Quinolines and quinoline N-oxides as important structural motifs exhibit antitumor and antimalarial activity [1-3]. In addition, the quinoline N-oxide core has been found in drugs that activate microsomal Na/K-ATPase [4]. 2-Arylquinoline N-oxide derivatives are important structural motifs with applications in the areas of drug discovery [5-7]. Hence, methods that allow for regioselective construction of C—C bonds to quinolines have attracted continuous attention. In particular, introduction of aryl substituent to the C2-position of quinoline derivatives is an important strategy in heterocyclic synthesis that represents a significant synthetic challenge. As a result, considerable efforts have been made towards their synthesis and functionalization [8-10].
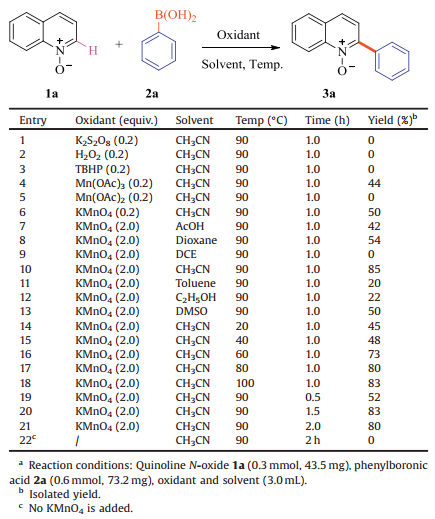
Transition-metal catalyzed cross-coupling is now recognized to be one of the most powerful C—C bond forming reactions. Of the methods available for the synthesis of 2-arylquinoline derivatives, the Pd-catalyzed Suzuki-Miyaura type coupling reaction is one of the most convenient methods for preparing biaryl compounds [11-13]. Using aryboronic acids as coupling partners, transitionmetal Palladium catalyzed C2 arylations of haloquinolines have recently been reported (Scheme 1, Eq. (1)) [14-21]. Nallasamy's group reported that 2-arylquinolines was synthesized via ONO pincer type Pd(Ⅱ) complexes as a catalyst system using 2-chloroquinolines and arylboronic acids as coupling partners [22]. However, employing halogenated quinolines for SuzukiMiyaura type coupling has its own drawback as it requires prefunctionalization of quinolines. Haloquinolines are hard to access due to chemo and regioselectivity issues. A direct arylation of a variety of electron-deficient heterocycles with arylboronic acids has also been developed using silver nitrate in the presence of persulfate. But this method provided the two regioisomer products in moderated yields with bad regioselectiity when pyridine and quinoline were employed [23]. Moreover, the relatively high price and considerable toxicity of catalysts limit its application. Direct C—H cross-coupling is highly advantageous, as this avoids involvement of prefunctionalized starting materials. Recently, more methods have been developed based on metal-catalyzed or metal-free catalyzed direct C—H bond functionalization in which heteroaromatic
|
Download:
|
| Scheme 1. Synthesis of 2-aryl N-heterocycle derivatives with boronic acids. | |
N-oxides are employed as versatile substrates [24-31]. In 2012, a direct arylation of pyridine N-oxides with arylboronic acids through C—H functionalization has been reported by our group, and this reaction was performed at room temperature using catalytic silver(Ⅰ) nitrate in the presence of potassium persulfate, and a ligand-free Pd(OAc)2-catalyzed selective arylation of pyridine N-oxides using potassium (hetero)aryltrifluoroborates as coupling partners via C—H bond activation was achieved in the presence of TBAI (Scheme 1, Eq. (2)) [32, 33]. Cu(acac)2-catalyzed direct C—H arylation of pyridine N-oxides with arylboronic esters has been developed by Wu's group, leading to a wide range of 2-arylpyridines in a one-pot synthesis with moderate to good yields without an additional reductant (Scheme 1, Eq. (3)) [34]. In 2015, Antonchick's group described a direct C—H bond functionalization of quinoline N-oxides to 2-arylated quinoline derivatives by employing quinoline N-oxides with arylboronic acids as substrates, and this transformation was realized without metal in DMSO solvent at 110 ℃ for 2-24 h (Scheme 1, Eq. (4)) [35]. Although this method could make a direct arylation of pyridine N-oxides, and achieve an improved regioselectivity, a high reaction temperature, long reaction time, and an excess of arylboronic acid (3.0 equiv.) were required. Moreover, the scope of the cross-coupling reaction was limited.
