b Innovative Drug Research Centre, Chongqing University, Chongqing 401331, China
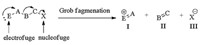
Grob fragmentations are the direct breakage of heterolytic sigma bonds that have been widely studied since their discovery in the 1950s [1], which are the key steps in numerous syntheses of natural products [2] involving the very important and intriguing diterpene Vinigrol [2a]. Among Grob fragmentations, 1, 3-diheterofunctionalized compounds are typically substrates featuring a nucelophilic atom with a negative charge or lone electron pair (electrofuge) and a leaving group (nucleofuge) in a 1, 3-relationship, undergoing heterolytic fragmentation to form three fragments: a positive fragment Ⅰ (the "electrofuge"), an unsaturated neutral fragment Ⅱ, and a negative ion Ⅲ (the "nucleofuge") (Fig. 1). Usually, the positive ion could be a carbonium ion; the neutral fragment could be an alkene and the negative fragment could be a tosyl ion. Bases or nucleophiles induced fragmentations are most common in the reactions. However, Brønsted and Lewis acids mediated or catalyzed Grob fragmentations are relatively rare due to the side reactions triggered by the carbonium ion intermediate in the substrates [3].
|
Download:
|
| Figure 1. Grob fragmentation of a general 1, 3-difunctionalized substrate. | |
2. Results and discussion
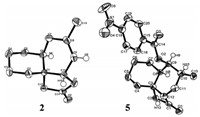
In the course of our study towards the synthesis of sesquiterpenes containing a decalin skeleton, an unexpected and unprecedented Grob fragmentation was uncovered (Scheme 1). In order to remove the silyl group, the vinylogous β-silyloxycyclobutanone 1 [4] was treated with 40% aqueous HF in CH3CN at 0 ℃ for 24 h [5], expecting to obtain the desired target alcohol product 3. However, this reaction led to an unexpected product 2 in 90% yield, in which the cyclobutanone was converted to the γ-lactone and the silyloxy group was removed. The structure of 2 was confirmed by single-crystal X-ray analysis (Fig. 2). Actually, the ketone carbonyl group and the silyloxy group are in a 1, 5-relationship. To the best of our knowledge, this is the first example of Grob fragmentation of a 1, 5-difunctionalized substrate under acidic conditions and we envisioned that the double bond plays key role in this long-distance Grob fragmentation [6].

|
Download:
|
| Scheme 1. Unusual transformation of vinylogous β-silyloxy-cyclobutanone 1 to γ-lactone 2. | |
|
Download:
|
| Figure 2. X-ray structures of compounds 2 and 5. | |
In order to identify the role of the double bond and the reaction mechanism in this fragmentation, therefore, substrate 4 without double bond was synthesized and treated with the same conditions as described in Scheme 1, the desired target alcohol product was obtained smoothly. Then, in the presence of 4-nitrobenzoyl chloride and pyridine, the ester 5 was obtained in 82% yield over two steps (Scheme 2). As the result illustrated above we can see that the double bond is undoubtedly key and important in this fragmentation. The structure of 5 was confirmed by singlecrystal X-ray analysis (Fig. 2).

|
Download:
|
| Scheme 2. Preparation of cyclobutanone derivative 5. | |
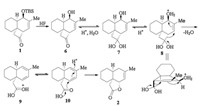
According to the result above, we proposed a plausible mechanism of this fragmentation (Scheme 3). Initially, the TBS (tert-butyldimethylsilyl) group was removed by fluoride ion to afford the alcohol 6. Under aqueous acidic conditions, the ketone carbonyl group hydrolyzed to the hydrate intermediate 7. Then, after protonation of the hydroxyl group, water acted as a leaving group and the four-membered ring of 8 opened to generate the 1, 3-butadiene intermediate 9 involving a carboxylic acid group. From the transition state of 8 we can see that the lone electron pair on O (H), the C-OH2+, and carbon—carbon bond of CH—C(OH)OH are anti-periplanar for maximal orbital overlap and the double bond can transmit electrons in the fragmentation. Finally, by proton transfer one double bond of the diene 10 could be protonated and was attacked by the internal carboxylic acid group to form the cislactone 2. In this fragmentation the double bond behaves like a shuttle bus which can transmit the electrons from one side to another [7]. Unsaturated decalinic lactones obtained are important building blocks and key synthetic intermediates in many organic syntheses [8].
|
Download:
|
| Scheme 3. Proposed mechanism. | |
3. Conclusion
In conclusion, we have disclosed an unprecedented formal oxy transposition of vinylogous β-silyloxycyclobutanone (a 1, 5-difunctionalized substrate) into unsaturated decalinic γ-lactone under acidic conditions by Grob fragmentation and we propose a plausible mechanism of this reaction. We envision that this unique fragmentation will provide new opportunities for organic synthesis.
