b Joint Laboratory for Extreme Conditions Matter Properties, Southwest University of Science and Technology and Research Center of Laser Fusion, Mianyang, 621010, China;
c Science and Technology on Plasma Physics Laboratory, Research Center of Laser Fusion, China Academy of Engineering Physics, Mianyang 621900, China
Supercapacitors, which have been widely used in various areas, such as memory back-up, electric vehicles, power quality management, battery improvement, high performance energy storage/conversion devices and renewable energy applications, have attracted growing attention owing to their ultrahigh power density, fast charging, long-life cycle and excellent safety properties [1, 2]. A variety of materials have been used to build high-performance supercapacitors to obtain high energy storage capability [3-8]. Because of its well-developed microstructure, high specific surface area and relatively high packing density, porous carbon material is one of the most well-studied and widely used as a supercapacitor electrode material. With unique three-dimensional (3D) structure and outstanding electrochemical properties, graphene aerogels have promised potential applications for energy storage. Recently, much research had been focused on enhancement of the electronic properties of graphene materials through doping nitrogen element by using various reducing agents such as pyrrole, dopamine, thiocarbohydrazide, phenylenediamine, melamine, amino acids and urea [9-22]. The obtained N-doped graphene aerogels showed improved excellent electrochemical performance. However, the environmental destructiveness of preparation, high cost of raw materials and complicated manufacturing limited its further applications in the market. Chitosan (CS) was produced through the deacetylation of chitin, which was one of the most abundant and renewable natural polymers, and was biocompatible and biodegradable [23, 24]. In addition, the chitosan consisted of a large number of amino-groups that could be used to synthesize N-doped carbon aerogels with excellent supercapacitor performance [25].
Herein, we used the chitosan as the nitrogen-doped agent to fabricate the nitrogen-doped graphene aerogels (NGAs) by a rapid method. The synthesized NGAs could have the surface area as large as 692 m2/g. Especially, it was found that the porous structure and specific capacitance of NGAs could be adjusted by the change of the carbonizing temperature. The prepared NGAs exhibited a high specific capacitance and outstanding cyclability due to the high specific surface area and hierarchical pore size and distribution.
2. Results and discussionAs shown in Fig. 1a and b, a good-formability black hydrogel with a cylinder shape was formed by using chitosan as functionalizing agents in GO aqueous solution in a vacuum oven at 90 ℃ for 6 h. In the contrast experiment, GO suspension could not form hydrogels in the same conditions (90 ℃ for 6 h), indicating GO suspension and chitosan were indispensable in the formation of hydrogels and chitosan was conducive to selfassembly of GO nanosheets to form three-dimensional hydrogels acting as a binder. After supercritical CO2 drying, NGOAs would be obtained. Then, the NGOAs were carbonized at 900 ℃ in nitrogen atmosphere for 4 h and the NGAs (Fig. 1c) was gained by removing the residual oxygen-containing groups on graphene nanosheets. NGAs had a relatively high mechanical strength as shown in Fig. 1d. Even with load of 200 g, the obtained NGA (50 mg) gave no obvious deformation and collapsing, which indicated the mechanical strength properties of the aerogels. The height and bottom diameters of the prepared regular cylinder sample (Fig. 1d) were 20.6 mm and 14.5 mm, respectively. Hence, it could be calculated that the pressure on the sample was 11.9 KPa. The structure evolution of the NGAs in the synthetic process was shown in Fig. 1e. The carboxyl group of the GO molecule could form an amide bond with the amino-group of CS, which helped to form a 3D network consisting of chitosan molecules and GO nanosheets. After carbonization, the carbon, nitrogen and oxygen atoms decomposed from the chitosan planar networks connected together in situ to form graphene-like structure, resulting in the formation of the nitrogen-doped graphene aerogel.

