b University of Chinese Academy of Sciences, Beijing 100049, China;
c Department of NMR Technology Services, Public Technical Service Center, Shanghai Institute of Materia Medica, Chinese Academy of Sciences, Shanghai 201203, China;
d State Key Laboratory of Quality Research in Chinese Medicine, Institute of Chinese Medical Sciences, University of Macau, Macao 999078, China;
e School of Life Science and Technology, ShanghaiTech University, Shanghai 201203, China
The genus Inula (Asteraceae) comprises more than one hundred species [1]. Sesquiterpenoids were considered as the characteristic constituents of this genus with diverse bioactivities such as cytotoxic, anti-inflammatory, and antifungal activities [2]. Inula cappa, a medicinal herb known as "Yang er ju" in Chinese, is widely distributed in the southern part of China. Its whole plant or roots have long been used for the treatment of rheumatoid arthritis, malaria, dysentery, and hepatitis [3]. Previous investigations of this plant reported the isolation of germacrane-type sesquiterpene lactones [4, 5], along with inositol derivatives [6-8], flavonoids [9], and phenolic glycosides [10, 11]. The highly oxygenated germacrane-type sesquiterpene lactones showed potent cytotoxic activity [5, 12, 13].
As part of our continuing search for bioactive constituents with novel structures from natural resources, a detailed investigation of I. cappa was carried out, resulting the isolation of two new highly oxygenated germacrane-type sesquiterpene lactones, ineupatolides D and E (1 and 2), and three known analogs (3-5, Fig. 1). This paper describes the isolation and structure elucidation of these isolates, and their cytotoxic activity against six human tumor cell lines as well.

|
Download:
|
| Figure 1. Structures of compounds 1-5. | |
2. Results and discussion
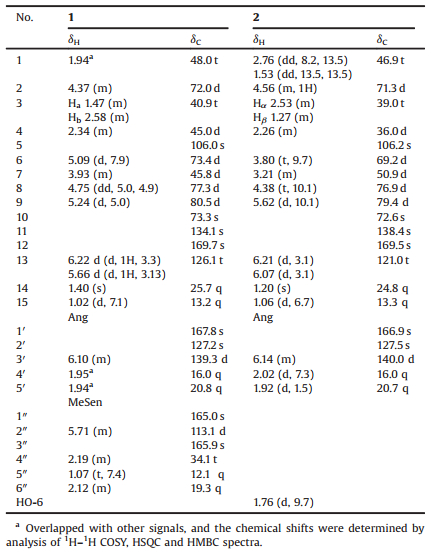
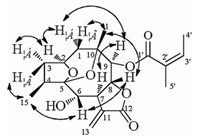
Compound 1 was obtained as a white amorphous powder. Its molecular formula was determined as C26H36O9 by HR-ESIMS (m/z 515.2253 for C26H36O9Na, calcd. m/z 515.2257), and implied an unsaturation equivalence of nine (see Supporting information). The IR spectrum suggests the presences of hydroxy (3439 cm-1), carbonyl (1716 cm-1), and double bond (1645 cm-1) groups. The 13C and DEPT NMR spectra gave 26 resonances ascribed to 6 methyls, 4 methylenes, 8 methines, and 8 quaternary carbons (Table 1). Among them, three ester carboxyls (δC 165.0, 167.8 and 169.7), and one exocyclic olefinic group (δC 126.1 and 134.1), were identified. The 1H NMR spectrum displayed 'signals for six methyls (δH 1.02, 1.07, 1.40, 1.94, 1.95, 2.12), two olefinic protons (δH 5.71, m; 6.10, m), and two exocyclic olefinic protons (δH 6.22, d, J = 3.3 Hz; 5.66, d, J = 3.3 Hz) (Table 1). In the HMBC spectrum (Fig. 2), both methyls at δH 1.94 and 1.95 showed correlations to the carbonyl carbon at δC 167.8 and two olefinic carbons at δc 127.2 and 139.3, indicating the existence of an angelate moiety. The 1H-1H correlations between H3-5" (δH 1.07, t, J = 7.4 Hz) and H2-4" (δH 2.19, m), and the HMBC correlation from the characteristic proton at δH 5.71 (1H, m) to C-4" (δc 34.1) and C-6" (δc 19.3), suggested the existence of a 4-methylsenecioyl residue. Apart from these two substituents, the left 15 carbons were indicative of the same sesquiterpene skeleton with that of dvaricin B, a known germacraenolide isolated from this