The 4, 4-difluoro-4-bora-3a, 4a-diaza-s-indacenes (BODIPY) dyes, known as the "little sister" of porphyrins because of the similar dipyrromethene backbone they possess [1, 2], have gained much attention in the field of labeling, liquid-crystalline materials, chemical sensors, electroluminescence, energy transfer cassettes and dye-sensitized solar cells recently [3-12]. The wide range of the applications of these dyes have a very close relationship with their excellent properties, such as facile functionalization of their backbones, outstanding spectroscopic properties with maximum molar extinction coefficient (εmax) larger than 80 ×103 L mol-1 cm-1 and quantum yield (ΦF) larger than 0.5, insensitive to the solvent polarity, together with excellent thermal and chemical stability [13-16]. Due to the advantages aforementioned as well as the suitable frontier energy levels of the BODIPY core, it has been regarded as promising candidate as electron-deficient group in the regime of organic photovoltaic in the past decade. By rationally tailoring the structures, such as extending the p-system by attaching or fusing electron-donating aromatic building blocks at the α and β positions, reinforcing intermolecular interaction by modifying the fragments at the meso position, power conversion efficiency (PCE) ofnear5%havebeenrealized[17-27].Veryrecently, the BODIPYshavebeen usedas the building blocks toconstruct nonfullerene acceptors and a moderate PCE of 1.5% has been achieved when blend with P3HT [28].
In this work, we concentrate on the structural modification of the BODIPY-based dimers with the goal of effectively modulating the energy levels, absorption as well as the optoelectronic properties in the solid. To realize this, we synthesized a series of novel BODIPY based dyes (Ph (m-BODIPY)2, T (m-BODIPY)2, BDT (Tm-BODIPY)2, BDT (β-metBODIPY)2 and BDT (β-metBODIPY)2 (Fig. 1)), differing in the structure of the π-bridges and the positions they link to the BODIPY core as well as the substitutions on the BODIPY core. Our results imply that the π-bridges at the meso position have just slight effects on the photophysical properties of the molecules regardless of their electron-donor abilities and sizes. However, they do have significant effects on the aggregation behaviors in solid and optoelectronic properties in bulk heterojunction solar cells. Besides, variation of the positions bridges linked to the terminal BODIPY motifs and the dihedral angle between them have dramatically influences not only on the photophysical and electrochemical properties, but on the packing behaviors in the solid state, thus the optoelectronic properties of the molecules.

|
Download:
|
| Figure 1. Chemical structure of five BODIPY based dyes | |
2. Results and discussion
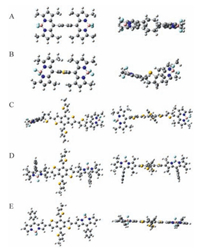
Fig. 2 shows the optimal conformations of the five molecules at the ground state simulated by Guassian 09 program at the B3LYP/ 6-31G level. In the cases of Ph (m-BODIPY)2, T (m-BODIPY)2, and BDT (T-m-BODIPY)2, the dihedral angles between the bridge units and the BODIPY planes are larger than 70°. The twisted arrangement between the bridge units and the terminal BODIPY motifs is independence of the structures of the bridges. Compared with BDT (T-m-BODIPY)2, the dihedral angle between the BODIPY and 4, 8-bis (5-(2-ethylhexyl) thiophen-2-yl) benzo[1, 2-b: 4, 5-b'] dizhiophene (BDT) plane reduce to 50° in the case of BDT (β-metBODIPY)2, implying better planarity can be achieved when the BDT bridge is selected to link at BODIPY's β position. Moreover, the comparison between BDT (β-metBODIPY)2 and BDT (β-BODIPY)2 reveals that the severely steric congestion can be released further when the four methyl groups on the BODIPY plane are replaced by H atoms. Therefore, the geometries of the targeted molecules can be tuned by a series of structural evolution, thus modulating the photo-physical and optoelectronic properties consequently.
