b School of Petroleum and Chemical Engineering, Dalian University of Technology, Panjin 124221, China
The development of convenient and efficient methods for synthesis of 5-vinyl-2-norbornene (VNB) has attracted considerable attention because VNB can be easily transformed into 5-ethylidene-2-norbornene (ENB) via a simple isomerization reaction (Scheme 1) [1]. ENB was widely utilized as the best thirdmonomer in the manufacture of ethylene-propylene rubber (EPR) [2]. Over the past decades, some methods have been reported for the synthesis of VNB [1]. From an economic point of view, the Diels–Alder reaction of cyclopentadiene (CPD) with 1, 3-butadiene (BD) has emerged as a valuable method for the synthesis of VNB [1, 3].

|
Download:
|
| Scheme 1. Isomerization of VNB to ENB. | |
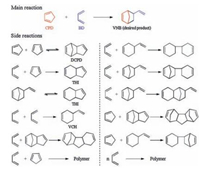
The starting materials CPD and BD can act as both diene and dienophile in Diels–Alder reaction, thereby making the reaction system very complex. Many by-products, including dicyclopentadiene (DCPD), tetrahydroindene (THI), and 4-vinylcyclohexene (VCH), are generated during the Diels–Alder reaction of CPD with BD (Scheme 2). Therefore, a general process for the synthesis of VNB through Diels–Alder reaction always suffers from the drawbacks of low yield and low selectivity. To suppress the formation of these undesired by-products, large amounts of solvents and/or inhibitors are commonly used [3]. Although many efforts have been devoted to the Diels–Alder reaction, the yield and selectivity of VNB remain relatively low. Moreover, the use of inhibitors often results in high additional costs and separation difficulties. Therefore, how to efficiently improve the Diels–Alder reaction for the synthesis of VNB remains an important challenge.
|
Download:
|
| Scheme 2. Main and side reactions. | |
The unique advantages of supercritical carbon dioxide (scCO2), such as low cost, non-flammability, chemical stability, zero surface tension, low toxicity, large diffusion coefficient, and analogous solubility with liquids, have made it an ideal solvent in different fields [4, 5]. In 1988, the Diels–Alder reaction of methyl acrylate with cyclopentadiene in scCO2 was firstly reported by Kim and Johnston [5k]. Subsequently, many Diels–Alder reactions with different substrates, such as the Diels–Alder reaction of isoprene with methyl acrylate [5g], the regioselective Diels–Alder reaction of methyl acrylate with 2-tert-butyl-1, 3-butadiene [5h], and the stereoselective Diels–Alder reaction of cyclopentadiene with a chiral dienophile [5c], have been investigated in scCO2. However, the Diels–Alder reaction of CPD with BD in scCO2 to synthesize VNB has not been reported thus far. In the course of our research on the application of carbon dioxide as a starting material or a solvent [6], we found that the Diels–Alder reaction of CPD with BD can also proceed effectively in scCO2 without any additional inhibitor to provide VNB in satisfactory yield with high selectivity. The results are presented in this paper.
2. Results and discussionIn the initial studies conducted, scCO2 and several traditional solvents including THF, hexane, and methanol were investigated at 205 ℃. The results are summarized in Table 1 (Table 1, entries 1–5). As shown in Table 1, THI was observed as the main by-product in the reaction. Interestingly, the reaction could occur under solventfree conditions (entry 1); the highest conversion of CPD (64%) was achieved in this case. However, large amounts of unidentified compounds were generated (20% total yield) along with the desired product VNB (21% yield). A slightly decreased conversion of CPD and an almost similar yield of VNB were observed when the nonpolar solvent hexane was examined (entry 2: CPD conversion, 59%; VNB yield, 22%). The conversion of CPD and the yields of VNB, THI, and unidentified compounds were all lower than those observed in hexane when the polar solvents THF and methanol were investigated (THF: 43% conversion of CPD, 15% and 18% yields of VNB and THI, respectively; methanol: 24% conversion of CPD, 9% and 10% yields of VNB and THI, respectively). These results indicate that a nonpolar solvent is advantageous to the target product. The scCO2 was finally examined, and the results were shown in entry 5. Up to 47% conversion of CPD and 25% yield of VNB were observed; the main by-product THI and the unidentified by-products were identified in 17% and 6% total yields, correspondingly. Although the conversion of CPD was lower than that found in hexane, a relatively high yield and selectivity of VNB were observed (entry 5, 25% yield and 52% selectivity). Furthermore, the unidentified compounds were generated in relatively low total yield (6%) in scCO2. However, the conversion of CPD and the yields of VNB, THI, and unidentified compounds all decreased when 4-oxo-2, 2, 6, 6-tetramethylpiperidinooxy was used as an inhibitor (entry 6, 34% conversion of CPD, 17% and 13% yields of VNB and THI, respectively). It was considered that the special physical properties of scCO2, such as zero surface tension, large diffusion coefficient, and analogous solubility with liquids, inhibited the occurrence of side reactions.