|
Download:
|
| Figure 1. Schematic illustration for the synthesis of the NGA: (a) GO suspension, (b) nitrogen-doped graphene oxide hydrogels, (c) nitrogen-doped graphene aerogel (NGA), (d) NGA with the load of 200 g and (e) structure evolution in the synthetic process. | |
The interior microstructures and morphologies of the obtained NGAs were characterized by SEM and TEM observations, as shown in Fig. 2. It could be seen that each of NGAs possessed a few macropores with diameters of several microns. This macroporous structure could form a great deal of interconnected network, which could work as electrolyte reservoirs and effectively shorten the ion diffusion length, to make the NGAs with improved performances at high current densities [26]. The XRD patterns of NGAs were shown in Fig. 3a. All the NGAs displayed a broad diffraction peak at about 24.7° and a weak diffraction peak at 43.2°, which could be attributed to the graphite-like structure (002) and (100), respectively. The broad XRD peaks of the NGAs indicated the disordered stacking of graphene nanosheets. As shown in Fig. 3b, the Raman spectra of NGAs contained both strong D bands at about 1355.6 cm-1 and strong G bands at about 1590.3 cm-1. The G band was a characteristic feature of graphitic carbon layers corresponding to the tangential vibration of the carbon atoms, whereas the D band was a typical sign of the presence of defective graphitic carbon. However, all the NGAs exhibited roughly similar Raman spectra as expected. Meanwhile, measurements of the specific surface area and BJH pore size distributions of the NGAs were carried out, as shown in Fig. 3c and d. It could be seen that the adsorption-desorption isotherms belonged to typical type Ⅳ, exhibiting an obvious hysteresis loop between the medium pressure and high pressure regions (P/P0 = 0.2-1), indicating their mesoporous characteristics The slow rise at the starting of the sorption isotherms indicated that microporosity had no significant contribution to the surface area of the as-prepared NGAs. A sharp increase at higher relative pressures could be attributed to the N2 adsorption in large mesopores or macropores. As shown in Fig. 3d, all the NGAs had a broad pore size distribution in the size range of 2-100 nm (mainly concentrated 5-50 nm). It clearly revealed that the as-prepared NGAs were highly porous and showed a typical hierarchical pore texture. As shown in Table 1, the specific surface area were 309, 692 and 472 m2/g for NGA-800, NGA-900 and NGA-1000, respectively. The pore volume of NGAs were 0.85, 3.02 and 1.94 cm3/g for NGA-800, NGA-900 and NGA-1000, respectively. In one word, the NGA-900 had the highest specific surface area and pore volume.

|
Download:
|
| Figure 2. SEM images of (a) NGA-800, (b) NGA-900, (c) NGA-1000, respectively, and TEM image of (d) NGA-900. | |

|
Download:
|
| Figure 3. XRD (a), Raman spectra (b), N2 adsorption-desorptionis otherms (c) and pore-size distributions (d) of NGA-800, NGA-900 and NGA-1000, respectively. | |
|
|
Table 1 Porosity parameters and element composition by XPS of NGAs. |
The element compositions of the prepared samples were determined by XPS measurement (Fig. 4) and the content of different elements were listed in Table 1. Based on the XPS results, nitrogen doping of NGAs were confirmed, which were mainly from chitosan. The N content of NGAs was in the range of 4.26-5.06 (at %). Apparently, the O content of NGAs decreased with the increase of the carbonizing temperature. In order to further interpret the evolution of surface chemical properties and the atom binding states of the prepared NGAs, the XPS measurements were performed as shown in Fig. 5, which showed the C1s and N1s deconvolution spectra of NGA-800 (a, d), NGA-900 (b, e) and NGA-1000 (c, f), respectively. In case of C1s of NGA-900, three peaks at 284.6, 287.6 and 289.4 eV corresponding to C═C/C—C in sp2-hybridized domains, C═O (carbonyl) and O═C—O (carboxyl) groups were observed. Besides, the appearance of the new characteristic peak corresponding to C—N bond (285.8 eV) indicated the successful doping of nitrogen. The N1s of NGA-900 were determined by 398.4, 400.6 and 404.3 eV, which could be attributed to pyridinic N, pyrrolic N and pyridine-N-oxide, respectively [18, 27-29]. All the high resolution XPS spectra demonstrated the effective removal of oxygen-containing groups and doping of nitrogen by chitosan in the preparation process compared to GO.