4. ExperimentalAll reactions were monitored by thin-layer chromatography (TLC). All organic extracts were washed with brine before being dried over Na2SO4. For column chromatography, silica gel (200-300 mesh) and light petroleum ether (PE, b.p. 60-90 ℃) were used. Melting points (mp) were measured on a Kofler hot stage and are uncorrected. FTIR spectrum was recorded on a Nicolet MAGNA-560 FTIR spectrometer. 1H NMR, 13C NMR and DEPT135 NMR spectra were recorded on a Bruker AV400 instrument; Chemical shifts are reported as δ using residual solvent as an internal standard. High resolution mass spectral analyses (HRMS) were measured on a Varian 7.0 TFTICR MS spectrometer by means of ESI.
4.1. Preparation of 5-methyl-6, 6a, 7, 8, 9, 10-hexahydro-1H-naphtho [1, 8a-b]furan-2(3aH)-one (2)To a solution of 1 (20 mg, 0.094 mmol) in acetonitrile (5 mL) was added the aqueous HF solution (0.2 mL). The mixture was stirred for 24 h at 0 ℃. The reaction mixture was concentrated in vacuo and the residue was purified through flash column chromatography (petroleum ether/ethyl acetate = 4/1) to afford the final product (17 mg) with 90% yield after concentration. White solid. Mp: 132-134 ℃; 1H NMR (400 MHz, CDCl3): δ 5.53 (d, 1H, J = 1.8 Hz), 4.52 (s, 1H), 2.75 (d, 1H, J = 17.3 Hz), 2.21 (d, 1H, J = 17.2 Hz), 2.04 (qd, 2H, J = 18.2, 6.2 Hz), 1.79 (m, 1H), 1.76 (s, 3H), 1.72-1.63 (m, 1H), 1.61-1.55 (m, 3H), 1.48-1.37 (m, 3H), 1.28-1.22 (m, 1H); 13C NMR (100 MHz, CDCl3): δ 176.3, 140.2, 116.8, 81.1, 40.8, 40.7, 33.5, 32.3, 32.1, 27.2, 23.6, 22.9, 22.5; IR (neat): 1774 cm-1; HRMS (ESI) exact mass calcd. for: C13H18O2Na (m+Na)+ 229.1199, found 229.1196.
4.2. Preparation of 4-methyl-2-oxodecahydro-1H-cyclobuta[d] naphthalen-5-yl 4-nitrobenzoate (5)To a solution of 4 (12 mg, 0.037 mmol) in acetonitrile (3 mL) was added the aqueous HF solution (0.2 mL). The mixture was stirred for 24 h at 0 ℃. The reaction mixture was concentrated in vacuo. To the residue was added ethyl acetate (10 mL). The organic solution was washed with aqueous saturated aqueous NaHCO3 (8 mL), brine (8 mL) and dried over Na2SO4 and concentrated. To the crude product was added dicholomethane (2 mL), pyridine (8.0 mL, 0.1 mmol) and 4-nitrobenzoyl chloride (13 mg, 0.07 mmol). The mixture was stirred for 4 h at room temperature. The reaction mixture was concentrated in vacuo and the residue was purified through flash column chromatography (petroleum ether/ethyl acetate = 20/1) to afford the final product (11 mg) with 82% yield after concentration. White solid. Mp: 140-142 ℃; 1H NMR (400 MHz, CDCl3): δ 8.34-8.29 (m, 2H), 8.24-8.19 (m, 2H), 5.23 (dd, 1H, J = 7.1, 3.7 Hz), 3.17 (dd, 1H, J = 16.9, 2.1 Hz), 2.94 (m, 1H), 2.70 (dd, 1H, J = 16.9, 3.8 Hz), 2.11 (m, 1H), 2.06-1.95 (m, 3H), 1.88-1.78 (m, 1H), 1.72-1.63 (m, 2H), 1.55-1.46 (m, 3H), 1.37 (m, 1H), 1.28-1.22 (m, 1H), 1.02 (d, 3H, J = 6.8 Hz); 13C NMR (100 MHz, CDCl3): δ 209.5, 164.3, 150.6, 135.8, 130.7, 123.7, 80.6, 60.2, 57.2, 38.0, 37.7, 32.8, 31.0, 26.2, 25.1, 23.6, 23.5, 19.4; IR(neat): 1776, 1718 cm-1; HRMS (ESI) exact mass calcd. for:C20H23NO5Na (m + Na)+ 380.1468 found 380.1464.