|
|
Table 1 Diels-Alder reaction of CPD with BD in different solvents.a |
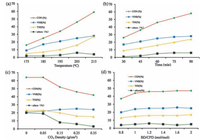
After the unique advantage of scCO2 used in the Diels–Alder reaction between CPD and BD as the reaction medium was demonstrated, some efforts were made to optimize the reaction conditions, including the reaction temperature, reaction time, CO2 density, and BD/CPD molar ratio. The effect of temperature on the reaction is shown in Fig. 1a. The yields of VNB and THI increased with the elevated temperature, but the influence of the temperature on the yield of THI was more obvious than that on VNB yield. For example, THI was obtained in only 7% yield at 185 ℃, which was lower than half of the yield of VNB (17%). However, almost the same yield of THI (27%) was observed as the yield of VNB (28%) when the temperature was increased to 215 ℃. Notably, the amounts of unidentified compounds also increased with the enhanced temperature. Similarly, the conversion of CPD increased with the enhanced temperature. Obviously, the conversion of CPD and the yields of VNB and THI all increased with the enhanced temperature. Considering the effect of temperature on the conversion of CPD and the yields of VNB and THI, 205 ℃ was selected as the reaction temperature.
|
Download:
|
| Figure 1. Influence of different factors on the reaction. Standard reaction conditions: CPD (15 mmol, 0.99 g), BD (18 mmol, 0.97 g) in the presence of 12.5 g CO2 at 205 ℃ for 60 min; all reactions were carried out in 50 mL autoclave. All the GC yields were based on CPD. | |
A series of comparative experiments was conducted to evaluate the influence of reaction time on the conversion and yields, and the results are summarized in Fig. 1b. When the Diels–Alder reaction between CPD and BD was performed at 205 ℃ for a prolonged time (from 30 min to 90 min) in scCO2, both the yield of VNB and the conversion of CPD increased (VNB yield: from 17% to 28%; CPD conversion: from 26% to 58%). The conversion of CPD increased sharply at the initial 60 min, and then the increase slowed down from 60 min to 90 min. This finding indicated that the influence of reaction time on the yield of THI was larger than that on the yield of VNB (VNB yield, from 17% to 28%; THI yield, from 9% to 24%). Moreover, the prolonged reaction time resulted in an increased amount of unidentified compounds. Relatively good results were obtained when the reaction mixture was treated for 60 min.
The next optimization step involved CO2 density variations, as detailed in Fig. 1c. When the reaction of CPD with BD was performed at 205 ℃ for 60 min in the absence of any solvents, the yields of VNB and THI were observed around 20% (VNB yield, 21%; THI yield, 23%). Unidentified compounds were also obtained with 20% total yield under solvent-free conditions. With CO2 density gradually approaching supercritical condition (from 0 g/cm3 to 0.25 g/cm3), the conversion of CPD and the total yield of unidentified compounds declined obviously (conversion of CPD, from 64% to 46%; total yield of unidentified compounds, from 20% to 6%); the yield of VNB increased from 21% to 25%, whereas the yield of THI decreased from 23% to 16%. With further increase of CO2 density from 0.25 g/cm3 to 0.35 g/cm3, the yields of VNB and THI remained unchanged. Meanwhile, the conversion of CPD and the total yield of unidentified compounds decreased slightly (conversion of CPD, from 46% to 42%; total yield of unidentified compounds, from 6% to 3%). Therefore, scCO2 with 0.25 g/cm3 density was selected as solvent in subsequent studies.
To assess the effect of BD/CPD molar ratio on the reaction, we conducted further comparative experiments with BD/CPD molar ratio ranging from 0.8 to 2.0, and the results are shown in Fig. 1d. When the BD/CPD molar ratio increased from 0.8 to 1.2, the conversion of CPD and the yields of VNB and THI simultaneously increased (VNB yield, from 20% to 24%; THI yield, from 13% to 16%; CPD conversion, from 37% to 44%). However, with the continued increase of BD/CPD molar ratio from 1.2 to 2.0, the conversion of CPD and the yields of VNB and THI were not changed. These results indicated that the optimal BD/CPD molar ratio was 1.2.