|
Download:
|
| Figure 4. XPS survey spectra of NGA-800, NGA-900 and NGA-1000, respectively. | |

|
Download:
|
| Figure 5. C1s XPS spectra and N1s XPS spectra of NGA-800 (a, d), NGA-900 (b, e) and NGA-1000 (c, f), respectively. | |
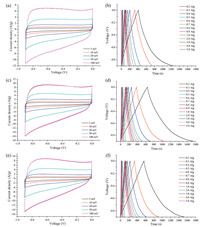
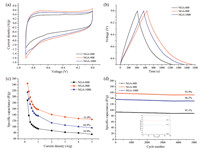
The electrochemical performances of NGAs were evaluated by cyclic voltammetry (CV) and galvanostatic charge/discharge (GCD) by using a conventional three-electrode system with a voltage window of -0.9 to 0 V in 6 mol/L KOH aqueous solution. As shown in Fig. 6a, c and e, all the CV curves exhibited the similar rectangle shape and good symmetry at the different scan rates, indicating their double-layer capacitor performance. The galvanostatic charge/discharge curves of NGAs were demonstrated in Fig. 6b, d and f and it could be found that the charge curves of NGAs were almost symmetric to their corresponding discharge counterparts, demonstrating the high reversibility. CV curves of all the NGAs at the scan rate of 5 mV/s were shown in Fig. 7a, and it could be observed that the NGA-900 had larger voltammetric current response compared with NGA-800 and NGA-1000, suggesting higher charge storage capability of NGA-900. The specific capacitances were calculated according to Ca = (It)/(mV), where Ca (F/g) was the gravimetric specific capacitance of the NGA samples, I (A) was the constant discharge current, t (s) was the discharge time, V(V) was the potential window of the cell, and m (g) was the mass of the active material in the work electrode [30]. Fig. 7b showed the GCD curves of NGA-800, NGA-900 and NGA-1000 at a current density of 0.2 A/g. The specific capacitances of all the samples at the current density of 0.2 A/g were calculated and the results indicated that NGA-900 had higher specific capacitance (244.4 F/g) than that of NGA-800 (188.4 F/g) and NGA-1000 (215.5 F/g). It was known that the capacitive behavior of graphene-based nanostructure materials used in supercapacitors was highly dependent on both the pore structure and the specific surface area [31, 32]. Thus, it could be because NGA-900 had an high pore volume of 3.02 cm3/g while maintaining the 3D hierarchical porous nanostructure of graphene with a large specific surface area of 692 m2/g. As seen from Fig. 7c, it could be seen that Ca values of all NGAs gradually decreased with the increase of the current density from 0.2 to 5 A/g. This could be attributed that at a low current density of 0.2 A/g, the K+ could easily diffuse into almost all available space of the NGAs, leading to a sufficient insertion reaction. However, the increase of the current density had a remarkable impact on the diffusion of K+ into the material. At a high current density of 5 A/g, the K+ could only approach the outer surface of the NGAs and the fewer K+ ions contacted the inner space of electrode for energy storage, so that the material located in the deep space had little contribution to the electrochemical capacitive behavior. However, the specific capacitance for NGA-900, which exhibited a high rate performance, had 51.0% retention of its initial value (from 244.4 F/g to 124.6 F/g), which was higher than that of NGA-1000 (44.9%) and NGA-800 (39.9%). Meanwhile, the rate capability of supercapacitors via CV measurements was also discussed. Fig. S1 exhibited Ca values of all the NGAs at different scan rates of 5, 10, 20, 50, and 100 mV/s. The Ca was calculated according to formula Ca = (∫IdV)/(2mΔVυ) in CV tests. Here, I (A) was the response current density, V (V) was the potential window, m (g) was the mass of active material and y (mv/s) was the scan rate. It was found that among all three samples, NGA-900 had the highest capacitance at the same scan rates. For instance, NGA-900 possessed a specific capacitance of about 236.4 F/g at 5 mV/s, which was higher than the specific capacitances of NGA-800 (161.8 F/g) and NGA-1000 (211.5 F/g). Moreover, compared with the rate capabilities of NGA-800 (71.5%) and NGA-1000 (71.4%), the specific capacitance for NGA-900 had 72.9% retention of its initial value when the scan rate was increased from 5 mV/s to 100 mV/s, suggesting good rate capability for NGA-900 as a supercapacitor. The cycling stability was measured at a current density of 1 A/g and the result was shown in Fig. 7d. It could be seen that the specific capacitance of the supercapacitor still retained 95.1% (98.6%), 96.2% (98.9%) and 95.9% (98.9%) of the initial capacity after 5000 (1000) cycles for NGA-800, NGA-900 and NGA-1000, respectively. The results suggested that the prepared NGAs electrodes exhibited good electrochemical reproducibility as supercapacitor electrode materials.