Aryboronic acids are highly stable, even in water and readily available in variety of substituted forms, making it a convenient precursor for building compounds of wide substrate scope. It is known that arylboronic acids decompose to aryl radicals in the presence of some oxidants, such as potassium persulfate (K2S2O8), Mn(OAc)3, di-tert-butyl peroxide (DTBP), Mn(acac)2, etc. [36-46]. Furthermore, we previously reported the direct and regioselective arylation of coumarins employing a wide range of arylboronic acids to produce 3-arylcoumarin using KMnO4/AcOH for an oxidant system [47]. Guided by the recent studies on using arylboronic acids as aryl sources, we turned our attention toward the activation of C—H bonds through a radical transfer pathway. Although direct arylation reaction of quinoline N-oxides has been investigated with many coupling partners, methods involving the reaction process of aryl radical are only in scarce. We decided to investigate the modular synthesis for 2-arylquinoline N-oxides by this method. Herein, we report such transformations starting from quinoline N-oxides with arylboronic acids that lead to 2-arylquinoline N-oxide derivatives using KMnO4 as an oxidant (Scheme 1, Eq. (5)).
2. Results and discussionOur studies began with optimization of conditions for the reaction between quinoline N-oxide 1a and arylboronic acid 2a as a model. Given aryl radical could be generated from arylboronic acids using K2S2O8 at higher temperature, quinoline N-oxide 1a reacted with arylboronic acid 2a in the presence of K2S2O8 (0.2 equiv.) as an oxidant in CH3CN at 90 ℃ for 1.0 h. Unfortunately, the desired product 3a was not formed (Table 1, entry 1). Various oxidants including H2O2, tert-butyl hydroperoxide (TBHP), Mn (OAc)3, Mn(OAc)2, and KMnO4 were investigated. To our delight, the product 3a could be obtained in 44% and 50% yields when the oxidants Mn(OAc)3 and KMnO4 were used, respectively (Table 1, entries 4 and 6). However, other oxidants were ineffective for the reaction (Table 1, entries 2, 3 and 5). The amount of oxidant was also examined. Increasing the amount of KMnO4 from 0.2 equiv. to 2.0 equiv. had beneficial effect and the product 3a was obtained in the highest yield (85%). Further, increasing in the quantity of KMnO4 to 3.0 equiv. had no improvement on the yield. When the quantity of KMnO4 was increased to 4.0 equiv., the product yield dropped dramatically (Table S1 in Supporting information, entries 1-5). The ratio of quinoline N-oxide 1a and arylboronic acid 2a was also screened. The result showed that the ratio of 1:2 was the optimal, and the yield was 85% (Table S2 in Supporting information, entries 1-4). Subsequently, a number of solvents including AcOH, dioxane, DCE, CH3CN, toluene, C2H5OH, and DMSO were examined, and these tests revealed that CH3CN was the best solvent (Table 1, entries 7-13). The solvent screening also indicated that the solvent played a key role in the reaction. Increasing the reaction temperature from 20 ℃ to 100 ℃, the optimal was 90 ℃ and it could provide 85% yield (Table 1, entries 10, 14-18). Finally, various reaction times were also tested, 1.0 h proved to the best appropriate and the yield was 85% (Table 1, entries 10, 19-21). In the absence of KMnO4 no desired product 3a was formed, which indicated that KMnO4 was crucial to this cross-coupling reaction (Table 1, entry 22).
|
|
Table 1 Optimization of reaction conditions.a |
With the optimized conditions in hand, we next set out to examine the scope of quinoline N-oxides and arylboronic acids, and the results are summarized in Fig. 1. Different arylboronic acids with substituents including -CH3, -C(CH3)3, -OCH3, -F, -Cl, -Br, etc. are well tolerated on the aromatic ring and their reactions afforded the target products in good to excellent yields (40%-85%), showing the broad scope of this reaction. Arylboronic acids bearing electron donating groups (3a-3e) could give better yields than analogues with electron-withdrawing groups (3g-3n). Unfortunately, when 2-methoxylphenyl boronic acid and 2, 4, 5-trimethylphenyl boronic acid were coupled with quinoline N-oxide, even after prolonged reaction time, the target products failed to be formed. These facts suggest that the steric hindrance of arylboronic acids plays a key role for this cross-coupling reaction. It is gratifying to obtain the desired product 3f with moderate yield (42%) when 1-naphthaleneboronic acid (NBA) was employed. Notably, arylboronic acids containing keto and aldehyde groups were well tolerated and offered 3m and 3n in moderate yields. However, arylboronic acid with strong electron withdrawing -CN group on reaction with quinoline N-oxide failed to produce the desired product. This may be due to the unstable nature of cyano aryl radical. Gratifying, heteroaromatic boronic acid, 2-furanboronic acid could also react smoothly with quinoline N-oxide to provide 2-arylquinoline N-oxide 3o.

