b Xiangya School of Pharmaceutical Sciences, Central South University, Changsha 410013, China
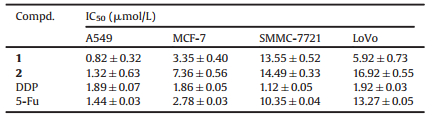
Selaginella doederleinii, belonging to the genus Selaginella, is a perennial herb, and is widely distributed in Guangxi Zhuang Autonomous Region, Guizhou and Yunnan Provinces of mainland China [1]. Traditionally, the whole plant has been used as a folk medicine to treat some kinds of cancers, sore throat and rheumatoid arthritis [2]. Several phytochemical studies of S. doederleinii led to reports of biflavonoids, lignans, and alkaloids, among which some exhibit diverse biological functions, including antioxidative, cytotoxic, and hypertensive activities [3-8], and the ethyl acetate and ethanol extracts from this plant show potent anticancer activity in vitro and in vivo [9-11]. In our previous work, some unique flavonoids were reported in some other species of genus Selaginella [12-20]. As part of ongoing search for novel and bioactive chemical constituents from this genus, the phytochemicals of S. doederleinii were investigated, and two new apigenin derivatives containing an aryl substituent at C-8 position, as well as ten known compounds were isolated. Here, the isolation and structural elucidation of two novel flavonoids, along with the evaluation of their cytotoxicity against four human cancer cell lines in vitro are described (Fig. 1).
|
Download:
|
| Figure 1. Structures of compounds 1-12. | |
2. Results and discussion 2.1. Chemistry
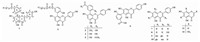
Compound 1 was obtained as a yellow amorphous powder. The ion at m/z 435.1076 in its positive HR-ESIMS spectrum (m/z calcd. for [M+H]+ 435.1074) gave a molecular formula of C24H19O8, which was in accordance with the 1H NMR and 13C NMR spectroscopic data (Table 1). The UV spectrum showed the Ⅰ band of 330 nm and Ⅱ band of 290 nm, and the positive result of the spay reagent of AlCl3, implying a flavone-like compound [21]. The 1H NMR spectrum of 1 (Table 1) suggested the presence of a set of para-substitued benzene ring (ring B) at δH 7.54 (d, 2H, J = 8.8 Hz, H-2'/6') and dH 6.75 (d, 2H, J = 8.8 Hz, H-3'/5') and a set of 1, 3, 4-trisubstituted benzene ring (ring D) [protons at δH 7.88 (dd, 1H, J = 8.0, 1.6 Hz, H-4"), δH 7.82 (d, 1H, J = 1.6 Hz, H-6") and δH 7.07 (d, 1H, J = 8.0 Hz, H-3")], and two isolated aromatic protons at δH 6.78 (s, 1H, H-3) and δH 6.37 (s, 1H, H-6), as well as two sets of intercoupling aliphatic protons at δH 4.25 (m, 2H, H-8") and δH 1.26 (t, 3H, H-9"). The 13C NMR spectrum (Table 1) showed a total of 24 carbon resonances, in addition to a apigenin skeleton (15 carbons), including an ester carbonyl resonance at δC 165.7, and a set of signals due to a 1, 3, 4-trisubstituted phenyl at δC 160.5, δC 134.4, δC 130.6, δC 127.4, dC 120.2, and δC 115.6, as well as two aliphatic carbons at δC 60.2, and δC 14.3. HMBC correlations (Fig. 2) between H-6" (δH 7.82) to C-8 (δC 104.1) confirmed the presence of the C-6"-C-8-linked in rings D and A, which suggested that the phenyl (ring D) was attached to C-8 of apigenin skeleton. The ethoxycarbonyl was linked to C-5" in ring D, which was strongly supported by the key HMBC correlations from H-4"/6" (δH 7.88, 7.82) to C-7" (δC 165.7). A hydroxyl was attached to C-2" in ring D, as indicated by the HMBC correlations from H-4"/6" (δH 7.88, 7.82) to C-2" (δC 160.4) and the downfield shift of the resonance of C-2" (δC 160.4) by 32.4 ppm. Hence, the structure of 1 was determined as ethyl 3-(5, 7-dihydroxy-2-(4-hydroxyphenyl)-4-oxo-4H-chromen-8-yl)-4-hydroxybenzoate, and named doederflavone A.