|
Download:
|
| Figure 6. Electrochemical capacitive performance of NGAs: Cyclic voltammograms of the supercapacitor based on NGAs at different scan rates and galvanostatic charge/ discharge curves of NGAs at different charging/discharging current density of NGA-800 (a, b), NGA-900 (c, d) and NGA-1000 (e, f), respectively. | |
|
Download:
|
| Figure 7. CV curves at 5 mV/s (a), GCD curves at 0.2 A/g (b), specific capacitances as a function of current density (c), cycling performance at 1 A/g (d). | |
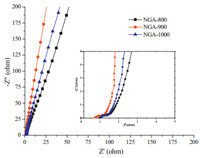
The electrode conductivity of NGAs was characterized by the electrochemical impedance spectroscopy (EIS) measurement. Herein, Nyquist plots were used to analyze EIS properties. Fig. 8 showed the Nyquist plot of the NGAs at the frequency range from 0.01 Hz to 100 kHz. The Nyquist plot could be divided to three parts, including a linear part at low frequency, an inclined portion about 45° at the middle frequency and an uncompleted semicircle part at high frequency. According to the EIS curves shown in Fig. 8, all the spectra had presented the vertical lines, parallel to the imaginary axis at the lower region in the Nyquist plot, indicating good capacitor behavior. At higher frequency (>10 kHz), the imaginary part (Z') of the impedance was near to zero and the real part of resistance (Z") was derived from the electrolyte and the contact resistance between the electrode and the current collector. The real-axis intercept in the high-frequency region is considered to be the equivalent series resistance (ESR) of the cell, which determines the charged/discharged rate of the cell, that is power capability [33, 34]. As seen from in Fig. 8, it showed the intersection points on the real axis, which were mainly associated with ohmic resistance of the electrolytes and internal resistance of the electrodes. Obviously, it could be seen that the ESR of NGA-900 was the lowest, meaning its better electrode conductivity. It was known that nitrogen atoms in graphene lattices could tailor local electronic structures, which was beneficial to enhance the band between the nitrogen atoms and K+ ions in the solution, resulting in plentiful K+ ions accommodated on the electrode surface and the enhancement of electrode conductivity [35]. Therefore, the best electrochemical capacitance performance of NGA-900 could be attributed to two factors. On one hand, NGA-900 had the highest N content as shown in Table 1. Plentiful nitrogen doping could reduce the ESR of NGA-900 and so enhance the electrode conductivity. On the other hand, NGA-900 possessed an largest pore volume of 3.02 cm3/g and specific surface area of 692 m2/g, which enhanced charge transport and storage.
|
Download:
|
| Figure 8. Nyquist impedance plots for NGAs in the frequency range of 100 kHz- 0.01 Hz. | |
3. Conclusion
Three-dimensional porous nitrogen-doped graphene aerogels (NGAs) by using GO and chitosan were synthesized by an easy and rapid method. The pore structure and capacitive performance of NGAs could be controlled by the carbonizing temperature. The NGA-900 exhibited the highest specific surface area and pore volume. As a supercapacitor electrode, NGA-900 exhibited a high specific capacitance of 244.4 F/g at a current density of 0.2 A/g, superior rate capability (51.0% capacity retention) and excellent cycling life (96.2% capacitance retained after 5000 charging/discharging cycles). Thus, the easy synthesis and the high electrochemical performance maked the nitrogen-doped graphene aerogel functionalized with chitosan an ideal candidate for supercapacitors.
