2 and 8 showed moderate cytotoxic activities.
|
Download:
|
| Figure 1. Structures of 1-21. | |
2. Results and discussion
The molecular formula of hyperisampsin N (1) was established as C38H50O7 by HR-ESIMS at m/z 641.3373 [M + Na]+. The 1H NMR spectrum of 1 (Table 1) showed five protons for a phenyl group [δH 7.13 (br d, 2H, J = 8.1 Hz; 7.28 (br t, 2H, J = 8.1 Hz); 7.42 (br t, 1H, J = 7.4 Hz)], two olefinic protons [δH 5.28 (t, 1H, J = 7.4 Hz) and 5.06 (t, 1H, J = 6.3 Hz)], an oxygenated methine [δH 4.89 (dd, 1H, J = 11.0, 3.9 Hz)], and nine methyl singlets (δH 1.67, 1.66, 1.59, 1.44, 1.36, 1.32, 1.27, 1.19, and 1.15). The 13C NMR (Table 1) and DEPT spectra of 1 displayed 38 carbon signals including four carbonyls (δC 207.7, 204.5, 203.8, and 193.2), ten olefinic carbons (δC 139.5, 135.6, 132.6, 131.7, 128.6, 128.6, 128.3, 128.3, 124.3, and 118.9), and three quaternary carbons and a methine located at the range of 70.0-90.0 ppm. These data showed high degrees of similarity to those of otogirinin B (4) [21], which suggested that 1 is also a PPAP with an 1, 2-dioxepane functionality.
|
|
Table 1 NMR data for compounds 1 and 2.a |
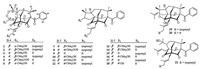
The planar structure of 1 was determined by extensive analyses of its HMBC and 1H-1H COSY spectra (Fig. 2). 1H-1H COSY correlations of H-4/H-5/H-6/H-7 and HMBC correlations from Me-37 and Me-38 to C-1, C-6, and C-10, from H-7 to C-8, C-9, and C-11, and from H-4 to C-2, C-3, and C-11 established the homoadamantyl framework of 1. In addition, HMBC interactions from Me-25 and Me-26 to C-4 and C-24 (δC 88.6), from Me-22 and Me-23 to C-20 (88.5) and C-21, and from H-19 to C-3 and C-11 together with 1H-1H COSY cross peak between H-19/H-20 indicated the presence of 1, 2-dioxepane ring system fused to the homoadamantyl core. Finally, the geranyl group was located at C-8 by HMBC correlations from H-27 to C-8, C-9, and C-11, and the remained benzoyl was located at C-1 in the same manner as those of 4 as considering the chemical shift of C-1 (δC 82.0). Consequently, compound 1 shared the same planar structure with 4.

|
Download:
|
| Figure 2. Key 1H–1H COSY and HMBC correlations of 1 and 2. | |
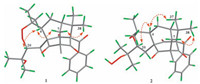
The relative configuration of 1 was determined by conducting NOESY experiment (Fig. 3). NOESY correlations from H-4 to H-20 and H-7b were observed (H-7b is overlapped with H-30 and H-32, but it is impossible to observe any NOESY correlation between H-4 and H-30 or H-32 for their large distances in molecular modeling), suggesting β-orientations both for H-4 and for H-20. The elucidated structure of 1 including relative configuration were identical to that of 6 [10], excepting the side chain at C-8, and a detailed comparison of their 13C NMR further supported the elucidated structure and relative configuration of 1.
|
Download:
|
| Figure 3. Key NOESY correlations of 1 and 2. | |
Hyperisampsin Q (2) showed an [M + Na]+ ion at m/z 657.3383 (C38H50O8Na), which was 16 mass units more as compared with 1 and 4. Detailed comparison of the NMR data of 2 with those of 4 revealed that 2 possesses a hydroperoxyl group at C-21 (δC 84.2), as evidenced by the deshielded chemical shift of C-21. In the NOESY spectrum of 2, cross-peaks for Me-38/H-7a, Me-37/H-4, and H-4/ H-20 implied that both H-4 and H-20 were α-oriented, which were identical with 4. Further comparison of 13C NMR of 2 and 5 [10] supported the elucidated structure and relative configuration of 2.