|
|
Table 1 1H NMR and 13C NMR data for compounds 1 and 2. |
Compound 2 was isolated as a yellow amorphous powder. Its HR-ESIMS exhibited a hydrogen adduct ion peak at m/z 477.1178 [M +H]+ (calcd. for C26H21O9, 477.1180), corresponding to the molecular formula C26H20O9. Compared to 1, the 1H NMR and 13C NMR data (Table 1) of 2 revealed that an additional methylene at C-8" and an unsaturated ketone carbonyl at C-7", which was attached to C-5" of ring A based on the key HMBC correlations between H-8" (δH 4.07) to C-9" (δC 168.4) and H-4"/6" (δH 7.91, 7.87) to C-7" (δC 191.9), respectively (Fig. 2). Thus, the structure of 2 was established as ethyl 3-(3-(5, 7-dihydroxy-2-(4-hydroxyphenyl)-4-oxo-4H-chromen-8-yl)-4-hydroxyphenyl)-3-oxopropanoate, and named doederflavone B.
|
Download:
|
| Figure 2. Selected 2D NMR correlations for compounds 1 and 2. | |
Comparsion of the spectroscopic and physical data with those reported allowed us to establish the structures of known compounds 3-12 as unciflavones E (3), unciflavone D (4), unciflavone F (5) [16], 6-(2-hydroxy-5-carboxyphenyl)-apigenin (6) [13], apigenin (7) [22], kaempferol (8) [23], isoschaftoside (9), schaftoside (10) [24], chromone (11) [25], 2, 6, 8-trimethylchromone (12) [26], respectively. Among them, compounds 8-10 were first reported from this genus, while 3-7, 11 and 12 were first reported from this plant.
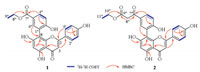
A tentative biosynthetic pathway for compounds 1 and 2 was proposed as shown in Scheme 1. These two compounds could be plausibly traced back to apigenin [27], which is coupled [28] with two polyketide products 1a and 1b to give compounds 1 or 2, respectively [29].
|
Download:
|
| Scheme 1. Putative biosynthetic pathway of compounds 1 and 2. | |
2.2. Cytotoxicity assay
New apigenin derivatives 1 and 2 were obtained to be evaluated for their cytotoxicity activity against human lung cancer A549, human breast cancer K562, human liver cancer SMMC-7721 and human colon cancer LoVo cell lines by using MTT method with DDP and 5-Fu as positive control (Table 2). Compounds 1 and 2 exhibited potent cytotoxicity against A549 with IC50 values of 0.82 mmol/L and 1.32 mmol/L, respectively. These results implied that compounds 1 and 2 might be candidates as leading compounds against A549 cancer cells, and may support the traditional uses of this plant in folk medicine.
|
|
Table 2 Cytotoxicity of compounds 1 and 2 against four human cancer cell lines in vitro. |
3. Conclusion
Phytochemical investigation of the whole herbs of S. doederleinii led to the isolation of two new flavonoids (1 and 2), together with ten known compounds (3-12). These two new flavonoids described herein feature an aryl substituent at C-8 of ring A of apigenin skeleton, which were further biologically evaluated their cytotoxicity activity against four human cancer cell lines in vitro, and exhibited considerable activity against A549 cancer cells. The findings could be enriched the flavonoids diversity and regarded as some further insight into the discovery of anticancer agent from natural products.
4. Experimental 4.1. General experimental proceduresUV spectra were measured on a UV-2600 spectrometer (SHIMADZU, Kyoto, Japan). NMR spectra were performed on a Bruker AV-500 MHz spectrometer and a Bruker AVHD-800 MHz spectrometer (Bruker, Karlsruhe, Germany) with tetramethylsilane (TMS) as an internal standard. HRESIMS data were collected with a Finnigan LTQ-FT (Thermo Fisher, MA, USA). Semi-preparative HPLC was performed on an Agilent 1200 unit equipped with a DAD detector and a YMC Pack ODS-A RP-18 column (10 mm, 250 × 10 mm, YMC Co. Ltd., Kyoto, Japan). Column chromatography (CC) was performed on Silica gel (Qingdao Marine Chemical Factory, Qingdao, China), macroporous resin (HPD-100, Hebei, China), and Sephadex LH-20 (TOYOPEARL TOSOH, Tokyo, Japan). Thin layer chromatography (TLC) was carried out with GF-254 plates (Qingdao Marine Chemical Factory, Qingdao, China). The spots were visualized using UV light at 254 and 365 nm and by spraying with AlCl3-EtOH (1:99, v/v) followed by heating. All solvents were analytical grade.