4. Experimental 4.1. Reagents and chemicalsThe graphite powder was from Shanghai Huayi (Group) Company. The chitosan powder (degree of deacetylation (DD) = 85%) was from Sinopharm Chemical Reagent Co., Ltd. Acetone (99.8%) and ethanol (99.8%) were provided by Chengdu Kelong Chemical Reagent Company. CO2 (99.9%) was purchased from Mianyang Changjun Gases Co., Ltd. All reagents and chemicals were used without further purification.
4.2. Preparation of nitrogen-doped graphene aerogelsIn a typical preparation, graphite oxide was prepared from natural graphite according to Hummers' method. 0.5 g graphite oxide was dispersed in 100 mL deionized water by ultrasonication for 3 h to acquire the grapheme oxide (GO) dispersion with a final concentration of 5 mg/mL. 0.6 g CS was added slowly into 30 mL GO dispersion under stirring at room temperature. Then, the mixture transferred into a 20 mL sealed glass phial at 90 ℃ for 6 h. The obtained hydrogels were washed with alcohol and acetone three times in sequence, and dried with supercritical CO2 to obtain nitrogen-doped graphene oxide aerogels (NGOAs). The NGOAs were heated to 900 ℃ at 5 ℃/min in N2 atmosphere and held for 3 h to yield nitrogen-doped graphene aerogels (NGAs). The different carbonizing temperature was selected as 800 ℃, 900 ℃ and 1000 ℃ (samples were denoted as NGA-800, NGA-900 and NGA-1000, respectively).
4.3. Materials characterizationField emission scanning electron microscopy (FE-SEM) was carried out in an Ultra 55 field emission scanning electron microscope operated at 15 kV. Transmission electron microscopy (TEM) images were measured on a Libra 200FE transmission electron microscope at 200 kV. X-ray photoelectron spectroscopy (XPS, AXIS Ultra DLD) was used to identify the chemical compositions and surface electron structure of the samples. Raman microscopy was obtained in an InVia laser Raman spectrometer with λ = 514.5 nm. X-ray diffraction (XRD) analysis was carried out in X' Pert PRO with Cu Kα radiation at λ = 0.15406 nm. The interplanar crystal spacing was calculated by Bragg equation (2dsin θ = nλ). Specific surface area was characterized by nitrogen adsorption/desorption measurement (AR-JW-BK112). The specific surface area and pore size distribution of the aerogels were commonly measured by the multipoint BET method on the basis of nitrogen adsorption-desorption isotherms at 77 K with a Quadrasorb SI instrument. The sample was degassed for at least 24 h at a temperature of 150 ℃ in vacuum in order to remove all the possible absorbed species.
4.4. Electrochemical measurementsElectrochemical experiments were performed on a CHI 660D electrochemical analyzer (CH Instruments, Chenhua Co., Shanghai, P. R. China). The electrochemical properties of the supercapacitors assembled by NGAs were investigated in 6 mol/L KOH electrolyte using three-electrode configuration at room temperature. The working electrodes were prepared by pressing the NGAs (about 2 mg), acetylene black and polytetrafluoroethylene with a weight ratio of 8:1:1 on a piece of Ni foam. Platinum foil and Hg/HgO electrodes were used as counter electrode and reference electrode, respectively. A potential window of -0.9 to 0 V vs. Hg/HgO reference electrode was applied to the electrochemical measurements.