To determine the absolute configurations of compounds 1 and 2, ECD calculations were performed for both of them. The absolute configurations of 1 and 2 were thus determined as 1R, 3R, 4R, 6S, 8S, 20R and 1R, 3R, 4S, 6S, 8S, 20S by comparison of their experimental ECD spectra with the calculated ECD curves (Fig. 4).

|
Download:
|
| Figure 4. Calculated and experimental ECD spectra of 1 and 2. | |
Compounds 2, 7, and 8 were tested for their cytotoxic activities against five human tumor cell lines, including a myeloid leukemia line (HL-60 cells), a hepatocellular carcinoma line (SMMC-7721), a lung carcinoma line (A-549), a breast cancer line (MCF-7), and a colon cancer line (SW480). Cis-platin (DDP) was used as positive control for antitumor activity (Table 2). Among them, compounds 2 and 8 showed moderate cytotoxic activities, with IC50 values over a range of 3.83-25.92 mmol/L. Compound 7 was inactive against all the tested cell lines at concentrations as high as 40 mmol/L.
|
|
Table 2 Cytotoxic activities of 2, 7, and 8 (IC50 in mmol/L). |
3. Conclusion
In conclusion, twenty one benzoylated PPAPs with homoadamantyl framework were isolated from H. sampsonii, including two new ones. As far as we know, there are only eight examples of PPAPs with a 1, 2-dioxepane moiety that fused to the homoadamantyl core structure, and compound 2 represents the fourth example of this compound class possessing two peroxide moieties. Moreover, compounds 2 and 8 exhibit significant cytotoxic activities toward HL-60 cell and moderate activities against others cell lines, which may depend on the hydroperoxyl group as compound 7 showed no cytotoxic activity at concentrations as high as 40 mmol/L.
4. Experimental 4.1. GeneralOptical rotations were determined with a Perkin-Elmer 341 polarimeter. UV, ECD, and IR spectra were measured using a Varian Cary 50, a JASCO-810 spectrometer, and a Bruker Vertex 70, respectively. NMR spectra were recorded on a Bruker AM-400 spectrometer, and the 1H and 13C NMR chemical shifts were referenced to the solvent or solvent impurity peak for CDCl3 (δH 7.26 and δC 77.0). High-resolution electrospray ionization mass spectra (HRESIMS) were obtained in the positive ion mode with a Thermo Fisher LC-LTQ-Orbitrap XL spectrometer. Semi-preparative HPLC was performed on an Ultimate 3000 pump, an Ultimate 3000 autosampler injector, and an Ultimate 3000 diode array detector (DAD) controlled by Chromeleon software (version 6.80) using a reverse-phase C18 column (5 mm, 10 × 250 mm, Welch Ultimate XB-C18). Column chromatography was performed using silica gel (100-200 and 200-300 mesh; Qingdao Marine Chemical Inc., China), ODS (50 mm, Merck, Germany), Sephadex LH-20 (Merck, Germany), and MCI gel (75-150 mm, Merck, Germany). Thin-layer chromatography (TLC) was performed with silica gel 60 F254 (Yantai Chemical Industry Research Institute) and RP-C18 F254 plates (Merck, Germany).
4.2. Plant materialThe aerial parts of H. sampsonii were collected from the Da-bie Mountain areas of Hubei Province, China, in October 2011, and were identified by associate professor Jianping Wang. A voucher specimen (ID 20111008) has been deposited with the Herbarium of Materia Medica, Faculty of Pharmacy, Tongji Medical College of Huazhong University of Science and Technology, China.