|
Download:
|
| Figure 1. 2-Arylquinoline N-oxides were synthesized from quinoline N-oxides and arylboronic acids, and yields are isolated yield. | |
As a part of the study we next examined reativity of quinoline N-oxide derivatives. Various substituted quinoline N-oxides were also found to be amenable to this direct C—H functionalization reaction. Arylation of quinoline N-oxides bearing methyl and methoxyl groups proceeded smoothly leading to 2-arylquinoline N-oxides scaffolds 3p, 3q, and 3t in 78%-83% yields. It was noteworthy that 6-bromoquinoline N-oxide and 3-bromoquinoline N-oxide were well tolerated, providing handles for further functionalization. Unfortunately, when quinoline N-oxide possessing an electron-withdrawing group -NO2 at the C6 position was employed, the yield (39%) of desired product 3s dropped dramatically. Pleasingly, the coupling reaction of 3-bromoquinoline N-oxides with phenylboronic acid could proceed to afford the product 3u in 76% yield, which indicated that the steric effect of quinoline N-oxides played a weak role in this transformation.
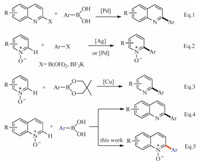
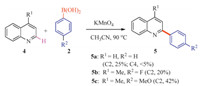
With these impressive results, we further focused on application of the same oxidant system for arylation of other heterocycles such as quinolines (Scheme 2). Quinolines could also react smoothly with arylboronic acids to provide the corresponding products 5a-5c in 20%-42% yields.
|
Download:
|
| Scheme 2. Synthesis of 2-arylquinolines from quinolines and arylboronic acids. Reaction conditions: Quinolines 4 (0.3 mmol), arylboronic acid 2 (0.6 mmol), KMnO4 (0.6 mmol, 94.8 mg) in 3.0 mL CH3CN solvent, 90 ℃ for 1.0 h. Yield are isolated yield. | |
Although a detailed reaction pathway remains to be unclarified, a plausible mechanism for the current KMnO4-mediated direct C2 arylation of quinoline N-oxides with arylboronic acids is depicted in Scheme 3, which is based on the radical mechanism proposed [41, 42]. Arylboronic acids have been reported to decompose into aryl radicals through a single-electron transfer in the presence of an oxidant [36-46, 48]. Initially, phenylboronic acid A was oxidized by one-electron oxidation to generate a phenyl radical B. The N-oxide moiety increases the electron-density of the electrondeficient pyridine ring system, and enhances the Brönsted acidity of the adjacent pyridyl C—H bonds. The given phenyl radical B attacked on C2 position of quinoline N-oxide to form the corresponding radical C. Subsequently, through a single-electron transfer (SET) process, the corresponding cationic intermediate D was provided. The generated intermediate D aromatized to afford the desired product E by a deprotonation step.
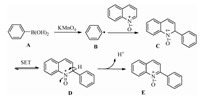
|
Download:
|
| Scheme 3. Proposed reaction mechanism. | |
3. Conclusion
In summary, we have successfully developed a direct and regioselective C2 arylation of quinoline N-oxides with arylboronic acids. This method provides an efficient protocol to construct regioselectively 2-arylquinoline N-oxides. The advantage of this reaction is high efficiency, moderated to good yield, and a broad functional groups tolerance.
4. ExperimentalGeneral procedure for synthesis of 2-arylquinoline N-oxide derivatives 3a-u: In a 50 mL Schlenk tube, quinoline N-oxides 1 (0.3 mmol), arylboronic acids 2 (0.6 mmol), and KMnO4 (0.6 mmol, 94.8 mg) in acetonitrile (3.0 mL) were added and the reaction was continued at 90 ℃ for 1.0 h (monitored by TLC). The reaction mixture was diluted with ethyl acetate, then washed with saturated sodium chloride solution for three times. The resulting organic phase was dried over anhydrous Na2SO4 and concentrated under vacuum. The crude product was purified by silica gel column chromatography using ethyl acetate/petroleum ether (1:3 to 2:1) as eluant to obtain the desired products 3a-u. All compounds were confirmed by IR, 1H NMR, 13C NMR, and MS.