Because the dimer of CPD, namely dicyclopentadiene (DCPD), should decompose to generate CPD at the selected reaction temperature (205 ℃) in scCO2 medium [7], we attempted to direct use of DCPD in the target Diels–Alder reaction. The results are listed in Table 2. The influence of reaction time and temperature on the conversion of DCPD and the yield of VNB was investigated in scCO2 with 0.25 g/cm3 density. The same optimal conditions were established as established in the Diels–Alder reaction of CPD with BD (scCO2 with 0.25 g/cm3, 60 min, 205 ℃). Almost the same results were obtained (entry 3) compared with those showed in Table 1 (entry 5). These results obtained indicated that DCPD could be directly utilized in the scCO2 medium to simplify the VNB synthesis procedure. To the best of our knowledge, this is the first successful example of direct use of DCPD for synthesis of VNB in a batch reactor.
|
|
Table 2 Direct use of DCPD for synthesis of VNB.a |
3. Conclusion
In conclusion, we have developed a convenient and efficient method to synthesize VNB through the Diels–Alder reaction of CPD with BD in scCO2 for the first time. The Diels–Alder reaction of CPD with BD proceeded smoothly in the absence of any inhibitor to produce VNB in relatively high yield compared with previous methods in a batch reactor (25% yield vs. 11%–16% yield) [3b]. The scCO2 not only acted as a solvent, but also acted as a thinner to suppress the side reactions occurring. The ready availability of starting materials, mild reaction conditions, experimental simplicity, and satisfactory yield should make the present method more useful in VNB synthesis.
4. Experimental 4.1. MaterialsAll the reagents used were commercially available and were purified by distillation at reduced pressure. Carbon dioxide with purity greater than 99.5% was purchased from Dalian Gas Co., Ltd. VNB (AR), THI (AR), and VCH (AR) were supplied by Tokyo Chemical Industry Co., Ltd. (TCI). DCPD (purity > 99.5%) was purchased from Jinlin Petroleum (Group) Co., Ltd. CPD was generated from DCPD via thermal decomposition [8]. Toluene, THF, methanol, and hexane were provided by Tianjin Kemiou Chemical Co., Ltd. GC analysis was performed using an Agilent 7820 equipped with an HP-5 capillary column (see the Supporting information).
4.2. General procedure for Diels–Alder reactionsThe sealed 50 mL autoclave was purged thrice with N2. CPD (0.99 g, 15 mmol), BD (1.0 g, 18 mmol), and CO2 (12.5 g) were then charged into autoclave at -20 ℃. The autoclave was placed in a 205 ℃ oil bath and was maintained constant for 60 min. Upon completion of the desired reaction time, the autoclave was allowed to cool to -20 ℃ in an ethylene glycol bath. The remaining gas was slowly vented, and toluene (0.2 g) was then charged into the autoclave. The remaining liquid in the autoclave was then transferred to a gas chromatography (GC) for further detection. The yields of VNB and THI, as well as the other products, were all calculated based on CPD.
AcknowledgmentsWe are grateful to the National Natural Science Foundation of China (Nos. 21373041, 21372035 and NSFC-IUPAC program, No. 21361140375) for their financial support.
Appendix A. Supplementary dataSupplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.cclet.2016.12.018.
| [1] | Yu.G. Osokin, Vinylnorbornene: preparation, chemical transformations, and use in organic synthesis and polymer chemistry. Vinylnorbornene synthesis and isomerization to ethylidenenorbornene (Review). Pet. Chem. 47 47 (2007) 1–11. DOI:10.1134/S096554410701001X |
| [2] |
(a) P. S. Ravishankar, Treatise on EPDM, Rubber Chem. Technol. 85 (2012) 327– 349; (b) E. K. Easterbrook, R. Allen, Ethylene-propylene Rubber, 1999, pp. 260–283. |
| [3] |
(a) K. Cao, X. Liu, Y. Zhang, et al. , Insights into the Diels-Alder reactions between cyclopentadiene and 1, 3-butadiene with high temperature and high pressure, Ind. Eng. Chem. Res. 54 (2015) 7565–7570; (b) Z. X. Jiang, S. Z. Guo, Z. B. Yang, H. X. Ma, Sequential experimentation of synthesis of vinyl norbornene, Chin. Petrochem. Technol. 32 (2003) 847–852; (c) Z. Zhu, Z. Li, C. Cui, the synthesis method of 5-vinyl-2-norbornene, CN Patent 1580015, 2004. (d) M. Ogawa, Process for producing vinylnorbornene and/or tetrahydroindene, U. S. Patent 4219688, 1980. (e) M. Matsuno, Method for the production of vinyl norbornene, U. S. Patent 4079091, 1978. –2, U. S. Patent 3728406, 1973. (f) C. H. M. A. Vrinssen Cramers, Process for the preparation of 5-vinylnorbornene-2, U. S Patent 3728406 1973. |