AcknowledgmentThis work was financially supported by the National Natural Science Foundation of China (No. 51502274), the Doctoral Research Fund of Southwest University of Science and Technology (Nos. 15zx7137, 16zx7142) and the Research Fund for Joint Laboratory for Extreme Conditions Matter Properties (Nos.13zxjk04, 14tdjk03).
| [1] | P. Simon, Y. Gogotsi. Materials for electrochemical capacitors. Nat. Mater. (2008) 845–854. |
| [2] | X.J. Lu, H. Dou, X.G. Zhang. Mesoporous carbon nanospheres inserting into graphene sheets for flexible supercapacitor film electrode. Mater. Lett. (2016) 304–307. |
| [3] | Y.X. Xu, G.Q. Shi. Assembly of chemically modified graphene:methods and applications. J. Mater. Chem. (2011) 3311–3323. |
| [4] | X.L. Wu, A.W. Xu. Carbonaceous hydrogels and aerogels for supercapacitors. J. Mater. Chem. A (2014) 4852–4864. |
| [5] | X. Zhang, H.T. Zhang, C. Li, et al., Recent advances in porous graphene materials for supercapacitor applications. RSC Adv. (2014) 45862–45884. |
| [6] | B. Luo, L.J. Zhi. Design and construction of three dimensional graphene-based composites for lithium ion battery applications. Energy Environ. Sci. (2015) 456–477. |
| [7] | S. Nardecchia, D. Carriazo, M.L. Ferrer, M.C. Gutierrez, F. del Monte. Three dimensional macroporous architectures and aerogels built of carbon nanotubes and/or graphene:synthesis and applications. Chem. Soc. Rev. (2013) 794–830. |
| [8] | S. Mao, G.H. Lu, J.H. Chen. Three-dimensional graphene-based composites for energy applications. Nanoscale (2015) 6924–6943. |
| [9] | V.H. Luan, H.N. Tien, L.T. Hoa, et al., Synthesis of a highly conductive and large surface area graphene oxide hydrogel and its use in a supercapacitor. J. Mater. Chem. A (2013) 208–211. |
| [10] | H.B. Feng, Y.M. Li, J.H. Li. Strong reduced graphene oxide-polymer composites:hydrogels and wires. RSC Adv. (2012) 6988–6993. |
| [11] | Y. Zhao, J. Liu, Y. Hu, et al., Highly compression-tolerant supercapacitor based on polypyrrole-mediated graphene foam electrodes. Adv. Mater. (2013) 591–595. |
| [12] | X.H. Song, L.P. Lin, M.C. Rong, et al., multifunctional 3D nitrogen-doped graphene aerogel. Carbon (2014) 174–182. |
| [13] | H.C. Gao, Y.M. Sun, J.J. Zhou, R. Xu, H.W. Duan. Mussel-inspired synthesis of polydopamine-functionalized graphene hydrogel as reusable adsorbents for water purification. ACS Appl. Mater. Interfaces (2013) 425–432. |
| [14] | H. Bai, K.X. Sheng, P.F. Zhang, C. Li, G.Q. Shi. Graphene oxide/conducting polymer composite hydrogels. J. Mater. Chem. (2011) 18653–18658. |
| [15] | L.B. Xing, S.F. Hou, J.L. Zhang, et al., S co-doped graphene hydrogels with thiocarbohydrazide for electrode materials in supercapacitor. Mater. Lett. (2015) 97–100. |
| [16] | Y. Liu, H.H. Wang, J. Zhou, et al., Graphene/polypyrrole intercalating nanocomposites as supercapacitors electrode. Electrochim. Acta (2013) 44–52. |
| [17] | X.X. Sun, P. Cheng, H.J. Wang, et al., Activation of graphene aerogel with phosphoric acid for enhanced electrocapacitive performance. Carbon (2015) 1–10. |
| [18] | X.P. Shi, J.Y. Zhu, Y. Zhang, et al., Ndoped graphene aerogels and their application in supercapacitors. RSC Adv. (2015) 77130–77137. |