|
Download:
|
| Figure 2. The optimal geometries of the five BODIPY derivatives of Ph (m-BODIPY)2 (A), T (m-BODIPY)2 (B), BDT (T-m-BODIPY)2 (C), BDT (β-metBODIPY)2 (D) and BDT (β-BODIPY)2 (E) using Gaussian 09 program. | |
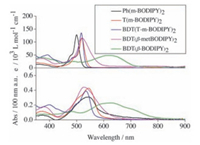
Fig. 3 depicts the absorption spectra of the five molecules both in dilute CH2Cl2 solution and in the solid state. The relevant data are summarized in Table 1. Obviously, the absorption spectra of the five molecules in the solution cover a wide range of the visual light region from 400 nm to 700 nm in solution. Specifically, for Ph (mBODIPY)2, T (m-BODIPY)2, and BDT (T-m-BODIPY)2, they all exhibit similar narrow Gaussian-shaped S0→S1 absorption bands with the λmax at about 500 nm and εmax larger than 100 ×103 L mol-1 cm-1, besides, a pronounced shoulder peak on the high-energy side of the main peak can also be observed, which is attributed to the vibrational transition [14, 29]. Their optical phenomena are similar to those of BODIPY monomer, which implies the poor electronic communication between the π-bridges and the BODIPY planes due to their highly twisted conformations. However, in contrast, the absorption spectrum of BDT (β-metBODIPY)2 is broader and the bandedge red-shifts significantly compared with the meso linked counterpart (BDT (T-m-BODIPY)2) although its λmax red-shifts only a little (520 nm vs. 517 nm), this result indicates that when BDT group is selected to link the BODIPY motifs at their β positions (2, 6-positions) directly, it can conjugate with the BODIPY planes to some degree, despite that the existing of the methyl groups on the pyrrole rings deteriorate the electronic interaction between the bridge and the BODIPY cores, which is consistent with the theoretical results. Furthermore, as the steric hindrance is removed effectively when the methyl groups are removed, a broad intramolecular charge-transfer (ICT) peak which results from the strong donor-acceptor (D-A) interaction between the BDT bridge and the BODIPY moieties, with a εmax of 50 ×103 L mol-1 cm-1 appears at about 617 nm in BDT (β-BODIPY)2. When spuncast into thin films, their absorption spectra all red-shift and broaden compared with the solution counterparts due to the molecular aggregation and interaction in the solid [30, 31]. Moreover, for BDT (β-BODIPY)2, a shoulder peak could clearly be seen at 700 nm, which is related to better π-stacking in the solid due to its more planar conformation than BDT (β-metBODIPY)2. The optical band gaps are calculated to be 2.09, 2.11, 2.19, 1.99 and 1.54 eV for Ph (m-BODIPY)2, T (m-BODIPY)2, BDT (T-m-BODIPY)2, BDT (β-metBODIPY)2 and BDT (β-metBODIPY)2 respectively, according the formula: Egopt = 1240/λedge [32]. Here, λedge refers to the onset values of the absorption spectra of the molecules in the thin films.
|
Download:
|
| Figure 3. Absorption spectra of the five dimers with concentration of 10-6 mol L-1 in CH2Cl2 solution (upper) and in the thin films (lower). | |
|
|
Table 1 A summary of the optical and electrochemical properties of the five molecules. |
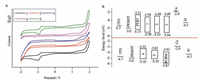
Cyclic voltammetry (CV) was applied to investigate the electrochemical properties of the five small molecules. The corresponding CV traces are plotted in Fig. 4A. According to the onset of the oxidation and reduction potentials as well as the empirical equations: EHOMO =-e(4.4 + Eox); ELUMO =-e(4.4 + Ered), the highest occupied molecular orbital (HOMO) and the lowest unoccupied molecular orbital (LUMO) energy levels are estimated to be-5.50 and-3.59 eV, -5.53 and-3.49 eV, -5.54 and-3.49 eV, -5.10 and-3.32 eV, and-5.10 and-3.62 eV for Ph (m-BODIPY)2, T (m-BODIPY)2, BDT (T-m-BODIPY)2, BDT (β-metBODIPY)2 and BDT (β-BODIPY)2, respectively. The electrochemical band gaps of Ph (mBODIPY)2, T (m-BODIPY)2, BDT (T-m-BODIPY)2, BDT (β-metBODIPY)2 and BDT (β-BODIPY)2, are calculated to be 1.91, 2.04, 2.05, 1.78 and 1.48 eV, respectively, according to the equation: Egcv = ELUMO-EHOMO, these values are well agree with the corresponding Egopt values mentioned above. Judging from the electrochemical data, one can find that the substitutions at the meso position has very slight impact on the frontier orbital energies, thus leading to comparable bandgaps for Ph (m-BODIPY)2, T (m-BODIPY)2 and BDT (T-m-BODIPY)2. When the two BODIPY monomers are selected to link at their 2, 6-positions via the same π-bridges, the HOMO energies rise up conspicuously (about 0.4 eV) in the case of BDT (β-metBODIPY)2 and BDT (β-BODIPY)2. Interestingly, the planar BDT (β-metBODIPY)2 possesses much lower LUMO energy (-3.62 eV) than the twisted BDT (β-metBODIPY)2 (-3.32 eV). All the modulations of the frontier energies resulting from the structural modification leads to the decrease of the bandgap value from BDT (T-m-BODIPY)2 (2.05 eV) to BDT (β-BODIPY)2 (1.48 eV).
