b Key laboratory of Magnetic Resonance in Biological Systems, State Key Laboratory of Magnetic Resonance and Atomic and Molecular Physics, Wuhan Institute of Physics and Mathematics, Chinese Academy of Sciences, Wuhan, Hubei 430071, China
The thermal stability of triple helical structure plays a critical role in collagen biosynthesis, function and degradation [1, 2]. Collagen, as the predominant component of connective tissues such as bone, skin, tendons, ligaments, cartilage and basement membranes, provides structural integrity and mechanical strength for human body [2]. Collagen displays a unique triple helical structure, consisting of three polypeptide chains with the repetitive Gly-X-Y amino acid sequences [3]. The close packing of the three chains requires Gly to be every third residue, while proline and hydroxyproline at the X and Y positions are considered to stabilize the triple helical structure [4]. The thermal stability of collagen has been found to be correlated with the environment of the organism, ranging from 6 ℃ in ice fish to 46 ℃ in thermophilic worms [5, 6]. The melting temperature of monomer collagen in mammals was estimated to be ~35 ℃, slightly less than body temperature [7]. The self-association of individual triple helices into fibrils offers collagen enhanced stability and great tensile strength to support stress in tissues [2].
Collagen has been suggested to possess different local stabilities along the 1014-residue long triple helix [8-10]. Weak triple helix with less stability has been observed in an imino acid poor region adjacent to the matrix metalloproteinase cleavage site, allowing the scissile bonding to be exposed for the enzyme attack [11, 12]. Mutations in collagen have also been observed to affect the stability of collagen, leading to abnormalities in collagen structure associated with connective tissue diseases with various clinical phenotypes [13, 14]. Micro-unfolding with dynamic loss of local triple helix structure has been proposed as an important factor for collagen fibril formation [11, 15]. Understanding the sequence dependence of the global and local stability of collagen triple helix will greatly contribute to the development of improved collagen biomaterials and effective therapies for collagen-related disorders.
CD characterization of the thermal stability of synthetic collagen mimic peptides has provided useful insights into the function and pathology of collagen [8]. CD studies of host-guest peptides indicated that the degree of triple helix destabilization depended on the identity of the substituting residue in the order of destabilization as Ala, Ser < Cys < Arg < Val < Glu, Asp, which was significantly correlated with the severity of Osteogenesis Imperfecta (OI) mutations [16]. CD characterization of collagen-like heterotrimers mimicking the presence of Gly mutations in one, two or three chains showed that the frequency of substitutions at a particular position affected the thermal stability of heterotrimer triple helices [17]. CD provides a powerful approach to measure the thermal stability of collagen peptides, however, the expensive instrumentation has made CD with limited access in developing countries.
We therefore have developed an alternative approach to detect the thermal stability of collagen mimic peptides without the need of expensive instrument. Fluorescence spectroscopy is widely used with easy access all around the world because of its inexpensive instrumentation, low operation cost, easy operation, and high sensitivity. Fluorescence spectroscopy has been utilized to study urea-induced protein unfolding [18]. We have recently developed an efficient fluorescent self-quenching assay to monitor the helix composition of heterotrimeric collagen-like peptides [19]. Here we have evaluated the potential of fluorescence spectroscopy in the application of measuring the thermal stability of triple helical collagen mimic peptides. We have demonstrated that fluorescence spectroscopy could measure the thermal stability of collagen mimic peptides with low concentrations under different circumstances. This highly sensitive fluorescence self-quenching assay will greatly expedite the studies of sequence-dependent properties of collagen mimic peptides.
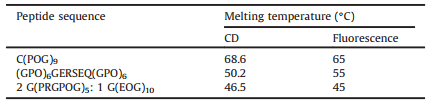
2. Results and discussion 2.1. Design of dye-labeled collagen mimic peptidesThis fluorescence method requires a fluorescent dye to label collagen mimic peptides, which can be easily achieved at the end of the solid-state synthesis of the peptide. We have chosen two widely used commercial dyes, FITC (fluorescein isothiocyanate) and FAM (carboxyfluorescein) as demonstration. We have applied this approach on three model peptides to represent three typical cases in collagen studies (Table 1). Peptide C(POG)9 was designed to model the classic (Gly-Pro-Hyp)n repetitive triplets in collagen; peptide (GPO)6GERSEQ(GPO)6 was designed to model a Gly-Ser mutation leading to Osteogenesis Imperfecta; a heterotrimer peptide composed of G(PRGPOG)5 and G(EOG)10 was designed to model heterotrimer collagens containing two α1 and one α2 chains (Table 1).