2-Phenylquinoline 1-oxide 3a: Colorless solid, mp 145-146 ℃ (EtOAc) [lit [26] mp 148.5-149.7 ℃]. IR (KBr, cm-1): ν 1462, 1377, 1352, 1302. 1H NMR (400 MHz, CDCl3): δ 8.86 (d, 1H, JH-H = 8.8 Hz), 7.97 (d, 2H, JH-H = 8.3 Hz), 7.87 (d, 1H, JH-H = 8.1 Hz), 7.82-7.76 (m, 2H), 7.65 (t, 1H, JH-H = 7.2 Hz), 7.55-7.45 (m, 4H). 13C NMR (100 MHz, CDCl3): δ 145.1, 142.2, 133.4, 130.6 (CH), 129.6 (CH), 129.5 (CH), 128.4 (CH), 128.3 (CH), 127.9 (CH), 125.4 (CH), 125.3, 123.3 (CH), 120.3 (CH). MS (ESI): m/z [M+H]+ found: 222.3, calcd. for C15H12NO:222.1.
AcknowledgmentsThis work is supported by the Department of Henan Province Natural Science and Technology Foundation (No. 142102210410), Natural Science Foundation in Henan Province Department of Education (No. 17A150005), the Program for Innovative Research Team from Zhengzhou (No. 131PCXTD605) and Project of Youth Backbone Teachers of Henan University of Technology.
Appendix A. Supplementary dataSupplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.cclet.2017.01.016.
| [1] | S. Vshyvenko, M.R. Reisenauer, S. Reisenauer, et al., Synthesis and biological evaluation of unnatural derivatives of narciclasine:7-aza-nornarciclasine and its N-oxide. Bioorg. Med. Chem. Lett. (2014) 4236–4238. |
| [2] | R. Tahar, L. Vivas, L. Basco, et al., Indolone-N-oxide derivatives:in vitro activity against fresh clinical isolates o plasmodium falciparum. stage specificity and in vitro interactions with established antimalarial drugs. J. Antimicrob. Chemother. (2011) 2566–2572. |
| [3] | L.M. Werbel, S.J. Kersten, W.R. Tumer. Structure-activity relationships of antimalarial indolo[3, 2-c]quinolines[1.2]. Eur. J. Med. Chem. (1993) 837–852. |
| [4] | V.P. Andreev, E.G. Korvacheva, Y.P. Nizhnik. Effect of pyridine and quinolilne Noxides on microsomal Na. K-ATPase activity. Pharm. Chem. J. (2006) 347–348. |
| [5] | P.W. Smith, P.A. Wyman, P. Lovell, et al., New quinoline NK3 receptor antagonists with CNS activity. Bioorg. Med. Chem. Lett. (2009) 837–840. |
| [6] | T. Rodrigues, D. Reker, J. Kunze, et al., Revealing the macromolecular targets of fragment-like natural products. Angew. Chem. Int. Ed. (2015) 10516–10520. |
| [7] | L. Strekowski, M. Say, M. Henary, et al., Synthesis and activity of substituted 2-phenyl quinoline-4-amines. antagonists of immunostimulatory CpGoligodeoxynucleotides. J. Med. Chem. (2003) 1242–1249. |
| [8] | R. Kumar, I. Kumar, R. Sharma, et al., Catalyst and solvent-free alkylation of quinoline N-oxides with olefins:a direct access to quinoline-substituted α-hydroxy carboxylic derivatives. Org. Biomol. Chem. (2016) 2613–2617. |
| [9] | J.J. Zhao, P. Li, C.G. Xia, et al., Metal-free regioselective C-3 nitrotion of quinoline N-oxides with tert-butyl nitrite. RSC Adv. (2015) 32835–32838. |
| [10] | K. Sun, X.L. Chen, X. Li, et al., H-phosphonate-mediated sulfonylation of heteroaromatic N-oxides:a mild and metal-free one-pot synthesis of 2-sulfonyl quinolines/pyridines. Chem. Commun. (2015) 12111–12114. |
| [11] | A. Suzuki. Recent advances in the cross-coupling reactions of organoboron derivatives with organic electrophiles. 1995-1998. J. Organomet. Chem. (1999) 147–168. |
| [12] | J. Hassan, M. Sevignon, C. Gozzi, et al., Aryl-aryl bond formation one century after the discovery of the Ullmann reaction. Chem. Rev. (2002) 1359–1469. |