|
Download:
|
| Figure 4. (A) CV curves of the five molecules in dilute CH2Cl2 solutions; (B) The energy diagram of the five molecules, PTB7, PC71BM as well as other components used in the normal solar cells for comparisons. For clarity, from 1 to 5 represent Ph (m-BODIPY)2, T (m-BODIPY)2, BDT (T-m-BODIPY)2, BDT (β-metBODIPY)2 and BDT (β-BODIPY)2 here, respectively. | |
Given to the appropriate energy levels of the five small molecules (Fig. 4B), solar cells with the structure of ITO/poly (3, 4-ethylenedioxythiophene):poly (styrenesulfonate) (PEDOT:PSS)/PTB7:Ph (m-BODIPY)2 (or T (m-BODIPY)2 and BDT (T-m-BODIPY)2)/Ca (20 nm)/Al (80 nm) and ITO/poly (3, 4-ethylenedioxythiophene): poly (styrenesulfonate) (PEDOT:PSS)/PC71BM: BDT (β-metBODIPY)2 (or BDT (β-metBODIPY)2)/Ca (20 nm)/Al (80 nm) were constructed to evaluate Ph (m-BODIPY)2, T (m-BODIPY)2 and BDT (T-m-BODIPY)2 as the acceptor materials and BDT (β-metBODIPY)2 and BDT (β-BODIPY)2 functioning as the donor materials. Poly[[4, 8-bis[(2-ethylhexyl) oxy]benzo[1, 2-b:4, 5-b']dithiophene-2, 6-diyl]]3-fluoro-2-[(2-ehtylhexyl) carbonyl]-thieno[3, 4-b]thiophenediyl]] (PTB7) was employed as the donor here not only because of its appropriate HOMO and LUMO energy but its excellent light capturing ability from 550 nm to 700 nm [33]. Unfortunately, Ph (m-BODIPY)2 exhibits poor solubility even in dilute CHCl3, consequently, no perfect films can be obtained from the solution mixture of PTB7: Ph (m-BODIPY)2. Different conditions, including processing solution, donor: acceptor weight ratio, concentration, the amount of additive and thermal annealing were optimized to achieve the best performances for the others. During these procedures, we found that all the systems exhibited best performances when using o-DCB as the processing solvent with a D:Aweight ratio of 1:1. Commonly used solvent additive such as DIO and 1-CN were used to optimize the performances of the devices further, however, no positive effect was observed, unfortunately. The best photovoltaic parameters for each molecule are given in Table 2. The corresponding J-V curves are displayed in Fig. 5 as well. Compared with the negligible PCE of PTB7: BDT (T-m-BODIPY)2 based system ( < 0.04%), PTB7: T (m-BODIPY)2 based one exhibits higher Jsc (0.10 vs. 0.72 mA cm-2) and an extremely high Voc of 1.12 V is also achieved, to the best of our knowledge, this Voc is the highest value reported from PTB7 based solar cells andfinally, a PCE of 0.24% is reached. The similar Voc values of BDT (β-metBODIPY)2: PC71BM and BDT (β-BODIPY)2: PC71BM based devices are in accordance with their close HOMO energy. However, BDT (β-metBODIPY)2 based devices exhibit lower Jsc and FF than BDT (β-BODIPY)2 based one. On the one hand, the absorption band of BDT (β-metBODIPY)2 covers only a narrow region from 450 nm to 600 nm while that of BDT (β-metBODIPY)2 extends even to 800 nm, which contributes to the full use of the sunlight, since about 50% of the solar photons have energies corresponding to a wavelength of 600-1000 nm, on the other, the presence of the tilt angles between the BODIPY planes and BDT plane hinders the molecules from packing orderly in the solid state. As it is known that the packing ordering of the molecules in the solid has a close correlation with the transportation of the charge carriers, thus the Jsc and the PCE of the solar cells [34, 35].
|
Download:
|
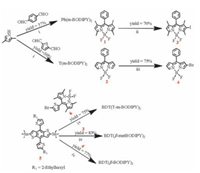
| Figure 5. Synthesis routines of the dimers. Reaction conditions: (ⅰ) TFA, DDQ, (iPr)2EtN, BF3-Et2O, N2, CH2Cl2, yield: 37% and 30% for Ph (m-BODIPY)2 and T (mBODIPY)2, respectively; (ⅱ) NIS, CH2Cl2, yield: 70%; (ⅲ) Br2, CH2Cl2, yield: 75%; (ⅳ) Pd (PPh3)4, N2, 120 ℃, toluene, 12 h, yield: 75%, 83% and 77% for BDT (T-m-BODIPY)2, BDT (β-metBODIPY)2 and BDT (β-BODIPY)2, respectively. | |
|
|
Table 2 Performances of the five molecules based solar cells under optimal conditions. |
3. Conclusion
A series of BODIPY-based dimers have been synthesized and characterized. Our results clearly demonstrate that the modification at the meso position has only slight effect on the absorption and electrochemical properties of the dimers, their aggregation behaviors and optoelectronic behaviors can be modulated effectively, however. The thiophene π-bridges one gives superior performances to other analogues when blended with PTB7 in organic solar cells. The Voc of 1.12 V is among the highest Voc values in single-junction solution-processed organic solar cells. The modulation of the photophysical behaviors has also been realized by changing the positions the π-bridges link to the BODIPY cores and the dihedral angle between them. The reduction of the dihedral angle from BDT (β-metBODIPY)2 to BDT (β-BODIPY)2 not only improves the conjugation, brings down the LUMO energy and red-shifts the absorption spectrum, but facilitates the ordered stacking of BDT (β-BODIPY)2 in the solid, thus leading to an improvement of light-harvesting ability and charge carriers transformation behaviours, which are corresponding to the enhancement of the performance of the solar cells from 0.4% to about 1%. All the results reveal that the modulation of the photophysical properties of BODIPY dyes is achievable by judicious structural modification, and BODIPY dyes are promising candidates for application in organic solar cells, functioning as both the donor and the acceptor materials.