plant [14]. The key HMBC correlations from H-6 (δH 5.09, d, J = 7.9 Hz) to the carbonyl carbon of the 4-methylsenecioyl group, and from H-9 (δH 5.24, d, J = 5.0 Hz) to the carbonyl carbon of the angelate group suggested that these two substituent groups were located at C-6 and C-9, respectively. The relative configuration was inferred from the ROESYexperiment (Fig. 2). The observed correlations of H-6/H-8, H-6/Me-15, H-2/H-4, H-2/Me-14, Me-14/H-9, and H-9/H-7 indicated the orientation of H-2 (α), H-4 (α), H-6 (β), H-7 (α), H-8 (β), H-9 (α), Me-14 (α), and Me-15 (β), respectively. The absolute configuration of compound 1 was determined by CD spectrum (Fig. 2) based on the Geissman rule [15, 16]. The negative Cotton effect at 259 nm for the nπ* transition of α-methylene γ-lactone chromophore suggested C-7, C-8 trans-fusion of the γ-lactone with a 7R configuration. Additionally, the positive Cotton effect at 229 nm can result from the 4-methyl seneciolate chromophore at C-6 with S-configuration [16]. Therefore, compound 1 was finally proposed to be (2S, 4S, 5S, 6S, 7R, 8R, 9S, 10R)-2, 5-epoxy-5, 10-dihydroxy-6-(4-methylsenecioyloxy)-9-angeloyloxy-germacran-8, 12-olide, and named ineupatolide D.
|
|
Table 1 1H (500 MHz, δ in ppm, J in Hz) and 13C NMR (125 MHz, δ in ppm) data of compounds 1 and 2 in CDCl3. |

|
Download:
|
| Figure 2. Key HMBC correlations (H→C) and ROESY correlations (H↔H) of compound 1; and CD spectrum of compound 1. | |
Compound 2, obtained as a white amorphous powder, was given a molecular formula of C20H26O7 by HRESIMS (m/z 401.1569 for C20H26O7Na, calcd. m/z 401.1576), corresponding to an unsaturation equivalence of eight. Its 1H and 13C NMR data showed a high similarity to those of 1, suggesting it as a germacrane-type sesquiterpene lactone with an angelate substituent group (Table 1). Two double bonds and two carbonyls in this molecule accounted for four of eight indices of hydrogen deficiency, thus the remaining four required 2 to be tetracyclic. The location of the angelate group at C-9 was inferred from the observed HMBC correlation from H-9 (δH 5.62) to the carbonyl carbon (δc 166.9) of the angelate group. The up-field chemical shift of H-6 (δH 3.65, t, J = 9.7), together with its correlation with HO-6 (δH 1.76, d, J = 9.7) in the 1H-1H COSY spectrum, revealed the presence of a hydroxy group at C-6. All the above evidences suggested that an additional oxygen bridge was formed between C-5 and C-10. Such a proposed structure explained well the high similarity of NMR data for compounds 1 and 2, and was supported by the molecular formula of 2. Its relative configuration was inferred from the ROESY experiment (Fig. 3). The observed correlations of H-8/H-6, H-6/Me-15, Me-15/H-3α, Me-14/H-8, Me-14/H-1α, H-1α/H-9, H-9/H-7, H-1α/H-2, and H-2/H-3α indicated the orientation of H-2 (α), H-4 (α), H-6 (β), H-7 (α), H-8 (β), H-9 (α), Me-14 (β), Me-15 (β), respectively. To minimize unfavorable steric interactions with the β-oriented 2, 5-epoxide bridge and M-14, the 5, 10-epoxide bridge was inferred to be a-orientation. In the CD spectrum of compound 2, the negative Cotton effect 246 nm indicated C-7, C-8 trans-fusion of the γ-lactone with a 7R configuration (Supporting information Fig. S17). Therefore, compound 2 was established as (2S, 4S, 5R, 6 S, 7R, 8R, 9S, 10R)-2, 5-epoxy-5, 10-epoxy-6-hydroxy-9-angeloyloxygermacran-8, 12-olide, and named ineupatolide E.