| [19] | L.B. Xing, S.F. Hou, J. Zhou, et al., Three dimensional nitrogen-doped graphene aerogels functionalized with melamine for multifunctional applications in supercapacitors and adsorption. J. Solid State Chem. (2015) 224–232. |
| [20] | H.L. Guo, P. Su, X.F. Kang, S.K. Ning. Synthesis and characterization of nitrogendoped graphene hydrogels by hydrothermal route with urea as reducingdoping agents. J. Mater. Chem. A (2013) 2248–2255. |
| [21] | T. Wang, L.X. Wang, D.L. Wu, et al., Hydrothermal synthesis of nitrogen-doped graphene hydrogels using amino acids with different acidities as doping agents. J. Mater. Chem. A (2014) 8352–8361. |
| [22] | G.Q. Tang, Z.G. Jiang, X.F. Li, et al., Three dimensional graphene aerogels and their electrically conductive composites. Carbon (2014) 592–599. |
| [23] | W.S.W. Ngah, S.A. Ghani, A. Kamari. Adsorption behaviour of Fe(Ⅱ) and Fe(Ⅲ) ions in aqueous solution on chitosan and cross-linked chitosan beads. Bioresour. Technol. (2005) 443–450. |
| [24] | D. Klemm, B. Heublein, H.P. Fink, A. Bohn. Cellulose:fascinating biopolymer and sustainable raw material. Angew. Chem. Int. Ed. (2005) 3358–3393. |
| [25] | P. Hao, Z.H. Zhao, Y.H. Leng, et al., Graphene-based nitrogen self-doped hierarchical porous carbon aerogels derived from chitosan for high performance supercapacitors. Nano Energy (2015) 9–23. |
| [26] | Z.R. Geng, H. Wang, R.T. Wang, et al., Facile synthesis of hierarchical porous carbon for supercapacitor with enhanced electrochemical performance. Mater. Lett. (2016) 1–5. |
| [27] | F. Xu, M. Minniti, P. Barone, et al., Nitrogen doping of single walled carbon nanotubes by low energy N2+ ion implantation. Carbon (2008) 1489–1496. |
| [28] | M. Zhou, F. Pu, Z. Wang, S.Y. Guan. Nitrogen-doped porous carbons through KOH activation with superior performance in supercapacitors. Carbon (2014) 185–194. |
| [29] | H.B. Ren, X.P. Shi, J.Y. Zhu, et al., Facile synthesis of N-doped graphene aerogel and its application for organic solvent adsorption. J. Mater. Sci. (2016) 6419–6427. |
| [30] | Y.T. Wang, A.H. Lu, H.L. Zhang, W.C. Li. Synthesis of nanostructured mesoporous manganese oxides with three-dimensional frameworks and their application in supercapacitors. J. Phys. Chem.C (2011) 5413–5421. |
| [31] | G. Gryglewicz, J. Machnikowski, E. Lorenc-Grabowska, G. Lota, E. Frackowiak. Effect of pore size distribution of coal-based activated carbons on double layer capacitance. Electrochim. Acta (2005) 1197–1206. |
| [32] | S. Yoon, J. Lee, T. Hyeon, S.M. Oh. Electric double-layer capacitor performance of a new mesoporous carbon. J. Electrochem. Soc. (2000) 2507–2512. |
| [33] | M.D. Stoller, S. Park, Y.W. Zhu, J.H. An, R.S. Ruoff. Graphene-based ultracapacitors. Nano Lett. (2008) 3498–3502. |
| [34] | R. Imran Jafri, N. Rajalakshmi, S. Ramaprabhu. Nitrogen doped graphene nanoplatelets as catalyst support for oxygen reduction reaction in proton exchange membrane fuel cell. J. Mater. Chem. (2010) 7114–7117. |
| [35] | D. Hulicova-Jurcakova, M. Seredych, G.Q. Lu, T.J. Bandosz. Combined effect of nitrogen-and oxygen-containing functional groups of microporous activated carbon on its electrochemical performance in supercapacitors. Adv. Funct. Mater. (2009) 438–447. |
 2017, Vol. 28
2017, Vol. 28 