4. Experimental 4.1. MaterialsAll the materials used were received from commercial sources (Acros, Sigma, TCI or Stream) and were used without further purification. Toluene was distilled from benzophenone ketyl under the protection of nitrogen prior to use in order to get rid of the trace amount of water. Compounds 1, 2 and 6 were synthesized according to the reported literatures [21, 36, 37]. 2, 6-Bis (trimethyltin)-4, 8-bis (5-(2-ethylhexyl) thiophen-2-yl) benzo[1, 2-b: 4, 5-b'] dizhiophene (BDT-2Sn, compound 3) were purchased from Suna Tech Inc and used as received.
4.2. General characterization methodsNMR spectra were measured on a Brucker AVANCE 300 MHz or 400 MHz, the samples were dissolved in chloroform-d or dichloromethane-d2.
Mass spectra (MALDI-TOF-MS) were conducted on a Bruker BIFLEX Ⅲ mass spectrometer (Matrix: CCA). Elemental analysis was conducted on a flash EA1112 analyser to analyse the content of C, H, and N, respectively.
UV-vis absorption spectra were recorded on Schimadzu UV 2600 absorption spectrometer. The concentration of the solutions used here was 10-6 mol L-1, and the solid samples were prepared by spin-casting the solution on the quartzes. Cycle voltammetry (CV) was measured on a computer-controlled Zennium electrochemical workstation at a scan rate of 100 mV s-1. A glassy carbon electrode, a Pt wire and a Ag/AgCl electrode were used as the working, counter and reference electrodes, respectively. The molecules were dissolved in degassed anhydrous CH2Cl2 to achieve a concentration of 10-4 mol L-1 with 0.1 mol L-1 tetra-n-butylammonium hexafluorophosphate (Bu4NPF6) as the supporting electrolyte.
4.3. Synthetic procedures (The routines are depicted in Fig. 5)Synthesis of Ph (m-BODIPY)2: To a well-dried two neck round bottle with 100 mL CH2Cl2, 2, 4-dimethylpyrrole (1.6 mL, 15.5 mmol) and terephthalaldehyde (519.9 mg, 3.88 mmol) were charged. After N2 was purged for 1 h, a small amount of trifluoroacetic acid (TFA) was added, and the solution turned purple quickly. After one hour, 2, 3-dichloro-5, 6-dicyano-1, 4-benzoquinone (1.76 g, 7.76 mmol) was added at once. After 2 h, (i-Pr)2EtN (15 mL) was added to the reaction mixture followed by BF3-Et2O (15 mL) immediately. The reaction was quenched by adding 100 mL water 1 h later. The mixture was extracted with CH2Cl2 and the organic phase was collected and evaporated under vacuum. Finally, the crude product was purified by column chromatography (eluent: CH2Cl2/petroleum = 3/2) to give a red solid as desired product (818.6 mg, yield: 37%). 1H NMR (300 MHz, CDCl3): δ 7.52 (s, 4H), 6.01 (s, 4H), 2.58 (s, 12H), 1.55 (s, 12H). 13C NMR (75 MHz, CDCl3): δ 155.4, 143.0, 142.1, 135.1, 131.5, 128.0, 121.1, 14.6, 14.3. MS (MALDI-TOF): calculated for 570.27, found m/z 570.2 (M+). Elemental anal. calcd. for C32H32B2F4N4 (%): C, 67.40; H, 5.66; N, 9.83. Found (%) C, 67.58; H, 5.46; N, 9.85.
Synthesis of T (m-BODIPY)2: The procedure was similar to that of Ph (m-BODIPY)2, except that thiophene-2, 5-dicarbaldehyde (543.8 mg, 3.88 mmol) was used instead of terephthalaldehyde. A reddish solid (670.7 mg, yield: 30%) was gotten after purification. 1H NMR (300 MHz, CDCl3): δ 7.16 (s, 2H), 6.05 (s, 4H), 2.56 (s, 12H), 1.88 (s, 12H). 13C NMR (75 MHz, CDCl3): δ 156.1, 143.4, 134.9, 133.9, 132.3, 127.4.121.6, 14.6, 13.5. MS (MALDI-TOF): calcd. for 576.23, found m/z 576.1 (M+). Elemental anal. calcd. for C30H30B2F4N4S (%): C, 62.53; H, 5.25; N, 9.72. Found (%) C, 62.58; H, 5.16; N, 9.75.