4.2. Plant materialThe whole herbs of S. doederleinii were collected in the town of Wutong, Lingui district, Guangxi, China, in July 2013 and authenticated by Prof. Zhen-Ji Li (Xiamen University, China). A botanical specimen of this species (20130710) was deposited at the Xiangya School of Pharmaceutical Sciences, Central South University.
4.3. Extraction and isolationWhole herbs of S. doederleinii (10.0 kg) were exhaustively extracted with 75% EtOH twice under reflux (2 × 60 L, 3 h/each). The extracts were combined and concentrated under vacuum to give a viscous mass (150 g), which was suspended in H2O (1.5 L) and partitioned with petroleum ether, EtOAc and n-BuOH, successively, to yield EtOAc fraction (90 g) and n-BuOH fraction (28 g).
The EtOAc fraction was separated by silica gel CC and eluted with CH2Cl2-MeOH-H2O (from 1:0:0 to 0:1:1) to obtain eight fractions (Fr.1-8). Applying Fr. 5 (18.3 g) to Sephadex LH-20 CC and eluted with H2O-MeOH (from 50:50 to 0:100) to give six subfractions Fr. A-F, of which Fr. B-D were separated repeatedly by CC over Sephadex LH-20, eluting with H2O-MeOH, followed by semi-preparative RP-HPLC (YMC-Pack ODS-A; 250 × 10 mm; 10 mm; 3 mL/min) using a mobile phase of acetonitrile (ACN)-H2O. Subfraction Fr. B was purified by semi-preparative RP-HPLC (ACN:H2O, 40:60, v/v) to yield compounds 5 (7.5 mg, tR = 20.4 min) and 6 (4.9 mg, tR = 23.5 min), while Fr. C was purified by semipreparative RP-HPLC (ACN:H2O, 50:50, v/v) to yield 7 (12.8 mg, tR = 16.5 min), 4 (5.2 mg, tR = 19.2 min), 3 (3.2 mg, tR = 21.7 min), and 11 (12.8 mg, tR = 25.6 min). Compounds 12 (4.5 mg, tR = 15.4 min), 8 (6.8 mg, tR = 18.2 min), 1 (4.8 mg, tR = 20.2 min), and 2 (7.9 mg, tR = 23.5 min) were obtained from Fr. D by using semi-preparative RP-HPLC (ACN:H2O, 65:35, v/v).
The n-BuOH fraction was loaded on a macroporous adsorbent resin (HPD-100) column, and eluted successively with H2O, 20% EtOH, 50% EtOH, 70% EtOH, 95% EtOH to obtain five corresponding fractions Fr. A-E. Fr. B (12.4 g) was subjected to CC over Sephadex LH-20 (MeOH/H2O in gradient) and then further to semipreparative HPLC (YMC-Pack ODS-A; 250 × 10 mm; 10 mm; 3 mL/ min; 15% ACN/H2O), compounds 9 (9.5 mg, tR = 14.6 min), 10 (8.2 mg, tR = 16.9 min) were obtained.
Doederflavone A (1): Yellow amorphous powder; UV (MeOH) λmax (log ε) = 260 (6.41) and 327 (6.21) nm; 1H/13C NMR data see Table 1; HR-ESIMS m/z 435.1076 [M+H]+, calculated for C24H19O8, 435.1074.
Doederflavone B (2): Yellow amorphous powder; UV (MeOH) λmax (log ε) = 275 (6.41) and 335 (6.14) nm; 1H/13C NMR data see Table 1; HRESIMS m/z 477.1178 [M+H]+, calculated for C26H21O9, 477.1180.