AcknowledgmentWe are grateful for financial support from National Natural Science Foundation of China (NSFC, Nos. 21302177, 21672049 and 21672030).
Appendix A. Supplementary dataSupplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.cclet.2016.12.017.
| [1] |
(a) C. A. Grob, W. Baumann, Die 1, 4-Eliminierung unter Fragmentierung, Helv. Chim. Acta 38(1955) 594-610; (b) C. A. Grob, P. W. Schiess, Heterolytic fragmentation. A class of organic reactions, Angew. Chem. Int. Ed. Engl. 6(1967) 1-15; (c) C. A. Grob, Mechanisms and stereochemistry of heterolytic fragmentation, Angew. Chem. Int. Ed. Engl. 8(1969) 535-546; (d) P. Weyersthal, H. Marschall, Fragmentation reactions, in: B. M. Trost, I. Fleming, E. Winterfeldt (Eds. ), Comprehensive Organic Synthesis, Pergamon Press, Oxford, 1991, pp. 1041-1070; (e) K. Prantz, J. Mulzer, Synthetic applications of the carbonyl generating Grob fragmentation, Chem. Rev. 110(2010) 3741-3766. |
| [2] |
(a) T. J. Maimone, J. Shi, S. Ashida, P. S. Baran, Total synthesis of vinigrol, J. Am. Chem. Soc. 131(2009) 17066-17067; (b) J. Q. Dong, H. N. C. Wong, Biomimetic total synthesis of (±)-pallavicinolide A, Angew. Chem. Int. Ed. 48(2009) 2351-2354; (c) C. Xu, Z. Liu, H. Wang, et al. , Rapid construction of [5-6-7] tricyclic ring skeleton of calyciphylline alkaloid daphnilongeranin B, Org. Lett. 13(2011) 1812-1815; (d) N. Yamamoto, H. Fujii, S. Imaide, et al. , Synthesis of (-)-homogalanthamine from naltrexone, J. Org. Chem. 76(2011) 2257-2260; (e) X. S. Xu, Z. W. Li, Y. J. Zhang, X. S. Peng, H. N. C. Wong, Total synthesis of (±)-pallambins C and D, Chem. Commun. (2012) 8517-8519; (f) H. Suizu, D. Shigeoka, H. Aoyama, T. Yoshimitsu, Total synthesis of clavilactone B: a radical cyclization-fragmentation strategy, Org. Lett. 17(2015) 126-139. |
| [3] |
(a) R. A. Holton, R. M. Kennedy, Stereochemical requirements for fragmentation of homoallylic epoxy alcohols, Tetrahedron Lett. 25(1984) 4455-4458; (b) M. De Giacomo, R. M. Bettolo, R. Scarpelli, Unprecedented Grob-type fragmentation of 5-dioxolan-bicyclo[4. 2. 0] octan-2-ones into 3-(methoxycarbonylmethyl)cyclohexanones, Tetrahedron Lett. 38(1997) 3469-3470; (c) J. Barluenga, M. Álvarez-Pérez, K. Wuerth, F. Rodríguez, F. J. Fañanás, Acidcatalyzed Grob fragmentation reactions of acetonides derived from terpenes, Org. Lett. 5(2003) 905-908; (d) F. A. Khan, Ch. Nageswara Rao, Grob fragmentation of norbornyl α-diketones: a route to α-ketoenols and aromatic compounds, J. Org. Chem. 76(2011) 3320-3328; (e) S. H. Mahadevegowda, F. A. Khan, Grob-type fragmentation of 5-oxabicyclo[2. 1. 1] hexane system: a strategy for synthesis of annulated and 2, 2, 5-trisubstituted tetrahydrofurans, Tetrahedron 69(2013) 8494-8504; (f) F. Malihi, D. L. J. Clive, C. C. Chang, Synthetic Studies on CP-225, 917 and CP-263, 114: access to advanced tetracyclic systems by intramolecular conjugate displacement and[2, 3]-wittig rearrangement, J. Org. Chem. 78(2013) 996-1013; (g) S. He, R. P. Hsung, W. R. Presser, Z. X. Ma, B. J. Haugen, An approach to cyclohepta[b]indoles through an allenamide (4+3) cycloaddition-Grignard cyclization-Chugaev elimination sequence, Org. Lett. 16(2014) 2180-2183. |