Synthesis of compound 3: N-Iodo-succinimid (NIS) (300 mg, 1.33 mmol) was dissolved in 30 mL anhydrous CH2Cl2, then the solution was added dropwise into the solution of compound 1 (429.0 mg, 1.33 mmol) in CH2Cl2. After the reaction was completed, water (50 mL) was added, then the organic layer was extracted by CH2Cl2, the organic phase was evaporated to dry under vacuum. The crude material was purified by silica gel column chromatography (eluent: CH2Cl2/petroleum = 1/2). A red solid was collected after purification. (419.0 mg, yield: 70%). 1H NMR (300 MHz, CDCl3): δ 7.49-7.51 (m, 3H), 7.25-7.27 (m, 2H, overlapped with CDCl3), 6.04 (s, 1H), 2.64 (s, 3H), 2.57 (s, 3H), 1.38 (s, 6H).
Synthesis of compound 4: Liquid bromine (1 mL, excess) was dissolved in 50 mL anhydrous CH2Cl2, then the solution was added dropwise slowly into the solution of compound 2 (804.2 mg, 3.00 mmol) in CH2Cl2 (100 mL). The reaction was monitored by TLC every 30 min, after all the compound 2 was consumed, the organic layer was washed with aqueous solution of sodium thiosulfate, then the organic layer was extracted with CH2Cl2, the organic phase was collected and evaporated to dry by a rotator evaporation equipment. Purification was conducted by silica gel column chromatography (eluent: CH2Cl2/petroleum = 2/5) to afford a red solid (780.7 mg, yield: 75%) as the product. 1H NMR (400 MHz, CDCl3): δ 7.94 (s, 1H), 7.73 (s, 1H), 7.51-7.57 (m, 1H), 7.45-7.49 (m, 4H), 6.93-6.94 (d, J = 4 Hz, 1H), 6.82 (s, 1H), 6.53-6.54 (d, 1H, J = 4 Hz).
Synthesis of BDT (T-m-BODIPY)2: Compound 5 (180.9 mg, 0.20 mmol) and compound 6 (204.5 mg, 0.50 mmol), and 10 mL anhydrous toluene was added into a dry Schlenk tube. After purged with argon for 30 min, Pd (PPh3)4 (20 mg, 0.017 mmol) was charged. Then the mixture was heated at 110 ℃ for 12 h. After cooling to room temperature, the mixture was poured into water and extracted with CH2Cl2 three times. The organic phase was collected and washed with brine and water and then dried over Na2SO4. The residue was then loaded on silica gel column chromatography (eluent: CH2Cl2/petroleum = 1/1) and recrystallized from EtOH/ CHCl2 (1/10, v/v) to produce BDT (T-m-BODIPY)2 as a reddish powder (185.2 mg, 75%). 1H NMR (400 MHz, CD2Cl2): δ 7.63 (s, 2H), 7.25-7.27 (m, 4H), 6.87-6.90 (m, 4H), 5.98 (s, 4H), 2.79-2.82 (m, 4H), 2.43 (s, 12H), 1.67 (s, 12H), 1.60-1.64 (m, 2H), 1.30-1.40 (m, 16H), 0.77-0.89 (m, 12H). 13C NMR (100 MHz, CDCl3): δ 155.9, 145.6, 143.6, 143.3, 142.7, 138.0, 137.3, 134.4, 134.0, 132.6, 131.4, 127.5, 127.2, 125.6, 122.3, 121.1, 118.6, 116.4, 41.7, 34.4, 32.6, 29.1, 26.0, 23.2, 14.6, 14.1, 13.6, 10.9. MS (MALDI-TOF): Calcd. for 1234.42, found m/z 1234.0 (M+). Elemental anal. calcd. for C68H72B2F4N4S6 (%): C, 66.11; H, 5.87; N, 1.75. Found (%) C, 66.02; H, 6.00; N, 1.81.
Synthesis of BDT (β-metBODIPY)2: The synthetic procedure was similar to that of BDT (T-m-BODIPY)2, except that compound 3 (225.0 mg, 0.50 mmol) was used instead of compound 6. Purification on silica gel column chromatography (eluent: CH2Cl2/ petroleum = 2/3) following by recrystallization from a mixture of EtOH/CH2Cl2 (10/1, v/v) yielded BDT (β-metBODIPY)2 as a brownreddish powder (203.1 mg, 83%). 1H NMR (300 MHz, CDCl3): δ 7.46-7.48 (m, 6H), 7.35 (s, 2H), 7.28-7.31 (m, 4H, overlapped with CDCl3), 7.24-7.28 (m, 2H, overlapped with CDCl3), 6.83-6.84 (d, 2H, J = 3.3 Hz), 6.03 (s, 2H), 2.81-2.83 (d, 4H), 2.63 (s, 6H), 2.59 (s, 6H), 1.63-1.65 (m, 2H), 1.41 (s, 6H), 1.39 (s, 6H), 1.26-1.32 (m, 16H), 0.88-0.94 (m, 12H). 13C NMR (100 MHz, CDCl3): δ 156.2, 154.1, 145.2, 143.9, 143.4, 142.9, 142.1, 140.3. 137.9, 137.4, 135.4, 132.2, 130.8, 129.4, 129.1, 128.1, 127.9, 126.6, 125.5, 122.8, 121.7, 41.7, 34.2, 32.6, 29.0, 25.8, 23.2, 14.9, 14.6, 14.0, 13.5, 12.6, 10.9. MS (MALDITOF): calculated for 1222.51, found m/z 1222.0 (M+). Elemental anal. calcd. for C72H76B2F4N4S6 (%): C, 70.69; H, 6.26; N, 4.58. Found (%): C, 70.62; H, 6.30; N, 4.35.