|
Download:
|
| Figure 3. Key ROESY correlations (H ↔ H) of compound 2. | |
Three known sesquiterpenes were identified as dvaricin B (3) [17], nepalolide C (4) [18], and inculacappolide (5) [4], respectively, by comparing physical and spectroscopic data with those in literatures.
Compounds 1-5 were tested for their cytotoxic activity against A431, A549, BGC-823, HL-60, HT-29, and MCF-7 cancer cell lines. All of them showed moderate cytotoxic activity with IC50 values ranging from 2.1 to 36.3mmol/L (Table 2).
|
|
Table 2 Cytotoxicity of compounds 1-5 against human tumor cell lines. |
3. Conclusion
Two new germacrane-type sesquiterpene lactones, ineupatolides D and E (1 and 2), together with three known analogs (3-5), were isolated from the whole plant of I. cappa. The structures of compounds 1-5 are classified into highly oxygenated germacranetype sesquiterpene lactones. Compounds 1-3 possess a common skeleton with an ether linkage between C-2 and C-5, and compound 2 contained an additional ether linkage between C-5 and C-10. To the best of our knowledge, compound 2 represents the first example of germacrane-type sesquiterpene lactone with both a 2, 5-epoxide and a 5, 10-epoxide linkages.
4. Experimental 4.1. General experimental proceduresOptical rotations were measured on a Perkin-Elmer 341 polarimeter. IR spectra were recorded on a Nicolet Magna FT-IR 750 spectrophotometer using KBr disks. CD spectra were measured on a Jasco J-180 spectrophotometer. NMR spectra were recorded on Bruker AM-400 and INVOA-600 NMR spectrometers. The chemical shift (d) values are given in ppm with TMS as internal standard, and coupling constants (J) are in Hz. ESIMS and HR-ESIMS data were recorded on Waters 2695-3100 LC-MS and Waters Xevo TOF mass spectrometers. Silica gel (Qingdao Marine Chemical Industrials, Qingdao, China) was used for flash chromatography. MCI gel CHP20P (75-150mm, Mitsubishi Chemical Industries, Tokyo, Japan) and Sephadex LH-20 (Pharmacia Biotech AB, Uppsala, Sweden) were used for column chromatography (CC). TLC was carried out on precoated silica gel GF254 plates (Yantai Chemical Industrials, Yantai, China) and the TLC spots were viewed at 254 nm and visualized with 5% H2SO4 in EtOH containing 10 mg/mL vanillin. Analytical HPLC was performed on a Waters 2690 instrument with an Alltech ELSD 2000 detector. Preparative HPLC was performed on a Varian PrepStar system with an Alltech 3300 ELSD. Chromatographic separations were carried out on a Waters Sunfire® RP C18, 5mm, 30 mm × 150 mm column and a Waters Sunfire® RP C18, 5mm, 19 mm × 150 mm column, using a gradient solvent system comprised of H2O and CH3CN, with a flow rate of 25.0 and 10.0 mL/min, respectively.
4.2. Plant materialThe whole plants of I. cappa were collected in Yulin city, Guangxi province, People's Republic of China in September 2012, and identified by Professor Jin-Gui Shen from the Shanghai Institute of Materia Medica. A voucher specimen (No. 20131020) was deposited at the Herbarium of the Shanghai Institute of Materia Medica, Chinese Academy of Sciences.