| [4] |
(a) E. Reverchon, I. D. Marco, Supercritical fluid extraction and fractionation of natural matter, J. Supercrit. Fluids 38 (2006) 146–166; (b) P. Michel, Supercritical fluid applications: industrial developments and economic issues, Ind. Eng. Chem. Res. 39 (2000) 4531–4535; (c) A. Baiker, Supercritical fluids in heterogeneous catalysis, Chem. Rev. 99 (1999) 453–473; (d) P. G. Jessop, T. Ikariya, R. Noyori, Homogeneous catalysis in supercritical fluids, Chem. Rev. 99 (1999) 475–493. |
| [5] |
(a) E. J. Beckman, Supercritical and near-critical CO2 in green chemical synthesis and processing, J. Supercrit. Fluids 28 (2004) 121–191; (b) J. Qian, M. T. Timko, A. J. Allen, et al. , Solvophobic acceleration of Diels–Alder reactions in supercritical carbon dioxide, J. Am. Chem. Soc. 126 (2004) 5465–5474; (c) S. Fukuzawa, K. Metoki, S. Esumi, Asymmetric Diels–Alder reactions in supercritical carbon dioxide catalyzed by rare earth complexes, Tetrahedron 59 (2003) 10445–10452; (d) E. M. Glebov, L. G. Krishtopa, V. Stepanov, L. N. Krasnoperov, Kinetics of a Diels– Alder reaction of maleic anhydride and isoprene in supercritical CO2, J. Phys. Chem. A 105 (2001) 9427–9435; (e) R. Lee Thompson, R. Gla1ser, D. Bush, C. L. Liotta, C. A. Eckert, Rate variations of a hetero-Diels-Alder reaction in supercritical fluid CO2, Ind. Eng. Chem. Res. 38 (1999) 4220–4225; (f) S. K. I. M. Roberts, Supercritical and sub-critical fluid solvent effects on a Diels– Alder reaction, Chem. Eng. Comm. 171 (1999) 117–134; (g) B. Lin, A. Akgerman, Isoprene/methyl acrylate Diels–Alder reaction in supercritical carbon dioxide, Ind. Eng. Chem. Res. 38 (1999) 4525–4530; (h) A. R. Renslo, R. D. Weinstein, J. W. Tester, R. L. Danheiser, Concerning the regiochemical course of the Diels-Alder reaction in supercritical carbon dioxide, J. Org. Chem. 62 (1997) 4530–4533; (i) R. D. Weinstein, A. R. Renslo, R. L. Danheiser, J. G. Harris, J. W. Tester, Kinetic correlation of Diels–Alder reactions in supercritical carbon dioxide, J. Phys. Chem. 100 (1996) 12337–12341; (j) Y. Ikushima, N. Saito, Supercritical carbon dioxide as reaction medium: examination of its solvent effects in the near-critical region, J. Phys. Chem. 96 (1992) 2293–2297; (k) S. Kim, K. P. Johnston, Adjustment of the selectivity of a Diels–Alder reaction network using supercritical fluids, Chem. Eng. Comm. 63 (1988) 49–59. |
| [6] |
(a) J. Song, X. Feng, Y. Yamamoto, et al. , Selective synthesis of d-lactone via palladium nanoparticles-catalyzed telomerization of CO2 with 1, 3-butadiene, Tetrahedron Lett. 57 (2016) 3163–3166; (b) J. Sun, M. Bao, X. Feng, et al. , Carboxylative coupling reaction of fivemembered (chloromethyl) heteroarenes with allyltributylstannane catalyzed by palladium nanoparticles, Tetrahedron Lett. 56 (2015) 6747–6750; (c) X. Feng, A. Sun, S. Zhang, X. Yu, M. Bao, Palladium-catalyzed carboxylative coupling of benzyl chlorides with allyltributylstannane: remarkable effect of Palladium nanoparticles, Org. Lett. 15 (2013) 108–111; (d) Y. Dai, X. Feng, B. Wang, R. He, M. Bao, Preparation and application of airstable P, N-bidentate ligands for the selective synthesis of d-lactone via the palladium-catalyzed telomerization of 1, 3-butadiene with carbon dioxide, J. Organometals Chem. 696 (2012) 4309–4314; (e) X. Feng, M. Yan, X. Zhang, M. Bao, The SBA-15/SO3H nanoreactor as a highly efficient and reusable catalyst for diketene-based, four-component synthesis of polyhydroquinolines and dihydropyridines under neat conditions, Chin. Chem. Lett. 22 (2011) 643–646; (f) X. Feng, M. Yan, T. Zhang, Y. Liu, M. Bao, Preparation and application of SBA-15-supported palladium catalyst for Suzuki reaction in supercritical carbon dioxide, Green Chem. 12 (2010) 1758–1766. |
| [7] | The dicyclopentadiene (DCPD) will decompose to generate cyclopentadiene (CPD) at a temperature higher than its boiling point (170 ℃). |
| [8] | M. Korach, D.R. Nielsen, W.H. Rideout, Dihydroxycyclopentene. Org. Synth 42 (1962) 50–54. DOI:10.15227/orgsyn.042.0050 |
 2017, Vol. 28
2017, Vol. 28