| [13] | S.R. Chemler, D. Trauner, S.J. Danishefsky. The B-alkyl Suzuki-Miyaura crosscoupling reaction:development, mechanistic study. and applications in natural product synthesis. Angew. Chem. Int. Ed. (2001) 4544–4568. |
| [14] | S. Vuoti, J. Autio, M. Laitila, et al., Palladium-catalyzed Suzuki-Miyaura crosscoupling of various aryl halides using ortho-alkyl-substituted arylphosphanes and (ortho-alkylphenyl) alkylphosphanes under microwave heating. Eur. J. Inorg. Chem. (2008) 397–407. |
| [15] | J. Zhou, Q.F. Zhang, W.H. Zhao, et al., Chiral phosphoric acid-catalyzed asymmetric transfer hydrogenation of 3-trifluoromethylthioquinolines. Org. Biomol. Chem. (2016) 6937–6941. |
| [16] | N.M. Ali, A. McKillop, M.B. Mitchell, et al., Palladium-catalysed cross-coupling reactions of arylboronic acids with P-deficient heteroaryl chlorides. Tetrahedron (1992) 8117–8126. |
| [17] | J.Y. Jung, A. Taher, S. Hossain, et al., Highly active heterogeneous palladium catalyst for the Suzuki reaction of heteroaryl chlorides. Bull. Korean Chem. Soc. (2010) 3010–3012. |
| [18] | T. Tagata, M. Nishida. The direct synthesis of 3-amino-2-phenylpyridine by using palladium charcoal was reported. J. Org. Chem. (2003) 9412–9415. |
| [19] | M. Kuriyama, S. Natsuo, M. Shinozawa, et al., Ether-imidazolium carbenes for Suzuki-Miyaura cross-coupling of heteroaryl chlorides with aryl/heteroarylboron reagents. Org. Lett. (2013) 2716–2719. |
| [20] | R. Malacea, F. Chahdoura, M. Devillard, et al., ortho-(Dimesitylboryl) phenylphosphines:positive boryl effect in the palladium-catalyzed SuzukiMiyaura coupling of 2-chloropyridines. Adv. Synth. Catal. (2013) 2274–2284. |
| [21] | D.H. Lee, M. Choi, B.W. Yu, et al., Expanded heterogeneous Suzuki-Miyaura coupling reactions of aryl and heteroaryl chlorides under mild conditions. Adv. Synth. Catal. (2009) 2912–2920. |
| [22] | V. Arumugam, W. Kaminsky, D. Nallasamy. ONO pincer type Pd(Ⅱ) complexes:synthesis. crystal structure and catalytic activity towards C-2 arylation of quinoline scaffolds. RSC Adv. (2015) 77948–77957. |
| [23] | I.B. Seiple, S. Su, R.A. Rodriguez, et al., Direct C-H arylation of electron-deficient heterocylces with arylboronic acids. J. Am. Chem. Soc. (2010) 13194–13196. |
| [24] | S.H. Cho, S.J. Hwang, S. Chang. Palladium-catalyzed C-H functionalization of pyridine N-oxides:highly selective alkenylation and direct arylation with unactivated arenes. J. Am. Chem. Soc. (2008) 9254–9256. |
| [25] | L. Ackermann, S. Fenner. Direc arylations of electron-deficient (hetero)arenes with aryl or alkenyl tosylates and mesylates. Chem. Commun. (2011) 430–432. |
| [26] | S. Duric, C. Tzschucke. Synthesis of unsymmetrically substituted bipyridines by palladium-catalyzed direct C-H arylation of pyridine N-oxides. Org. Lett. (2011) 2310–2313. |
| [27] | L.C. Campeau, D.R. Stuart, J.P. Leclerc, et al., Palladium-catalyzed direct arylation of azine and azole N-oxides:reaction development. J. Am. Chem. Soc (2009) 3291–3306. |
| [28] | Z.Y. Wu, C. Pi, X.L. Cui, et al., Direct C-2 alkylation of quinoline N-oxides with ethers via palladium-catalyzed dehydrogenative cross-coupling reaction. Adv. Synth. Catal. (2013) 1971–1976. |
| [29] | E. Kianmehr, N. Faghih, S. Karaji, et al., Copper-catalyzed crossdehydrogenative coupling of pyridine N-oxides with cyclic ethers. J. Organomet. Chem. (2016) 10–13. |
| [30] | X.P. Chen, X.L. Cui, Y.J. Wu. C8-selective acylation of quinoline N-oxides with α-oxocarboxylic acids via palladium-catalyzed regioselective C-H bond activation. Org. Lett. (2016) 3722–3725. |