|
|
Table 1 Design and thermal stability of collagen mimic peptides characterized by CD and fluorescence. Peptides were modified by fluorescent dyes to facilitate the fluorescence determination. Specifically, FITC was used to label peptide C(POG)9 and (GPO)6GERSEQ(GPO)6, and FAM was used to label peptide G(PRGPOG)5. |
2.2. CD and fluorescence characterization of a classic collagen-like homotrimer peptide
First, the thermal stability of homotrimer peptide C(POG)9 was characterized by CD and fluorescence (Fig. 1). The peptide was prepared as 400mmol L-1 in 10 mmol L-1 PBS buffer at pH 7.4 for CD measurements. CD spectroscopy of the peptide at 4 ℃ displayed a high molecular residue ellipticity signal at 225 nm (MRE225 = 3836° cm2 dmol-1), indicating the formation of triple helix structure (Fig. 1A). The characteristic CD peak at 225 nm was lost at 86 ℃, showing the absence of triple helix after heating (Fig. 1A). Monitoring the ellipticity at 225 nm with increasing temperature showed a sharp thermal transition with Tm = 68.6 ℃ (Fig. 1B). The fluorescence emission spectra were carried out on the FITC-labeled peptide (FITC-C(POG)9) with a much lower concentration of 1mmol L-1 taking the advantage of the high sensitivity of fluorescence spectroscopy. The peptide showed a fluorescence peak at 517 nm at 18 ℃, while the fluorescence intensity became much higher at 70 ℃ than that at 18 ℃ (Fig. 1C). The low fluorescence intensity of the peptide at 18 ℃ indicated that formation of triple helix structure, as the close proximity of the FITC dye in the triple helix resulted in fluorescence self-quenching, while the higher fluorescence intensity of the peptide at 70 ℃ suggested the loss of triple helix structure, as the FITC dye was far away from each other in the unfolded state, resulting in little fluorescence self-quenching. The red-shift observed in the emission spectra may result from the conformational changes of the peptide from triple helix to monomer at higher temperatures. Monitoring the fluorescence at 517 nm with increasing temperature showed a sharp thermal transition with Tm = 65 ℃ (Fig. 1D). The thermal stability measured by CD spectroscopy was 3.6 ℃ higher than that measured by fluorescence spectroscopy, which may be due to the much higher concentration of the CD sample.

|
Download:
|
| Figure 1. Thermal stability of a classic collagen mimic peptide C(POG)9. CD wavelength scan of the peptide at 4 ℃ and 86 ℃ (A); CD thermal transitions of the peptide (B); fluorescence profiles of the peptide at 18 ℃ and 70 ℃ (C); fluorescence monitoring of thermal transitions of the peptide (D). | |
2.3. CD and fluorescence characterization of a collagen-like peptide mimicking an Osteogenesis Imperfecta mutation
Osteogenesis Imperfecta represents one of the most important connective tissue disorders resulting from mutations in collagen [20]. The thermal stability of peptide (GPO)6GERSEQ(GPO)6 modeling an Osteogenesis Imperfecta mutation was characterized by CD and fluorescence (Fig. 2). CD spectroscopy of the peptide at 4 ℃ displayed a high molecular residue ellipticity signal at 225 nm (MRE225 = 3900° cm2 dmol-1), showing the formation of triple helix structure (Fig. 2A). The loss of the characteristic CD peak at a high temperature of 86 ℃ suggested the absence of triple helix (Fig. 2A). Monitoring the ellipticity at 225 nm with increasing temperature showed a thermal transition with Tm = 50.2 ℃ (Fig. 2B). The fluorescence emission spectra were also carried out on the FITC-labeled peptide at both 18 ℃ and 70 ℃. The peptide showed a fluorescence peak at 517 nm at 18 ℃, while the fluorescence intensity became much higher at 70 ℃ than that at 18 ℃ (Fig. 2C). Similarly as peptide C(POG)9, peptide (GPO)6GERSEQ(GPO)6 displayed low fluorescence intensity at lower temperatures due to the fluorescence self-quenching effects resulting from the close proximity of the FITC dye in the triple helix structure. Monitoring the fluorescence at 517 nm with increasing temperature showed a thermal transition with Tm = 55 ℃ (Fig. 2D). The thermal stability measured by CD spectroscopy was 4.8 ℃ lower than that measured by fluorescence spectroscopy, suggesting that the dye labeling may have an enhanced effect on the stability of triple helix depending on the sequence.

|
Download:
|
| Figure 2. Thermal stability of a collagen peptide (GPO)6GERSEQ(GPO)6 mimicking an Osteogenesis Imperfecta mutation. CD wavelength scan of the peptide at 4 ℃ and 86 ℃ (A); CD thermal transitions of the peptide (B); fluorescence profiles of the peptide at 18 ℃ and 70 ℃ (C); fluorescence monitoring of thermal transitions of the peptide (D). | |
2.4. CD and fluorescence characterization of a heterotrimer peptide
Heterotrimer collagens represent a big group of collagen family. The thermal stability of heterotrimer model peptide composed of two G(PRGPOG)5 and one G(EOG)10 peptide chains was characterized by CD and fluorescence (Fig. 3). CD thermal transition curve of the heterotrimer peptide showed a sharp transition with Tm = 46.5 ℃ (Fig. 3A). Meanwhile, the fluorescence monitoring of the peptide showed a sharp thermal transition with Tm = 45 ℃ (Fig. 3B). The minor difference (1.5 ℃) between the thermal stabilities measured by CD and fluorescence methods demonstrated the applicability of fluorescence spectroscopy in the determination of triple helix stability.