4.3. Extraction and isolationThe dried and powdered whole plants of I. cappa (30 kg) were extracted with 95% EtOH (40 L × 3 and 7 days each) at room temperature. After evaporation of the solvent, the residue was dissolved in water (10 L) and partitioned with CH2Cl2 (10 L × 3) and EtOAc (10 L × 3), successively. The CH2Cl2 fraction was concentrated and then dissolved with 80% MeOH in water (5 L × 3). The MeOH layers were combined and concentrated, and then subjected to CC over MCI gel (EtOH/H2O 50%-95%) to yield fractions 1-3. Fraction 1 was subjected to CC over MCI gel eluted with a mixture of EtOH/ H2O (30%-50%), giving four fractions 1a-1d. Fraction 1c was subjected to CC over silica gel to give four fractions 1c1-1c4. Fraction 1c1 was further purified by preparative HPLC (CH3CN/H2O 60%-80%) to afford 1 (12 mg). Fraction 1d was subjected to CC over silica gel (CH2Cl2/MeOH 100:1, 50:1, 20:1, 10:1) to afford two fractions 1d1 and 1d2. Fraction 1d1 was purified by preparative HPLC (CH3CN/H2O 60%-80%) to yield 2 (8 mg). Fraction 1c2 was purified by preparative HPLC (CH3CN/H2O 60%-80%) to afford 3 (33 mg) and 5 (54 mg). Fraction 1b was subjected to CC over silica gel to give two fractions 1b1-1b2. Fraction 1b1 was further purified by preparative HPLC (CH3CN/H2O 60%-80%) to afford 4 (17 mg).
4.3.1. Ineupatolide D (1)White amorphous powder; [α]D20 -45:7 (c 0.1 in CHCl3); CD (MeOH) λmax (Δε), nm: 211 (1.01), 246 (-0.88); IR (KBr) νmax/cm-1 3439, 2971, 2925, 1770, 1716, 1645, 1459, 1383, 1281, 1231, 1142, 1044; 1H and 13C NMR: see Table 1; HR-ESIMS m/z 515.2253 [M + Na]+ (calcd. for C26H36O9Na, 515.2257).
4.3.2. Ineupatolide E (2)White amorphous powder; [α]D20 +128.0 (c 0.1 in CHCl3); CD (MeOH) lmax (Δε), nm: 211 (4.11), 227 (3.97), 265 (-2.95); IR (KBr) νmax/cm-1 3443, 2975, 2930, 1770, 1722, 1646, 1458, 1382, 1283, 1231, 1140, 1044; 1H and 13C NMR: see Table 1; HR-ESIMS m/z 401.1569 [M + Na]+ (calcd. for C20H26O7Na, 401.1576).
4.4. Cytotoxicity bioassaysThe isolates were evaluated for their anti-proliferative activity against A431, A549, BGC-823, HL-60, HT-29, and MCF-7 cancer cell lines using the 3-(4, 5-dimethylthiazol-2-yl)-2, 5-diphenyltetrazolium bromide (MTT) [14] and sulforhodamine B [19] methods, respectively. Doxorubicin was used as the positive control.
AcknowledgmentsFinancial support from the National Science and Technology Major Project "Key New Drug Creation and Manufacturing Program" (Nos. 2012ZX09301001-001, 2015ZX09103002), the National Natural Science Funds of China (Nos. 81302657, 81573305, 81473112), Youth Innovation Promotion Association CAS, the Ministry of Science and Technology (No. 2010DFA30980), the Chinese Academy of Sciences (No. KSZD-EW-Z-004-01), the Shanghai Commission of Science and Technology (No. 11DZ1970700, 12JC1410300), and the Research Fund of University of Macau (MYRG2014-00020-ICMS-QRCM and MYRG2015-00153-ICMS-QRCM) is gratefully acknowledged.