4.3. Extraction and isolationThe air-dried aerial parts of H. sampsonii (50 kg) were extracted with 95% EtOH, and the extract was partitioned successively with petroleum ether and CHCl3 against water. The petroleum ether soluble extract (800 g) was separated by chromatography on a silica gel column (5 kg, 20 × 120 cm; petroleum ether to acetone, 100:0 → 0:100) to furnish eight fractions (Fr. 1-Fr. 8). Fr. 3 was further purified by column chromatography (silica gel CC, 1 kg, 10 × 100 cm), eluting with a gradient of petroleum ether in isopropyl alcohol to yield three subfractions (Fr. 3.1-Fr. 3.3). Fr. 3.1 was separated over silica gel (petroleum ether to acetone, 100:1 → 5:1) to obtain ten further fractions (Fr. 3.1.1-Fr. 3.1.10). Fr.3.1.2 was purified over Sephadex LH-20 (MeOH) followed by ODS (MeOH·H2O, 80%) and finally by semi-preparative HPLC (MeCNH2O, 82%) to yield 3 (10 mg). Fr.3.1.4 was also purified over Sephadex LH-20 (MeOH) followed by ODS (MeOH-H2O, 80%) and finally by semi-preparative TLC (petroleum ether-acetone, 8:1) to obtain 19 (16 mg) and 20 (34 mg). Fr. 3.2 was separated over silica gel (petroleum ether to acetone, 20:1 → 5:1) to obtain six parts (Fr. 3.2.1-Fr. 3.2.6). These parts were subjected to Sephadex LH-20 (MeOH) and ODS (MeOH-H2O) and were then purified by semipreparative HPLC. Compounds 9 (2 mg) and 15 (7 mg) were obtained from Fr. 3.2.1, while 13 (8 mg) was obtained from Fr. 3.2.2, 12 (6 mg), 14 (8 mg), and 18 (5 mg) were purified from Fr. 3.2.3, and 4 (38 mg), and 5 (8 mg) were isolated from Fr. 3.2.3. Fr. 4 was applied to ODS (MeOH to H2O, 50% → 100%) and then to Sephadex LH-20 (MeOH) and repeated silica gel CC to obtain two mixtures (Ⅰ and Ⅱ). Mixture Ⅰ was purified by semi-preparative HPLC (silica gel, hexane-isopropanol, 97:3) to obtain 7 (4 mg) and 1 (2 mg), and mixture Ⅱ was purified by semi-preparative HPLC (MeCN-H2O, 50%) to obtain 11 (8 mg), 16 (6 mg), and 6 (25 mg). Fr. 6 was applied to ODS (MeOH to H2O, 50% → 100%) to obtain six subfractions (Fr. 6.1-Fr. 6.6). Fr. 6.3 was separated with Sephadex LH-20 (MeOH) and ODS (MeOH to H2O, 60% → 100%) to obtain three mixtures (Ⅲ-Ⅴ). Mixture Ⅲ was purified by semi-preparative HPLC (CH3CN-H2O, 88%) to obtain 10 (9 mg), while mixture Ⅳ was purified by semipreparative HPLC (CH3OH-H2O, 92%) to yield 21 (5 mg).
The CHCl3 soluble extract (approximately 1 kg) was also separated by chromatography on silica gel (6 kg, 20 × 120 cm; petroleum ether to acetone, 100:0 → 0:100) to furnish six parts (A-F). Fraction B was subjected to silica gel CC and was eluted with petroleum ether to acetone (50:1 → 1:1) to obtain seven subfractions (B.1-B.7). Subfraction B.2 was further subjected to Sephadex LH-20 (MeOH) and then MPLC (ODS, MeOH to H2O, from 40% to 100%) to obtain six additional subfractions (B.2.1-B.2.6). B.2.1 was further purified by Sephadex LH-20 (MeOH), silica gel CC, and finally by semi-preparative HPLC (CH3OH-H2O, 85%) to obtain 8 (30 mg). Similarly, 2 (12 mg) was obtained from B.2.4 (CH3CN-H2O, 90%) and 17 (17 mg) was obtained from B.2.6 (CH3OH-H2O, 92%).
4.3.1. Hyperisampsin N (1)Colorless oil, [α]D20 -31 (c 0.1, CHCl3); UV (MeOH) λmax (log ε) = 204 (4.34) and 245 (3.88) nm; ECD (MeOH) λmax (Δε) 208 (-1.6), 247 (+3.4) nm; IR νmax = 3414, 1736, and 1701 cm-1; for 1H NMR (400 MHz) and 13C NMR (100 MHz) data see Table 1; HRESIMS [M + Na]+ m/z 641.3373 (calcd. for C38H50O7Na, 641.3454).