Synthesis of BDT (β-BODIPY)2: The synthetic procedure was similar to that of BDT (T-m-BODIPY)2, except that compound 4 (182.5 mg, 0.50 mmol) was used instead of compound 6. Purification on silica gel column chromatography following by recrystallization from a mixture of EtOH/CH2Cl2 (8/1, v/v) yielded BDT (β-BODIPY)2 as a blue-black powder (171.1 mg, 77%). 1H NMR (300 MHz, CDCl3): δ 8.28 (s, 2H), 7.98 (s, 2H), 7.54-7.64 (m, 12H), 7.27 (s, 2H, overlapped with CDCl3), 6.91-6.98 (m, 6H), 6.57-6.58 (d, 2H, J = 2.7 Hz), 2.87-2.89 (d, 4H), 1.69-1.73 (m, 2H), 1.27-1.47 (m, 16H), 0.81-0.99 (m, 12H). 13C NMR (100 MHz, CDCl3): δ 147.3, 146.4, 145.2, 144.3, 143.1, 142.9, 142.1, 138.1, 137.9, 135.6, 134.5, 133.8, 132.0, 131.0, 130.9, 130.6, 128.2, 127.4, 125.9, 125.3, 122.9, 120.2, 41.8, 34.2, 32.6, 29.1, 25.9, 23.3, 14.0, 11.0. MS (MALDI-TOF): Calcd. for 1111.06, found m/z 1110.9 (M+). Elemental anal. calcd. for C64H60B2F4N4S4 (%): C, 69.18; H, 5.44; N, 5.04. Found (%): C, 69.22; H, 5.36; N, 5.15.
4.4. Fabrication and characterization of the organic solar cellsConventional organic solar cells with an architecture of: ITO/ poly (3, 4-ethylenedioxythiophene):poly (styrenesulfonate) (PEDOT:PSS)/active layer/Ca (20 nm)/Al (80 nm) were fabricated. The indium tin oxide (ITO) glasses were cleaned with detergent, deionized water, acetone and isopropanol and then treated in a Novascan PSD-ultraviolet-ozone chamber for 1 hour and a layer of 30 nm PEDOT: PSS (Baytron P VP AI 4083, Germany) was spincoated subsequently. After baking at 150 ℃ for 15 min in the air, the glasses were transferred into a glove box. Then a blend solution (1, 2-dichlorobenzene (o-DCB), chlorobenzene (CB) or chloroform (CF)) of PTB7:Ph (m-BODIPY)2 (orT (m-BODIPY)2 and BDT (T-mBODIPY)2) or BDT (β-metBODIPY)2 (or BDT (β-metBODIPY)2): PC71BM with various concentrations was spun-cast to form the photosensitive layer, donor/acceptor weight ratio, contents of 1, 8-diiodooctance (DIO) (or 1-chloronaphthalene (1-CN)), thermal annealing temperatures were optimized to achieve the best photovoltaic responses. The solutions were stirred at 60 ℃ overnight prior to use. The Ca/Al cathode was deposited by vacuum evaporation onto the photosensitive layer. The effective area was measured to be 6mm2. The current-voltage (J-V) measurement of the devices was measured using a Keithley 2400 Source Measure Unit in the glove box under white light illumination of simulated AM 1.5G, 100mWcm-2 using a xenon-lamp-based solar simulator (AAA grade, XES-70S1).
AcknowledgmentsThe authors gratefullyacknowledge the financial support of the National Natural Science Foundation of China (NSFC, Nos. 91433202, 91227112 and 21221002) and Chinese Academy of Sciences (CAS, No. XDB12010200).