Appendix A. Supplementary dataSupplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.cclet.2016.11.011.
| [1] | Editorial Committee of the Administration Bureau of Chinese Plant Medicine, Chinese Flora, Science Press, Beijing, 1979, pp. 248(in Chinese). |
| [2] | Q.L. Guo, J.S. Yang. Sesquiterpenes in Inula L. plants and their pharmacological activities. Nat. Prod. Res. Dev. (2005) 804–809. |
| [3] | Editorial Committee of the Administration Bureau of Chinese Plant Medicine, Chinese Flora, Science Press, Beijing, 1979, pp. 271(in Chinese). |
| [4] | H.G. Xie, H. Chen, B. Cao, H.W. Zhang, Z.M. Zou. Cytotoxic germacranolide sesquiterpene from Inula cappa. Chem. Pharm. Bull. (2007) 1258–1260. |
| [5] | F.Y. Wang, X.Q. Li, Q. Sun, et al., Sesquiterpene lactones from Inula cappa. Phytochem. Lett. (2012) 639–642. |
| [6] | F. Bohlmann, M. Ahmed, J. Jakupovic. Inositol angelates from Inula cappa. Phytochemistry (1982) 780–782. |
| [7] | Z.M. Zou, H.G. Xie, H.W. Zhang, L. Xu. Inositol angelates from the whole herb of Inula cappa. Fitoterapia (2008) 393–394. |
| [8] | J.W. Wu, C.P. Tang, S. Yao, et al., Anti-inflammatory inositol derivatives from the whole plant of Inula cappa. J. Nat. Prod. (2015) 2332–2338. |
| [9] | N.C. Baruah, R.P. Sharma, G. Thyagarajan, W. Herz, S.V. Govindan. New flavonoids from Inula cappa. Phytochemistry (1979) 2003–2006. |
| [10] | Y.L. Wang, Y.J. Li, A.M. Wang, et al., Two new phenolic glycosides from Inula cappa. J. Asian Nat. Prod. Res. (2010) 765–769. |
| [11] | Z.J. Wu, Y.H. Shen, W.D. Zhang. Two new phenolic glycosides from Inula cappa DC. Nat. Prod. Res. (2013) 719–722. |
| [12] | D.K. Kim, N.I. Baek, S.U. Choi, et al., Four new cytotoxic germacranolides from Carpesium divaricatum. J. Nat. Prod. (1997) 1199–1202. |
| [13] | M.R. Kim, B.Y. Hwang, E.S. Jeong, et al., Cytotoxic germacranolide sesquiterpene lactones from Carpesium triste var. manshuricum. Arch. Pharm. Res. (2007) 556–560. |
| [14] | J. Heilmann, M.R. Wasescha, T.J. Schmidt. The influence of glutathione and cysteine levels on the cytotoxicity of helenanolide type sesquiterpene lactones against KB cells. Bioorg. Med. Chem. (2001) 2189–2194. |
| [15] | W. Stöchlin, T.G. Waddell, T.A. Geissman. Circular dichroism and optical rotatory dispersion of sesquiterpene lactones. Tetrahedron (1970) 2397–2409. |
| [16] | D. Youssef, A.W. Frahm. Circular dichroism of C-7, C-6 trans-fused guaianolides of Centaurea scoparia. Phytochemistry (1996) 1107–1111. |
| [17] | R.N. Baruah, R.P. Sharma, G. Thyagarajan, et al., Unusual germacranolides from Inula eupatorioides. J. Org. Chem. (1980) 4838–4843. |
| [18] | Y.L. Lin, J.C. Ou, Y.H. Kuo, et al., four new sesquiterpene lactones from Carpesium nepalense. J. Nat. Prod. (1996) 991–993. |
| [19] | P.A. Skehan, R. Storeng, D. Scudiero, et al., New colorimetric cytotoxicity assay for anticancer-drug screening. J. Natl. Cancer Inst. (1990) 1107–1112. |
 2017, Vol. 28
2017, Vol. 28