|
Download:
|
| Figure 3. Thermal stability of a heterotrimer collagen mimic peptide. CD thermal transitions of the peptide (A); fluorescence monitoring of thermal transitions of the peptide (B). | |
3. Conclusions
The thermal stabilities of collagen-like peptides modeling three typical cases have been measured by both CD and fluorescence spectroscopy techniques. The variance of the thermal stabilities between these two techniques were estimated to be less than 5 ℃ (Table 1). It may result from the concentration difference as well as the effect of the dye labels. These results suggested that fluorescence spectroscopy could reasonably well measure the thermal stability of collagen mimic peptides. More importantly, we have demonstrated that the fluorescence spectroscopy can detect the triple helix stability at a much lower concentration than which was required by CD techniques. This far extends our capability to characterize the thermal stability of collagen triple helix. Since this assay only needs a tiny amount of peptides, it will greatly expedite the studies of sequence-dependent properties of collagen mimic peptides. Due to the easy access of fluorescence spectrometers, this highly sensitive approach has great potential in the application of determining the thermal stability of triple helix systems such as collagens, collectins, adiponectin, macrophage scavenger and C1q.
4. Experimental 4.1. Sample preparationAll peptides were synthesized by Chinese Peptide Company (Hangzhou, China), and their purities were confirmed by mass spectrometry. Fresh solutions with a starting concentration of 1 mg/ml were prepared for peptides C(POG)9 and (GPO)6GERSEQ (GPO)6 in 10 mmol L-1 PBS buffer at pH 7.4. The mixture of peptide FAM-G(PRGPOG)5 (denoted as peptide A) and G(EOG)10 (denoted as peptide B) at a molar ratio of 2:1 (300 mmol L-1 A and 150 mmol L-1 B, denoted as AB) was prepared in 10 m mol L-1 PBS buffer at pH 7.4.
4.2. Preparation of FITC-labeled peptidesPeptides C(POG)9 and (GPO)6GERSEQ(GPO)6 (1.0 mg) were dissolved in 2 mL 50 m mol L-1 Na2CO3-NaHCO3 buffer (pH 9.0, 140 m mol L-1 NaCl), respectively. FITC (2.0 mg) was dissolved in 2 mL 50 m mol L-1 carbonate buffer (pH 9.0, 140 m mol L-1 NaCl). The peptide and FITC solutions were mixed (the molar ratio of peptide: FITC as 1:12) and foil-wrapped to avoid the loss of fluorescence. The mixture was stirred constantly at room temperature for 16 h to complete the reaction. Finally, the product was repeatedly dialyzed against 10 m mol L-1 PBS buffer to remove excessive FITC. The fluorescence intensity of FITC in the dialysis buffer after exchange was monitored to assure the complete removal of FITC in the solution.
4.3. Circular dichroism spectroscopyCD spectra were measured on an Aviv model 400 spectrophotometer (Applied Photophysics Ltd., England). Cells with a path length of 2 mm were used, and the temperature of the cells was regulated using a Peltier temperature controller. The CD samples were prepared at a concentration of 1 mg mL-1 in 10 m mol L-1 PBS buffer at pH 7.4. Wavelength scans were recorded from 215 to 260 nm with a 0.5 nm increment per step and a 0.5 s averaging time. Each scan was repeated three times. Thermal stability was measured by monitoring the amplitude of the CD peak at 225 nm as a function of increasing temperature from 4 ℃ to 86 ℃ with an average heating rate of 0.4 ℃/min and 2 min equilibration time. The peptides were equilibrated for at least 24 h at 4 ℃ prior to the melting experiments. The first derivative of the thermal transition curve was calculated, and the melting temperature (Tm) was determined as the extrema of the first derivative.
4.4. Fluorescence spectroscopyFluorescence spectra were recorded on a FS5 fluorescence spectrometer by using a Xenon lamp as an excitation source (Edinburgh Instruments Ltd., England). The FITC-labeled peptides C(POG)9 and (GPO)6GERSEQ(GPO)6 and the heterotrimer peptide AB were all diluted to obtain a final concentration as 1 mmol L-1. The emission spectra were conducted for each peptide from 505 to 650 nm with a 1 nm increment per step at an excitation wavelength of 497 nm at two temperatures 4 ℃ and 70 ℃. Thermal unfolding was determined by real-time monitoring the fluorescence intensity as a function of increasing temperature from 18 ℃ to 70 ℃ or 84 ℃. The fluorescence spectra were recorded after incubation for 2 min at each temperature.