| [4] | Characterization of Compound 1: white solid. Mp: 40-42℃; 1H NMR (600 MHz, CDCl3): δ 5. 31(d, 1H, J=5. 2 Hz), 4. 39(d, 1H, J=4. 1 Hz), 3. 23(brs, 1H), 3. 05(dd, 1H, J=17. 4, 2. 7 Hz), 2. 40(dd, 1H, J=17. 4, 5. 6 Hz), 1. 86(m, 1H), 1. 78-1. 75(m, 2H), 1. 74(s, 3H), 1. 69-1. 59(m, 4H), 1. 25-1. 08(m, 2H), 0. 93(s, 9H), 0. 11(s, 3H), 0. 09(s, 3H); 13C NMR (150 MHz, CDCl3): δ 206. 2, 139. 1, 114. 6, 70. 6, 61. 4, 54. 2, 41. 3, 37. 9, 32. 5, 25. 9, 25. 1, 23. 6, 23. 0, 20. 3, 18. 2, -4. 3, -4. 9 ppm. IR (neat): 1776 cm-1; HRMS (ESI) exact mass calcd for: C19H33O2Si (m+H)+321. 2245, found 321. 2248. . |
| [5] | R.D. Crouch. Selective deprotection of silyl ethers. Tetrahedron (2013) 2383–2417. |
| [6] | B.A. Bhat, S.L. Maki, E.J. St, . Germain, P. Maity, S.D. Lepore. Annulation reactions of allenyl esters:an approach to bicyclic diones and medium-sized rings. J. Org. Chem. (2014) 9402–9407. |
| [7] |
(a) R. C. Fuson, The principle of vinylogy, Chem. Rev. 16(1935) 1-27; (b) G. Casiraghi, F. Zanardi, G. Appendino, G. Rassu, The vinylogous Aldol reaction: a valuable, yet understated carbon-carbon bond-forming maneuver, Chem. Rev. 100(2000) 1929-1972; (c) S. K. Bur, S. F. Martin, Vinylogous Mannich reactions: selectivity and synthetic utility, Tetrahedron 57(2001) 3221-3242; (d) S. A. Frank, D. J. Mergott, W. R. Roush, The vinylogous intramolecular Morita-Baylis-Hillman reaction: synthesis of functionalized cyclopentenes and cyclohexenes with trialkylphosphines as nucleophilic catalysts, J. Am. Chem. Soc. 124(2002) 2404-2405; (e) G. Casiraghi, L. Battistini, C. Curti, G. Rassu, F. Zanardi, The vinylogous Aldol and related addition reactions: ten years of progress, Chem. Rev. 111(2011) 3076-3154. |
| [8] |
(a) R. S. Sulake, C. Chen, Total synthesis of (+)-antroquinonol and (+)-antroquinonol D, Org. Lett. 17(2015) 1138-1141; (c) E. N. Pitsinos, N. Athinaios, V. P. Vidali, Enantioselective total synthesis of (-)-laurenditerpenol, Org. Lett. 14(2012) 4666-4669; (d) J. Merten, Y. Wang, T. Krause, O. Kataeva, P. Metz, Total synthesis of the cytotoxic 1, 10-seco-eudesmanolides britannilactone and 1, 6-O, Odiacetylbritannilactone, Chem. Eur. J. 17(2011) 3332-3334; (e) S. Mukherjee, A. P. Scopton, E. J. Corey, Enantioselective pathway for the synthesis of laurenditerpenol, Org. Lett. 12(2010) 1836-1838; (f) H. Fujioka, K. Nakahara, T. Oki, et al. , The first asymmetric total syntheses of both enantiomers of cryptocaryone, Tetrahedron Lett. 51(2010) 1945-1946; (g) P. D. O'Connor, U. B. Kim, M. A. Brimble, Synthesis of (±) wine lactone and its analogues by a Diels-Alder approach, Eur. J. Org. Chem. (2009) 4405-4411; (h) L. A. Paquette, H. C. Tsui, Expedient enantiocontrolled synthesis of a tetracyclic lactone structurally related to the kaurane diterpenoids, Synlett (1996) 129-130. |
 2017, Vol. 28
2017, Vol. 28 