4.4. Cytotoxicity testFour human cancer cell lines A549, MCF-7, SMMC-7721, and LoVo, were used in the cytotoxicity assay. All cells were cultured in DMEM medium (Hyclone, USA) supplemented with 10% fetal bovine serum (Hyclone, USA) in a 5% CO2 atmosphere at 37 ℃. The cytotoxicity assay was performed using MTT (3-(4, 5-dimethylthiazol-2-yl)-2, 5-diphenyltetrazolium bromide) method in 96-well microplates [30]. Then, 100 mL of each cell suspension was placed into each well of a 96-well cell culture plate and allowed adherence in 5% CO2 for 24 h at 37 ℃ before drug addition, while suspended cells were seeded just before drug addition with an initial density of 1 × 105 cells/mL. Each cancer cell was exposed to a test compound at concentrations of 0.3, 1, 3, 10, 30, 100 mmol/L in DMSO in triplicate for 72 h, with cisplatin (DDP) (Qilu pharmaceutical, China) and 5-fluorouracil (5-Fu) (Hefei Bomei Biotechnology Co., Ltd., China) as positive control. The OD value of each well was measured at a microplate spectrophotometer. Each assay was done in triplicate, and inhibition was expressed as IC50 value.
AcknowledgmentsThis research was financially supported by the National Natural Science Foundation of China (No. 31370370), Key Project of Application Technology Research and Development of Haikou (No. 2015-039), Project of Strategic Emerging Industry Technology Research and Major Scientific and Technological Achievements of Hunan (No. 2015GK1053), and Innovation Project for Graduate Students of Hunan (No. CX2013B100). The authors would like to thank Dr. Bang-Shao Yin (College of Chemistry and Chemical Engineering in Hunan Normal University) and Prof. Guo-Gen Liu (Modern Analysis and Testing Centre in Central South University) for the assisting with the NMR measurement, and Prof. Zheng Li (High Resolution Mass Spectrometry Laboratory of Advanced Research Center in Central South University) for the HR-ESIMS measurement.
Appendix A. Supplementary dataSupplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.cclet.2017.01.011.
| [1] | Nanjing University of Chinese Medicine, Zhong Yao Da Ci Dian, 2th ed. , Shanghai Scientific & Technical Publishers, Shanghai, 2006, pp. 831-832. |
| [2] | State Administration of Traditional Chinese Medicine, Zhong Hua Ben Cao, Shanghai Scientific & Technical Publishers, Shanghai, 1998, pp. 44. |
| [3] | L.R. Chao, E. Seguin, F. Tillequin, M. Koch. New alkaloid glycosides from Selaginella doederleinii. J. Nat. Prod. (1987) 422–426. |
| [4] | R.C. Lin, J. Peyroux, E. Seguin, M. Koch. Hypertensive effect of glycosidic derivatives of hordenine isolated from Selaginella doederleinii Hieron and structural analogues in rats. Phytother. Res. (1991) 188–190. |
| [5] | R.C. Lin, A.L. Skaltsounis, E. Seguin, F. Tillequin, M. Koch. Phenolic constituents of Selaginella doederleinii. Planta Med. (1994) 168–170. |
| [6] | N.Y. Lee, H.Y. Min, J. Lee, et al., Identification of a new cytotoxic biflavanone from Selaginella doederleinii. Chem. Pharm. Bull. (2008) 1360–1361. |
| [7] | S.G. Li, H. Yao, M.F. Zhao, et al., Determination of seven biflavones of Selaginella doederleinii by high performance liquid chromatography. Anal. Lett. (2013) 2835–2845. |
| [8] | S.G. Li, M.F. Zhao, Y.X. Li, et al., Preparative isolation of six anti-tumour biflavonoids from Selaginella doederleinii Hieron by high-speed countercurrent chromatography. Phytochem. Anal. (2014) 127–133. |
| [9] | R. Lian, J. Li, H.M. Ma, et al., Effect of ethanol extract of Selaginella doederleinii Hieron on the proliferation of nasopharyngeal carcinoma CNE-1 and C666-1 cells. Afr. J. Tradit. Complement. Altern. Med. (2013) 490–493. |