| [1] | J. Q. Feng, B. L. Liang, D. L. Wang, L. Xue, X. Y. Li, Novel fluorescent dyes with fused perylene tetracarboxlic diimide and BODIPY analogue structures, Org. Lett. 10 (2008)4437-4440. |
| [2] | G. Ulrich, R. Ziessel, A Harriman. The chemistry of fluorescent Bodipy dyes: versatility unsurpassed. Angew.Chem.Int.Ed. 47 (2008) 1184–1201. DOI:10.1002/(ISSN)1521-3773 |
| [3] | A. Kamkaew, K Burgess. Aza-BODIPY dyes with enhanced hydrophilicity. Chem.Commun. 51 (2015) 10664–10667. DOI:10.1039/C5CC03649F |
| [4] | S. Mula, S. Frein, V. Russo, et al., Red and blue liquid-crystalline borondipyrromethene dendrimers. Chem.Mater. 27 (2015) 2332–2342. DOI:10.1021/cm503577y |
| [5] | Y. Zhang, Y.G. Gao, Y.D. Shi, et al., . Chin.Chem.Lett. 26 (2015) 894–898. DOI:10.1016/j.cclet.2015.05.032 |
| [6] | P.C. Shi, X.D. Jiang, R.N. Gao, Y.Y. Dou, W.L Zhao. Synthesis and application of Vis/NIR dialkylaminophenylbuta-1, 3-dienyl borondipyrromethene dyes. Chin. Chem.Lett. 26 (2015) 834–838. DOI:10.1016/j.cclet.2014.11.010 |
| [7] | S.L. Zhu, J.T. Zhang, J. Janjanam, et al., Highly water-soluble BODIPY-based fluorescent probes for sensitive fluorescent sensing of zinc (â…¡). J.Mater.Chem. B 1 (2013) 1722–1728. DOI:10.1039/c3tb00249g |
| [8] | ${referAuthorVo.mingEn} K.Gräf, ${referAuthorVo.mingEn} T.Körzdörfer, ${referAuthorVo.mingEn} S.Kümmel, M Thelakkat. Synthesis of donor-substituted meso-phenyl and meso-ethynylphenyl BODIPYs with broad absorption. New J. Chem. 37 (2013) 1417–1426. DOI:10.1039/c3nj00157a |
| [9] | C.J. Qin, A. Mirloup, N. Leclerc, et al., Molecular engineering of new thienyl-Bodipy dyes for highly efficient panchromatic sensitized solar cells. Adv. Energy Mater. 4 (2014) 1400085. DOI:10.1002/aenm.201400085 |
| [10] | S. Kolemen, Y. Cakmak, ${referAuthorVo.mingEn} S.Erten-Ela, et al., Solid-state dye-sensitized solar cells using red and near-IR absorbing Bodipy sensitizers. Org.Lett. 12 (2010) 3812–3815. DOI:10.1021/ol1014762 |
| [11] | N. DiCesare, J.R Lakowicz. Fluorescent probe for monosaccharides based on a functionalized boron-dipyrromethene with a boronic acid group. Tetrahedron Lett. 42 (2001) 9105–9108. DOI:10.1016/S0040-4039(01)02022-6 |
| [12] | J. Karolin, L. B. A. Johansson, L. Strandberg, T. Ny, Fluorescence and absorption spectroscopic properties of dipyrrometheneboron difluoride (BODIPY) derivatives in liquids lipid membranes, and proteins, J. Am. Chem. Soc. 116 (1994)7801-7806. |
| [13] | A. Loudet, K Burgess. BODIPY dyes and their derivatives:syntheses and spectroscopic properties. Chem.Rev. 107 (2007) 4891–4932. DOI:10.1021/cr078381n |
| [14] | H. Lu, J. Mack, Y.C. Yang, Z Shen. Structural modification strategies for the rational design of red/NIR region BODIPYs. Chem.Soc.Rev. 43 (2014) 4778–4823. DOI:10.1039/c4cs00030g |
| [15] | R. Ziessel, G. Ulrich, A Harriman. The chemistry of Bodipy:a new El Dorado for fluorescence tools. New J.Chem. 31 (2007) 496–501. DOI:10.1039/b617972j |
| [16] | A. Bessette, G.S Hanan. Design, synthesis and photophysical studies of dipyrromethene-based materials:insights into their applications in organic photovoltaic devices. Chem.Soc.Rev. 43 (2014) 3342–3405. DOI:10.1039/c3cs60411j |
| [17] | B.M. Squeo, N. Gasparini, T. Ameri, et al., Ultra low band gap α, β-unsubstituted BODIPY-based copolymer synthesized by palladium catalyzed cross-coupling polymerization for near infrared organic photovoltaics. J.Mater.Chem.A 3 (2015) 16279–16286. DOI:10.1039/C5TA04229A |
| [18] | X.F. Zhang, Y.D. Zhang, L.C. Chen, Y Xiao. Star-shaped carbazole-based BODIPY derivatives with improved hole transportation and near-infrared absorption for small-molecule organic solar cells with high open-circuit voltages. RSC Adv. 5 (2015) 32283–32289. DOI:10.1039/C5RA02414E |
| [19] | L. G. Xiao, H. D. Wang, K. Gao, et al. , A-D-A type small molecules based on boron dipyrromethene for solution-processed organic solar cells, Chem. Asian J. 10 (2015)1513-1518. |