| [31] | X.P. Chen, X.L. Cui, Y.J. Wu. One-pot approach to 8-acylated 2-quinolinones via palladium-catalyzed regioselective acylation o quinoline N-oxides. Org. Lett. (2016) 2411–2414. |
| [32] | W.P. Mai, J.W. Yuan, Z.C. Li, et al., Silver-catalyzed 2-pyridyl arylation of pyridine N-oxides with arylboronic acids at room temperature. Synlett (2012) 145–149. |
| [33] | M.L. Li, X. Li, H.H. Chang, et al., Palladium-catalyzed direct C-H arylation of pyridine N-oxides with potassium aryl-and heteroaryltrifluoroborates. Org. Biomol. Chem. (2016) 2421–2426. |
| [34] | Y. Shen, J.X. Chen, M.C. Liu, et al., Copper-catalyzed direc C-H arylation of pyridine N-oxides with arylboronic esters:one-pot synthesis of 2-arylpyridines. Chem. Commun. (2014) 4292–4295. |
| [35] | L. Bering, A.P. Antonchick. Regioselective metal-free cross-coupling of quinoline N-oxides with boronic acids. Org. Lett. (2015) 3134–3137. |
| [36] | J. Wang, S. Wang, G. Wang, et al., Iron-mediated direct arylation with arylboronicacids throughan arylradicaltransfer pathway. Chem. Commun. (2012) 11769–11771. |
| [37] | Y. Fujiwara, V. Domingo, I.B. Seiple, et al., Practical C-H functionalization of quinines with boronic acids. J. Am. Chem. Soc. (2011) 3292–3295. |
| [38] | D. Arghya, M. Srimanta, M. Arun, et al., Iron-catalyzed direct C-H arylation of heterocycles and quinines with arylboronic acids. Eur. J. Org. Chem. (2013) 5251–5256. |
| [39] | P.S. Parvinder, A. Sravan Kumar, Y. Mahipal, et al., Iron-catalyzed crosscoupling of electron-deficient heterocycles and quinine with organoboron species via innate C-H functiionalization:application in total synthesis of pyrazine alkaloid botryllazine A. J. Org. Chem. (2013) 2639–2648. |
| [40] | A. Ilangovan, A. Polu, G. Satish. K2S2O8-mediated metal-free direct C-H functionalization of quinines using arylboronic acids. Org. Chem. Front. (2015) 1616–1620. |
| [41] | S.K. Guchhait, M. Kashyap, S. Saraf. Direct C-H bond arylation of (hetero)arenes with aryl and heteroarylboronic acids. Synthesis (2010) 1166–1170. |
| [42] | H. Wang, Y. Yu, X.H. Hong, et al., Mn(Ⅱ)/O2-promoted oxidative annulations of vinyl isocyanides with boronic acids:synthesis of multi-substituted isoquinolines. Chem. Commun. (2014) 13485–13488. |
| [43] | D. Liu, Y.X. Li, X.T. Qi, et al., Nickel-catalyzed selective oxidative radical crosscoupling:an effective strategy for inert Csp3H functionalizaiton. Org. Lett. (2015) 998–1001. |
| [44] | A.S. Demir, Ö. Reis, M. Emrullahoglu. Generation of aryl radicals from arylboronic acids by manganese(Ⅲ) acetate:synthesis of biaryls and heterobiaryls. J. Org. Chem. (2003) 578–580. |
| [45] | A.S. Demir, H. Findik. Potassium permanganate/carboxylic acid/organic solvent:a powerful reagent for enone oxidation and aryl couling reactions. Tetrahedron (2008) 6196–6201. |
| [46] | A.S. Demir, H. Findik, N. Saygili, et al., Manganese(Ⅲ) acetate-mediated synthesis of biarylsunder microwave irradiation. Tetrahedron (2010) 1308–1312. |
| [47] | J.W. Yuan, L.R. Yang, Q.Y. Yin, et al., KMnO4/AcOH-mediated C3-selective direct arylation of coumarins with arylboronic acids. RSC Adv. (2016) 35936–35944. |
| [48] | M. Tobisu, K. Koh, T. Furukawa, et al., Modular synthesis of phenanthridine derivatives by oxidative cycliaztion of 2-isocyanobiphenyls with organoboron reagents. Angew. Chem. Int. Ed. (2012) 11363–11366. |
 2017, Vol. 28
2017, Vol. 28