4.3.2. Hyperisampsin O (2)Colorless oil, [α]D20 +12 (c 0.2, MeOH); UV (MeOH) λmax (log ε) = 205 (4.06) and 245 (3.64) nm; ECD (MeOH) λmax (Δε) 220 (+2.5), 254 (+1.8), 315 (-1.2) nm; IR νmax = 3419, 1734, and 1700 cm-1; for 1H NMR (400 MHz) and 13C NMR (100 MHz) data see Table 1; HR-ESIMS [M + Na]+ m/z 657.3383 (calcd. for C38H50O8Na, 657.3403).
4.4. Computational detailsThe theoretical calculations of compounds 1 and 2 were performed using Gaussian 09. Conformational analysis was initially performed using Maestro in Schrödinger 2010 conformational searching together with the OPLS_2005 molecular mechanics methods. The optimized conformation geometries and thermodynamic parameters of all conformations were provided. The OPLS_2005 conformers were optimized at the B3LYP/6-31G(d, p) level. The theoretical calculation of ECD was performed using time-dependent density functional theory (TDDFT) at the B3LYP/ 6-31G(d, p) level in methanol with a PCM model. The calculated ECD curve was generated using SpecDis 1.51. Rvel was used in this work.
4.5. Cytotoxic assayThe cytotoxic activities were measured with MTs method as described in our previously literature [7]. Five human cancer cell lines including HL-60, SMMC-7721, A-549, MCF-7, and SW-480, together with a noncancerous cell line (Beas-2B human bronchial epithelial) were tested in the cytotoxic activity assay.
AcknowledgmentsWe thank the Analytical and Testing Center at Huazhong University of Science and Technology for assistance in testing of IR and ECD. This work was financially supported by the Program for New Century Excellent Talents in University, State Education Ministry of China (No. NCET-2008-0224), National Science and Technology Project of China (No. 2011ZX09102-004), the National Natural Science Foundation of China (Nos. 31370372, 81573316, 81641129 and 21502057), the Fundamental Research Fund for the Central Universities (No. 2016YXMS149) and the China Postdoctoral Science Foundation (No. 2015M572154).
Appendix A. Supplementary dataSupplementary data includes HRESIMS, IR, UV, CD, 1D and 2D NMR spectra, and details for ECD calculations and can be found, in the online version, at http://dx.doi.org/10.1016/j.cclet.2016.11.014.
| [1] | R. Ciochina, R.B. Grossman. Polycyclic polyprenylated acylphloroglucinols. Chem. Rev. (2006) 3963–3986. |
| [2] | J.J. Zhang, J. Yang, Y. Liao, et al., Hyperuralones A and B. new acylphloroglucinol derivatives with intricately caged cores from Hypericum uralum. Org. Lett. (2014) 4912–4915. |
| [3] | X.W. Yang, Y.Q. Ding, J.J. Zhang, et al., New acylphloroglucinol derivatives with diverse architectures from Hypericum henryi. Org. Lett. (2014) 2434–2437. |
| [4] | W.J. Tian, Y. Yu, X.J. Yao, et al., Norsampsones A-D. four new decarbonyl polycyclic polyprenylated acylphloroglucinols from Hypericum sampsonii. Org. Lett. (2014) 3448–3451. |
| [5] | Y. Liao, X. Liu, J. Yang, et al., Hypersubones A and B. new polycyclic acylphloroglucinols with intriguing adamantane type cores from Hypericum subsessile. Org. Lett. (2015) 1172–1175. |