| [20] | W.X. Liu, J.N. Yao, C.L Zhan. Performance enhancement of BODIPY dimer-based small-molecule solar cells using a visible-photon-capturing diketopyrrolopyrrole P-bridge. RSC Adv. 5 (2015) 74238–74241. DOI:10.1039/C5RA16725F |
| [21] | W.X. Liu, A.L. Tang, J.W. Chen, et al., Photocurrent enhancement of BODIPY-based solution-processed small-molecule solar cells by dimerization via the meso position. ACS Appl.Mater.Interfaces 6 (2014) 22496–22505. DOI:10.1021/am506585u |
| [22] | W.H. He, Y.B. Jiang, Y Qin. Synthesis and photovoltaic properties of a low bandgap BODIPY? Pt conjugated polymer. Polym.Chem. 5 (2014) 1298–1304. DOI:10.1039/C3PY01396K |
| [23] | S. P. Economopoulos, C. L. Chochos, H. A. Ioannidou, et al. , Novel BODIPY-based conjugated polymers donors for organic photovoltaic applications, RSC Adv. 3 (2013)10221-10229. |
| [24] | T. Bura, N. Leclerc, S. Fall, et al., High-performance solution-processed solar cells and ambipolar behavior in organic field-effect transistors with thienyl-BODIPY scaffoldings. J.Am.Chem.Soc. 134 (2012) 17404–17407. DOI:10.1021/ja3072513 |
| [25] | H.Y. Lin, W.C. Huang, Y.C. Chen, et al., BODIPY dyes with b-conjugation and their applications for high-efficiency inverted small molecule solar cells. Chem.Commun. 48 (2012) 8913–8915. DOI:10.1039/c2cc34286c |
| [26] | B. Kim, B. W. Ma, V. R. Donuru, H. Y. Liu, J. M. J. Fréchet, Bodipy-backboned polymers as electron donor in bulk heterojunction solar cells, Chem. Commun. 46(2010)4148-4150. |
| [27] | T. Rousseau, A. Cravino, T. Bura, et al., BODIPY derivatives as donor materials for bulk heterojunction solar cells. Chem.Commun. 167 (2009) 1673–1675. |
| [28] | A.M. Poe, A.M.D. Pelle, A.V. Subrahmanyam, et al., Small molecule BODIPY dyes as non-fullerene acceptors in bulk heterojunction organic photovoltaics. Chem.Commun. 50 (2014) 2913–2915. DOI:10.1039/c3cc49648a |
| [29] | H.L. Kee, C. Kirmaier, L.H. Yu, et al., Structural control of the photodynamics of boron-dipyrrin complexes. J.Phys.Chem.B 109 (2005) 20433–20443. DOI:10.1021/jp0525078 |
| [30] | J.H. Huang, C.L. Zhan, X. Zhang, et al., Solution-processed DPP-based small molecule that gives high photovoltaic efficiency with judicious device optimization. ACS Appl.Mater.Interfaces 5 (2013) 2033–2039. DOI:10.1021/am302896u |
| [31] | Y. Hayashi, N. Obata, M. Tamaru, et al., Facile synthesis of biphenyl-fused BODIPY and its property. Org.Lett. 14 (2012) 866–869. DOI:10.1021/ol2033916 |
| [32] | Z.B. Henson, G.C. Welch, der Poll T.van, G.C Bazan. Pyridalthiadiazole-based narrow band gap chromophores. J.Am.Chem.Soc. 134 (2012) 3766–3779. DOI:10.1021/ja209331y |
| [33] | Y. Y. Liang, Z. Xu, J. B. Xia, et al. , For the bright futureâ€"bulk heterojunction polymer solar cells with power conversion efficiency of 7. 4%, Adv. Mater 22 (2010) E135-E138. |
| [34] | T.S. Qin, W. Zajaczkowski, W. Pisula, et al., Tailored donor? acceptor polymers with an A-D1-A-D2 structure:controlling intermolecular interactions to enable enhanced polymer photovoltaic devices. J.Am.Chem.Soc 136 (2014) 6049–6055. DOI:10.1021/ja500935d |
| [35] | B. Jiang, X. Zhang, C.L. Zhan, et al., Benzodithiophene bridged dimeric perylene diimide amphiphiles as efficient solution-processed non-fullerene small molecules. Polym.Chem. 4 (2013) 4631–4638. DOI:10.1039/c3py00457k |
| [36] | W. H. Wu, H. M. Guo, W. T. Wu, S. M. Ji, J. Z. Zhao, Organic triplet sensitizer library derived from a single chromophore (BODIPY) with long-lived triplet excited state for triplet?triplet annihilation based upconversion, J. Org. Chem. 76 (2011)7056-7064. |
| [37] | L.J. Jiao, W.D. Pang, J.Y. Zhou, et al., Regioselective stepwise bromination of boron dipyrromethene (BODIPY) dyes. J.Org.Chem. 76 (2011) 9988–9996. DOI:10.1021/jo201754m |
 2017, Vol. 28
2017, Vol. 28