| [10] | J.Z. Wang, J. Li, P. Zhao, et al., Antitumor activities of ethyl acetate extracts from Selaginella doederleinii Hieron in vitro and in vivo and its possible mechanism. Evid. Based Complement. Altern. Med. (2015) 865714. |
| [11] | Y.X. Sui, S.G. Li, P.Y. Shi, et al., Ethyl acetate extract from Selaginella doederleinii Hieron inhibits the growth of human lung cancer cells A549 via caspasedependent apoptosis pathway. J. Ethnopharmacol. (2016) 261–271. |
| [12] | J.F. Liu, K.P. Xu, D.J. Jiang, et al., A new flavonoid from Selaginella tamariscina. Chin. Chem. Lett. (2009) 595–597. |
| [13] | J.F. Liu, K.P. Xu, F.S. Li, et al., A new flavonoid from Selaginella tamariscina (Beauv.) Spring. Chem. Pharm. Bull. (2010) 549–551. |
| [14] | H. Zou, K.P. Xu, F.S. Li, et al., Uncinataflavone. a new flavonoid with a methyl benzoate substituent from Selaginella uncinata. J. Asian Nat. Prod. Res. (2013) 408–412. |
| [15] | H. Zou, K.P. Xu, Z.X. Zou, et al., A new flavonoid with 6-phenyl substituent from Selaginella uncinata. J. Asian Nat. Prod. Res. (2013) 84–88. |
| [16] | H. Zou, K.P. Xu, F.S. Li, et al., Unciflavones A-F. six novel flavonoids from Selaginella uncinata (Desv.) Spring. Fitoterapia (2014) 328–333. |
| [17] | H.P. Long, H. Zou, F.S. Li, et al., Involvenflavones A-F. six new flavonoids with 3'-aryl substituent from Selaginella involven. Fitoterapia (2015) 254–259. |
| [18] | H. Zou, P.S. Xu, R. Liu, et al., Selacyclicbiflavone A. an unusual macrocyclic biflavone from Selaginella uncinata (Desv.) Spring. Tetrahedron Lett. (2016) 892–894. |
| [19] | H. Zou, M.L. Yi, K.P. Xu, X.F. Sheng, G.S. Tan. Two new flavonoids from Selaginella uncinata. J. Asian Nat. Prod. Res. (2016) 248–252. |
| [20] | Z.X. Zou, P.S. Xu, C.R. Wu, et al., Carboxymethyl flavonoids and a chromone with antimicrobial activity from Selaginella moellendorffii Hieron. Fitoterapia (2016) 124–129. |
| [21] | T. J. Mabry, K. R. Markham, M. B. Thomas, The ultraviolet spectra of flavones and flavonols, in: T. J. Mabry, K. R. Markham, M. B. Thomas (Eds. ), The Systematic Identification of Flavonoids, Springer, Berlin Heidelberg, 1970, pp. 44-71. |
| [22] | Y. Cao, J.J. Chen, N.H. Tan, et al., Structure determination of selaginellins G and H from Selaginella pulvinata by NMR spectroscopy. Magn. Reson. Chem. (2010) 656–659. |
| [23] | F. Hadizadeh, N. Khalili, H. Hosseinzadeh, R. Khair, - Aldine. Kaempferol from saffron petals. Iran. J. Pharm. Res. (2003) 251–262. |
| [24] | C. Xie, N.C. Veitch, P.J. Houghton, M.S. Simmonds. Flavone C-glycosides from Viola yedoensis MAKINO. Chem. Pharm. Bull. (2003) 1204–1207. |
| [25] | H.P. Long, F.S. Li, K.P. Xu, et al., Chemical constituents from Selaginella involven Spring. Chin. Tradit. Pat. Med. (2014) 995–1000. |
| [26] | L.Y. Ma, S.C. Ma, F. Wei, et al., Uncinoside A and B. two new antiviral chromone glycosides from Selaginella uncinata. Chem. Pharm. Bull. (2003) 1264–1267. |
| [27] | B.W. Shirley. Flavonoid biosynthesis:'new' functions for an 'old' pathway. Trends Plant Sci. (1996) 377–382. |
| [28] | J.T. Fan, Y.S. Chen, W.Y. Xu, et al., Rubiyunnanins A and B. two novel cyclic hexapeptides from Rubia yunnanensis. Tetrahedron Lett. (2010) 6810–6813. |
| [29] | B.X. Jia, J. Yang, X.Q. Chen, et al., Baeckeins A and B. two novel 6-methylflavonoids from the roots of Baeckea frutescens. Helv. Chim. Acta (2011) 2283–2288. |
| [30] | T. Mosmann. Rapid colorimetric assay for cellular growth and survival:application to proliferation and cytotoxicity assays. J. Immunol. Methods (1983) 55–63. |
 2017, Vol. 28
2017, Vol. 28