AcknowledgementsWe are grateful to the financial support from the National Natural Science Foundation of China (No. 21305056) and open fund of State Key Laboratory of Magnetic Resonance and Atomic and Molecular Physics (No. T151402).
| [1] | C.L. Jenkins, R.T. Raines. Insights on the conformational stability of collagen. Nat. Prod. Rep. (2002) 49–59. |
| [2] | M.D. Shoulders, R.T. Raines. Collagen structure and stability. Annu. Rev. Biochem. (2009) 929–958. |
| [3] | A. Rich, F.H. Crick. The structure of collagen. Nature (1955) 915–916. |
| [4] | J. Bella, M. Eaton, B. Brodsky, H.M. Berman. Crystal and molecular structure of a collagen-like peptide at 1.9 A resolution. Science (1994) 75–81. |
| [5] | B.J. Rigby. Amino-acid composition and thermal stability of the skin collagen of the Antarctic ice-fish. Nature (1968) 166–167. |
| [6] | F. Gaill, K. Mann, H. Wiedemann, J. Engel, R. Timpl. Structural comparison of cuticle and interstitial collagens from annelids living in shallow sea-water and at deep-sea hydrothermal vents. J. Mol. Biol. (1995) 284–294. |
| [7] | E. Leikina, M.V. Mertts, N. Kuznetsova, S. Leikin. Type Ⅰ collagen is thermally unstable at body temperature. Proc. Natl. Acad. Sci. U. S. A. (2002) 1314–1318. |
| [8] | B. Brodsky, G. Thiagarajan, B. Madhan, K. Kar. Triple-helical peptides:an approach to collagen conformation, stability. and self-association. Biopolymers (2008) 345–353. |
| [9] | A.V. Persikov, J.A.M. Ramshaw, A. Kirkpatrick, B. Brodsky. Amino acid propensitiesfor the collagentriple-helix. Biochemistry (2000) 14960–14967. |
| [10] | A.V. Persikov, J.A. Ramshaw, B. Brodsky. Prediction of collagen stability from amino acid sequence. J. Biol. Chem. (2005) 19343–19349. |
| [11] | J.X. Xiao, R.M. Addabbo, J.L. Lauer, G.B. Fields, J. Baum. Local conformation and dynamics of isoleucine in the collagenase cleavage site provide a recognition signal for matrix metalloproteinases. J. Biol. Chem. (2010) 34181–34190. |
| [12] | G.B. Fields. A model for interstitial collagen catabolism by mammalian collagenases. J. Theor. Biol. (1991) 585–602. |
| [13] | E. Makareeva, E.L. Mertz, N.V. Kuznetsova, et al., Structural heterogeneity of type Ⅰ collagentriple helix and its rolein osteogenesis imperfecta. J. Biol. Chem. (2008) 4787–4798. |
| [14] | J.X. Xiao, B. Madhan, Y.J. Li, B. Brodsky, J. Baum. Osteogenesis imperfecta model peptides:incorporationof residues replacing Glywithin a triple helix achieved by renucleation and local flexibility. Biophys. J. (2011) 449–458. |
| [15] | L. Ryhänen, E.J. Zaragoza, J. Uitto. Conformational stability of type Ⅰ collagen triple helix:evidence for temporary and local relaxation of the protein conformation using a proteolytic probe. Arch. Biochem. Biophys. (1983) 562–571. |
| [16] | K. Beck, V.C. Chan, N. Shenoy, et al., Destabilization of osteogenesis imperfecta collagen-like model peptides correlates with the identity of the residue replacing glycine. Proc. Natl. Acad. Sci. U. S. A. (2000) 4273–4278. |
| [17] | V. Gauba, J.D. Hartgerink. Synthetic collagen heterotrimers:structural mimics of wild-type and mutant collagen type Ⅰ. J. Am. Chem. Soc. (2008) 7509–7515. |
| [18] | H.M. Chen, E. Rhoades. Fluorescence characterization of denatured proteins. Curr. Opin. Struct. Biol. (2008) 516–524. |
| [19] | X.X. Sun, J. Fan, X. Li, et al., Colorimetric and fluorometric monitoring of the helix composition of collagen-like peptides at the nM level. Chem. Commun. (2016) 3107–3110. |
| [20] | P. H. Byers, W. G. Cole, Osteogenesis imperfecta, in: P. M. Royce, B. Steinmann (Eds. ), Connective Tissue and Its Heritable Disorders, Wiley-Liss, New York, 2002, pp. 385-430. |
 2017, Vol. 28
2017, Vol. 28