| [6] | W.J. Tian, Y.Q. Qiu, X.J. Yao, et al., Dioxasampsones A and B. two polycyclic polyprenylated acylphloroglucinols with unusual epoxy-ring-fused skeleton from Hypericum sampsonii. Org. Lett. (2014) 6346–6349. |
| [7] | H.C. Zhu, C.M. Chen, J. Yang, et al., Bioactive acylphloroglucinols with adamantyl skeleton from Hypericum sampsonii. Org. Lett. (2014) 6322–6325. |
| [8] | H.C. Zhu, C.M. Chen, Q.Y. Tong, et al., Hyperisampsins H-M. cytotoxic polycyclic polyprenylated acylphloroglucinols from Hypericum sampsonii. Sci. Rep. (2015) 14772. |
| [9] | H.C. Zhu, C.M. Chen, J. Yang, et al., Hyperhexanone A. a crucial intermediate from bicyclo[3.3.1]-to cyclohexanone monocyclic-polycyclic polyprenylated acylphloroglucinols. Tetrahedron (2016) 4655–4659. |
| [10] | Z.Y. Xiao, Y.H. Zeng, Q. Mu, W.K.P. Shiu, S. Gibbons. Prenylated benzophenone peroxide derivatives from Hypericum sampsonii. Chem. Biodivers. (2010) 953–958. |
| [11] | Y. Ishida, O. Shirota, S. Sekita, et al., Polyprenylated benzoylphloroglucinoltype derivatives including novel cage compounds from Hypericum erectum. Chem. Pharm. Bull. (2010) 336–343. |
| [12] | J.S. Zhang, Y.H. Zou, Y.Q. Guo, et al., Polycyclic polyprenylated acylphloroglucinols:natural phosphodiesterase-4 inhibitors from Hypericum sampsonii. RSC Adv. (2016) 53469–53476. |
| [13] | W.J. Tian, Y.Q. Qiu, X.J. Jin, et al., Novel polycyclic polyprenylated acylphloroglucinols from Hypericum sampsonii. Tetrahedron (2014) 7912–7916. |
| [14] | Z.B. Zhou, Y.M. Zhang, K. Pan, J.G. Luo, L.Y. Kong. Cytotoxic polycyclic polyprenylated acylphloroglucinols from Hypericum attenuatum. Fitoterapia (2014) 1–7. |
| [15] | X. Liu, X.W. Yang, C.Q. Chen, et al., Hypercohones A-C. acylphloroglucinol derivatives with homo-adamantane cores from Hypericum cohaerens. Nat. Prod. Bioprospect. (2013) 233–237. |
| [16] | L.H. Hu, K.Y. Sim. Sampsoniones C-H, a unique family of polyprenylated benzophenone derivatives with the novel tetracyclo[7.3.1.13, 11.03, 7] tetradecane-2, 12, 14-trione skeleton. from Hypericum sampsonii (Guttiferae). Tetrahedron Lett. (1999) 759–762. |
| [17] | G.E. Henry, H. Jacobs, C.M.S. Carrington, S. McLean, W.F. Reynolds. Prenylated benzophenone derivatives from Caribbean Clusia species (Guttiferae). Plukenetiones B-G and xerophenone A. Tetrahedron (1999) 1581–1596. |
| [18] | Y.H. Zeng, K. Osman, Z.Y. Xiao, S. Gibbons, Q. Mu. Four geranyl-bearing polyisoprenylated benzoylphloroglucinol derivatives from Hypericum sampsonii. Phytochem. Lett. (2012) 200–205. |
| [19] | F.G. Cruz, J.S.R. Teixeira. Polyprenylated Benzophenones with a tricyclo[4.3.1.13.8] undecane skeleton from Clusia obdeltifolia. J. Braz. Chem. Soc. (2004) 504–508. |
| [20] | Z.Y. Xiao, Q. Mu, W.K.P. Shiu, Y.H. Zeng, S. Gibbons. Polyisoprenylated benzoylphloroglucinol derivatives from Hypericum sampsonii. J. Nat. Prod. (2007) 1779–1782. |
| [21] | L.H. Hu, K.Y. Sim. Complex caged polyisoprenylated benzophenone derivatives sampsoniones A and B. from Hypericum sampsonii. Tetrahedron Lett. (1998) 7999–8002. |
 2017, Vol. 28
2017, Vol. 28